INTRODUCTION
Salmonella enterica subspecies enterica serovar Enteritidis (SE) has been a major causative agent of bacterial foodborne diseases through consumption of contaminated shell eggs and egg products for decades [Reference St Louis1–Reference Reporter4]. Increase in the number of human cases of egg-borne SE infection has led not only to on-farm SE intervention strategies by egg/poultry producers but also an increase in recommendations to consumers for the handling and eating of eggs and egg products. Consumers have been advised to avoid eating raw or undercooked eggs and homemade foods containing raw eggs, such as Caesar salad, eggnog, mayonnaise, and ice cream [Reference Steiner5, Reference Buckner6]. Boiling and refrigerating eggs are included in the recommendations for consumers: SE does not survive in liquid egg at 64°C for 1 min [Reference Humphrey7] and SE does not grow in the egg or egg yolk when stored below 10°C [Reference Kim8, Reference Humphrey9]. The Code of Practice for Lion Eggs [10], mandated that eggs be kept at an even temperature below 20°C on the farm, on vehicles during distribution from farms to egg packing centres, at packing centres, and at retail stores, catering premises and in the home. Thus, temperature control is considered of major importance in preventing the bacterial growth inside shell eggs and, in turn, minimizing the incidence of human salmonellosis via SE-contaminated eggs.
The Code of Practice for Lion Eggs also requires the egg packing centres to label a maximum ‘best-before’ date of no more than 21 days from packing, within a maximum ‘life of lay’ plus 27 days, on the shell and on the package. In Japan, the Japan Egg Dealers Association provides the standard maximum best-before date (17, 27 and 50 days for summer, spring/autumn and winter, respectively) based on the formula given by a previous study in the United Kingdom [Reference Humphrey11]. Egg producers then determine their own maximum best-before dates (for consumption of raw eggs) as about 2–3 weeks after egg collection. However, since the climate and environment are different between the United Kingdom and Japan, this calculation might not be appropriate to the situation in Japan.
The present study is to determine whether the above best-before date is suitable to the situation in Japan. Since the albumen is contaminated with SE more frequently than the yolk [Reference Humphrey11–Reference Shivaprasad13], the intact shell eggs were inoculated with SE into the perivitelline or thin albumen from the air cell. The inoculated eggs were then stored at 10, 20, or 30°C for 6 weeks, and examined for SE each week. Another study attempted to examine the inoculated eggs for SE during 10 weeks' storage at changing temperatures that mimic a field situation in Japan.
MATERIALS AND METHODS
Bacterial strain and culture condition
Frozen stock of SE HY-1 strain with rifampicin-resistance (SE HY-1rif) was plated onto DHL agar (Eiken, Tokyo, Japan) plates supplemented with 100 μg/ml rifampicin (DHL-rif). Several colonies were suspended in a heart infusion broth (Eiken), and SE HY-1rif was grown to mid-logarithmic phase with vigorous shaking for 6 h. The fresh culture of bacteria was then washed by centrifugation at 7000 g for 10 min twice, and suspended in the same volume of phosphate-buffered saline (PBS). For preparation of inoculum, the suspension was diluted to the desired concentration (<50 c.f.u./0·1 ml) in PBS as estimated by the optical density at 600 nm. Actual bacterial counts in the inoculum were determined retrospectively by colony counts of serial tenfold dilutions that were spread on DHL-rif plates.
Eggs
Fresh eggs were obtained from a local commercial layer farm, where the reared hens were not vaccinated with any commercial SE vaccines. Average size of eggs was 60 mm in height and 43 mm in width, weighing about 60 g.
Growth of SE in eggs during 6 weeks' storage at defined temperatures
A total of 360 fresh eggs were inoculated with 0·1 ml SE HY-1 rif suspension. The eggs were placed with the air-cell upwards, and the shell surface was disinfected with iodine and 70% ethanol. A hole was made in the shell of each egg with 18-gauge needle, and 0·1 ml of bacterial suspension was inoculated slowly into albumen through the air-cell membrane with a 23-gauge needle. The depth of inoculation was 8–11 mm from the egg shell as determined by several preliminary experiments, so that the inoculum reached the albumen without rupturing the vitelline membrane. The holes in the shell were filled with paraffin, and the eggs were equally divided into three groups (120 eggs each) and kept at 10, 20, or 30°C for 6 weeks. Twenty eggs were randomly taken from each group each week, and the viable counts of SE inside the eggs were determined by the most probable number (MPN) estimation. Briefly, the egg surface was disinfected as described, and the contents separated aseptically from the egg shells were homogenized in separate sterile plastic bags. Ten, 1·0, and 0·1 ml from each homogenate were tenfold diluted in 90 ml, 9 ml, and 0·9 ml, respectively, of trypticase soy broth containing 50 μg/ml rifampicin in triplicate, and incubated for 24 h at 37°C. A loopful of each broth was then plated onto DHL-rif and trypticase soy agar plates. After incubation for 24 h at 37°C, the resultant colonies were confirmed as SE by serological examinations using commercial diagnostic antiserum (Denka Seiken, Tokyo, Japan) for O9-antigen of SE. The viable counts of SE were enumerated based on a MPN table, and translated to c.f.u./egg (average weight of egg contents was 50 g). Assuming the heavily increased bacterial numbers, the original serial dilutions of the egg contents in trypticase soy broth were also plated onto DHL-rif plates, and the viable counts were also enumerated. This study was repeated twice: the initial inocula were 20 and 47 c.f.u./egg, and the depth of inoculation was 8 mm and 11 mm, respectively, for each set of experiments.
Growth of SE in eggs under conditions simulating the field situation
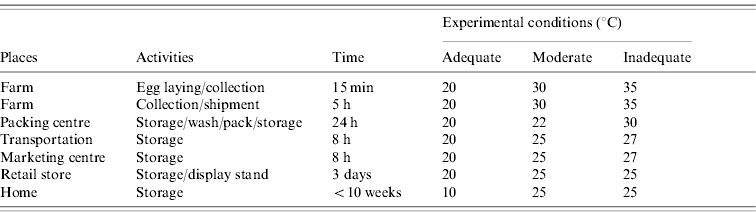
A total of 600 eggs were inoculated with SE HY-1 (27 c.f.u./egg) at a depth of 11 mm as described, and stored for 10 weeks at the three different conditions with changing temperatures set up according to the information obtained from egg-producing farms. The conditions mimicked two typical (adequate and inadequate) situations in Japan, and a moderate situation (Table). For the first 5 days of the experiment the inoculated eggs were exposed to the changing temperatures. An adequate temperature set (20°C) was incorporated for the cases simulating egg-producing farm-associated inline packaging facilities and for the winter season. On the other hand, an inadequate temperature set (25–35°C) was incorporated for simulating cases for the summer season. In these cases, eggs were assumed to be held under a low (adequate) or atmospheric (inadequate) temperature condition during transportation. Under the moderate condition, the temperature was changed to the range of 22–30°C, which is in between the adequate and inadequate conditions. After storage at the above changing temperatures, the eggs were then stored at 10°C (adequate) or 25°C (moderate and inadequate) for the rest of the experiment. A set of 20 eggs stored under each condition was taken each week and the egg contents were tested for bacterial growth as described above.
Table. Experimental conditions that simulate environmental temperatures for egg storage from farm to table
RESULTS
When 20 (=101·30) c.f.u. of SE were inoculated into perivitelline albumen (8-mm depth from the egg shell), the majority of eggs kept at 10°C and 20°C showed a slight increase in the viable counts of SE (<102 c.f.u./egg) after storage for 1 week, but the bacterial numbers tended to decrease over 5 weeks (Fig. 1 a, b). No eggs contained >103 c.f.u. during 6 weeks of storage at 10°C and 20°C. In contrast, storage at 30°C resulted in a rapid increase in bacterial numbers in eggs. The viable counts reached 102–103 c.f.u./egg in 50% of the eggs at 1 week, and the number of eggs with >106 c.f.u./egg consistently increased over 6 weeks (Fig. 1 c). The distribution of viable counts in eggs at this temperature diverged into two groups at 6 weeks: 11 eggs contained >1010 c.f.u./egg, and nine eggs were without SE. Mean SE growth at the three different temperatures is shown in Figure 1 d, which indicates that SE growth rapidly developed from 3 weeks when stored at 30°C whereas SE did not increase in the eggs that were stored at either 10°C or 20°C. Similar results were obtained from the repeat trial with inoculation of 47 (=101·67) c.f.u. into the albumen at 11-mm depth from the egg shell, except for one egg each with the higher number of SE (105 and 109 c.f.u./egg) at 5 and 3 weeks of storage at 10°C and 20°C, respectively (data not shown). After the viable counts reached about 103 c.f.u./egg in about 50% of the eggs at 1 week's storage at 30°C (data not shown), a constant increase in the number of eggs with >106 c.f.u./egg was observed. Finally, eight eggs contained >109 c.f.u./egg, while no SE was found in 12 eggs at 6 weeks.

Fig. 1. The fate of Salmonella enterica serovar Enteritidis (SE) during egg storage at (a) 10°C, (b) 20°C and (c) 30°C for 6 weeks. Each plot indicates the number of SE in each egg. A plot at 0 week indicates an initial inoculum dose. Averaged SE counts for each temperature are summarized in (d).
In the second experiment with changing temperatures, the bacterial counts were generally higher than those in the first experiment. When the eggs inoculated with 27 (=101·43) c.f.u. were kept at the adequate temperature (fluctuating between 10°C and 20°C), the bacterial counts increased up to <103 c.f.u./egg within 1 week, and apparently decreased gradually over 9 weeks (Fig. 2 a, d). However, two or three eggs routinely contained 106–1010 c.f.u. at 2–9 weeks. At the moderate temperature (fluctuating between 22°C and 30°C), the eggs with a greater than tenfold increase in the bacterial counts (>270 c.f.u./egg) increased from 8–10 eggs each in the first 3 weeks to 15–20 eggs in the following 7 weeks (Fig. 2 b). In these eggs, 2–5 eggs in the first 3 weeks and 15–18 eggs in the following 7 weeks contained >106 c.f.u./egg. The inadequate temperature (fluctuating between 25°C and 35°C) also caused a much higher increase in the bacterial counts than in the adequate condition, and similar to or slightly greater than the results of storage at the moderate condition. Ten to14 eggs in 1–3 weeks and 17–20 eggs in 4–10 weeks contained >270 c.f.u./egg (Fig. 2 c), of which 0–11 and 9–20 eggs, respectively, contained >106 c.f.u./egg. Mean numbers of SE in the inoculated eggs stored under the three different conditions are summarized in Figure 2 d, which also shows that the bacterial counts began to decrease under the adequate condition and to increase under the moderate and inadequate conditions at 3 weeks' storage.

Fig. 2. The fate of Salmonella enterica serovar Enteritidis (SE) during egg storage at (a) adequate, (b) moderate and (c) inadequate temperature for 10 weeks (see Table for temperature and times). Each plot indicates the number of SE in each egg. A plot at 0 week indicates an initial inoculum dose. Averaged SE counts for each temperature are summarized in (d).
DISCUSSION
The frequency of SE egg contamination is extremely low, which is reportedly <0·1% [Reference Humphrey11]. However, the risk for Japanese individuals to become infected is considered to be very high, since they consumed 330 eggs (including raw eggs) per person annually in 2004, which is second only to Mexico (341 eggs/person), according to the International Egg Commission. To reduce the risk, the control measure should focus on not only the prevention of SE infection in laying hens but also correct egg-handling by producers and consumers. For the latter purpose, the present study was conducted to provide the data that helped to establish the Japanese version of the Lion Quality Code of Practice.
One of the major factors responsible for the risk is the number of bacteria in the contaminated shell eggs. The SE counts were generally <10 in the contaminated eggs that were sampled immediately after laying [Reference Humphrey14], and <20 in the eggs sampled within 3 weeks after laying [Reference Humphrey and Whitehead15]. To investigate the bacterial growth in intact shell eggs, a number of studies have used eggs artificially inoculated with SE bacteria. Cogan et al.[Reference Cogan16] concluded that the SE growth profile in whole eggs (about 2 c.f.u./egg of SE was inoculated into the albumen) resembled that in naturally contaminated eggs. They also found that uninoculated eggs should occur in 9% of eggs inoculated with 2 c.f.u. and this percentage became smaller (<1%) when the eggs were inoculated with ⩾25 c.f.u. To ensure that all eggs were inoculated with viable bacteria, we employed inocula of 10–50 c.f.u./egg (20, 47, and 27 c.f.u./egg for three trials) in the present study. This allowed us to assume the worst-case scenarios as well as the actual occurrences in interpreting the results.
The temperature during egg storage is also an important factor that has been studied by many researchers. Interestingly, in the present study, the bacterial counts in the inoculated eggs tended to decrease over the experimental period after a slight increase from the inoculation doses to 102−103 c.f.u./egg at 1 week, when kept at 10°C or 20°C over 6 weeks or at the adequate temperature over 10 weeks (Figs 1a, b, d and 2a, d). This decrease of SE in inoculated eggs has not been described in the earlier studies. Humphrey [Reference Humphrey and Saeed17] showed that an exponential growth of SE eventually occurred in an increasing proportion of eggs after a lag phase with little or no change in the numbers of SE for 3–4 weeks' storage at 20°C following inoculation with <10 c.f.u. In another study by that group using naturally contaminated eggs, bacterial growth was observed after 3 weeks' storage at 20°C [Reference Humphrey11]. Kim et al. [Reference Kim8] also reported that SE in eggs inoculated into the albumen with <102 c.f.u. increased up to >108 c.f.u. after 20 days' storage at 21°C. In addition, the bacterial growth was observed in eggs stored under the moderate condition (fluctuating between 22°C and 30°C for 5 days, then fixed at 25°C) but not in the eggs stored at 20°C throughout the experiment in the present study (Figs 1b and 2b). Therefore, the temperature between 20°C and 25°C might make the results variable, depending on the source of eggs (differences in flock lines), the age of eggs after laying, the inoculum dose, and the bacterial strains used (for example, different susceptibility to an iron-restricted environment, such as albumen). On the other hand, the observed bacterial growth in the eggs stored at 30°C could be explained by the decreased integrity of the vitelline membrane. Humphrey et al. [Reference Humphrey and Whitehead15] found that the fluctuation of temperature between 18°C and 30°C affects the integrity of the vitelline membrane rapidly, which accelerates a leakage of the yolk contents, and in turn causes a rapid bacterial growth in the albumen after 6–10 days. Chen et al. [Reference Chen, Shallo Thesmar and Kerr18] also reported that egg storage at 4°C or 10°C might retard the ageing process of the eggs and maintain the integrity of vitelline membrane, which could inhibit the bacterial growth in the albumen of eggs with initial inocula of 102, 104, and 106 c.f.u./egg.
The position in the albumen for inoculation of SE has been reported to be important. Humphrey et al. [Reference Humphrey11] used eggs that were >2 weeks old, and found that inoculation of SE near the vitelline membrane caused good bacterial growth within 5 days, while no growth was found in eggs receiving SE deposited at the outer edge of the albumen or at a point equidistant between that point and the vitelline membrane, after incubation at ambient temperature. In the present study, where the eggs used were fresh, although we used different inoculation depths (8–11 mm) to compare the eventual bacterial growth between inoculations into perivitelline albumen and thin albumen, similar results were obtained in the trials. One egg each with >105 c.f.u. of SE observed in the replicated experiment (11-mm depth inoculation) with egg storage at 10°C and 20°C might be inoculated with SE into the yolk through the vitelline membrane, depending on the position of egg yolk in the shell eggs [Reference Clay and Board19]. Therefore, distance of the site of SE inoculum from the vitelline membrane might not influence the bacterial growth as much in fresh shell eggs, but seems to be important with regard to the probability of directly contaminating the yolk.
In the second experiment where the eggs were stored at adequate, moderate or inadequate conditions for 10 weeks, the increasing numbers of SE in the inoculated eggs were observed similarly between the eggs stored under the moderate and inadequate conditions (Fig. 2b–d). Since the eggs were stored at 25°C over 9 weeks, after the first 5 days at changing temperatures in these conditions, the long storage at 25°C seems to be the major factor that led to the bacterial growth. On the other hand, the population of eggs with >106 SE was larger under the adequate condition (Fig. 2 a) than at 10°C (Fig. 1a). Since the possibility of unexpected inoculation into the yolk by a 9-mm depth inoculation in this experiment is considered lower than that by an 11-mm depth inoculation in the first experiment, the more rapid increase of SE in the eggs kept at the changing temperatures than at the fixed temperatures is suggested to be not due to the inoculation into the yolk, but due to other unknown factor(s).
The results of the present study indicate that the best-before date of eggs, calculated based on data from the United Kingdom, is still considered suitable for the situation in Japan. Egg storage under an appropriate temperature can control the SE growth inside the contaminated eggs, although complete agreement with the results in the United Kingdom was not obtained. Egg storage at 10°C or even 20°C can prevent an exponential SE growth in the contaminated eggs for at least 6 weeks, which agrees with the implementation of the Lion Quality Code of Practice for eggs. Shell eggs were generally kept in the refrigerator after purchase at retailers in Japan and the United States, but, to our knowledge, not in the United Kingdom. However, this is not completely correlated with the fact that the number of SE isolates in the last decade in Japan showed an 83·0% decrease from 3830 to 653 [20, 21], which appears more effective than that in the United States (50·9% decrease from 10210 to 5012) [22] and the United Kingdom (62·7% from 17880 to 6677) [23]. Therefore, to further decrease the incidence of human salmonellosis derived from SE-contaminated eggs, temperature during the storage and transportation of eggs from farms to retailers should also be taken into consideration.
ACKNOWLEDGEMENTS
The authors appreciate the critical review of the manuscript by Dr Peter S. Holt of the Egg Safety and Quality Research Unit, USDA-ARS, Athens, Georgia.
DECLARATION OF INTEREST
None.







