INTRODUCTION
Transmission of hepatitis B virus (HBV) occurs through percutaneous or permucosal exposure to infective body fluids. Infection can resolve spontaneously or become chronic with sequelae including cirrhosis and liver cancer. A safe and effective vaccine to prevent HBV infection has been available since 1982. In countries where the HBV prevalence is high (⩾8% chronically infected), most infections are acquired perinatally or in childhood [Reference Grosheide and Van Damme1]. In contrast, in countries where the prevalence of HBV is low, most infections are acquired in adult life [Reference Haks, Bosman and van de Laar2–Reference Gay4]. In The Netherlands, the incidence and prevalence of HBV infection is very low, and >75% of infections with information are reported to have been acquired through sexual contact [Reference Haks, Bosman and van de Laar2]. HBV vaccination in The Netherlands is based upon selective vaccination of individuals at high risk of infection. Examining the epidemiology of HBV contributes to the evaluation of the effectiveness of this strategy.
Routine reporting of acute HBV infections is of limited value to gain insight in the epidemiology of HBV infection. First, for about one third of reports the route of transmission is not reported. This percentage is remarkably constant across countries with different reporting systems [Reference Haks, Bosman and van de Laar2, Reference Ramsay5, Reference Goldstein6] and may reflect limitations of all reporting systems or acquisition by unnoticed exposures. Second, the choice of the most likely transmission route by those reporting is based on their knowledge of risk factors and their assumed hierarchy when several are present. Last, since among adults heterosexual contact is almost ubiquitous as well as a known route of transmission of HBV, it could mask other transmission routes.
Therefore, analytical epidemiological studies are necessary to investigate transmission of HBV. Since the incidence of HBV is very low in The Netherlands, cohort studies are impracticable. We have conducted a population-based case-control study, aiming to describe routes of transmission of HBV within the Dutch population in order to inform vaccination policy.
MATERIALS AND METHODS
We carried out a population-based, frequency-matched case-control study. A case was defined as symptoms and serology for HBsAg and HBcIgM compatible with acute HBV infection in a resident in The Netherlands. Cases notified between May 1999 and July 2000 as part of the routine Dutch notification system for communicable diseases were included.
Controls were selected from the general population through random sampling. Men and persons aged 20–39 years were oversampled (200% and 175% respectively) in order to obtain a match with expected frequencies among cases.
Written informed consent was obtained from all participants. The study was approved by the Medical Ethics Committee of The Netherlands Organisation for Applied Scientific Research.
Data collection
Information on demographics, occupation, travel, parenteral exposures and sexual partners were collected through a self-administered questionnaire. For participants aged <12 years, parents were asked to complete the questionnaire. Ethical permission was not granted to ask questions on sexual partnerships to persons aged <18 years of age. Persons who did not agree to participate in the study were asked to complete a non-response form.
More detailed questions on sexual behaviour were asked in a second questionnaire, to all cases aged >17 years and to a sample of controls aged >17 years. For cases, the second questionnaire was administered by a public health nurse, whereas controls received the second questionnaire by mail. The sample of controls who were sent the second questionnaire included all men who have (had) sex with men (MSM); all persons with a partner of non-Dutch nationality; all persons with multiple partners in the previous 6 months; and a 12% sample of all persons with at least one sexual contact during the previous 6 months. Questions in the second questionnaire included: the number of partners in the previous 6 months [and for each of the most recent three partners in this period the type of partner (casual or steady)]; sex; age; country of birth of partner; condom use (never, sometimes, mostly, always); country where sexual contact took place; injecting drug use (IDU) and commercial sex work of partner; and other sexual contacts of the partner in the last year. All exposure questions for cases and controls referred to the period 6 months prior to the date of diagnosis or of completing the questionnaire, respectively. In order to account for potential seasonal variation of exposure to risk factors for HBV infection, the mailing of questionnaires to controls was divided in four mailings of each 1800 questionnaires, in September and December 1999, and March and June 2000.
Parenteral exposures were grouped into three categories: medical (having undergone one or more of the following: injection, biopsy, operation, wound suture, renal dialysis, blood transfusion, phlebotomy); other (acupuncture, needle-stick injury, contact with another person's blood, a human bite, tattooing, piercing) and possible parenteral exposures (manicure, pedicure, beauty parlour treatment, borrowing of a toothbrush or razor, hairdresser visit abroad). Any parenteral exposure was defined as presence of a parenteral exposure as defined above.
Countries were grouped according to the prevalence of HBsAg in low (<2%), medium (2–8%) or high (>8%) endemicity [7].
Sample size calculation
It was expected that about 200 cases of acute HBV per year would be eligible to take part in the study [Reference Veldhuijzen8]. We calculated the number of controls needed in each subgroup of sexual behaviour in order to be able to detect an odds ratio (OR) of ⩾3 for a risk factor with a prevalence of 10%, with an α of 0·05 and a power of 0·80. Subsequently, we estimated what percentage of controls, after oversampling of males and persons between 20 and 39 years, would belong to each subgroup. Finally, estimating the non-response to the first (general) and second (sexual history) questionnaire to be 1/3 and 1/6, respectively, we calculated that we needed to recruit 7200 controls.
Analysis
We used stata statistical software, version 8.1 (StataCorp, College Station, TX, USA), except for calculating P values for trend, which was done in Epi-Info (CDC/WHO, version 6.04 d). Effects of exposures on risk of acute HBV were estimated by the OR and its 95% confidence interval (CI).
To adjust for oversampling of men and those aged 20–39 among controls, weights were used in all univariate analysis. Cases were assigned a weight of ‘1’. For controls, weights were chosen such that the age group and sex distribution among controls would fit that of the Dutch population in 2000 (http://statline.cbs.nl/). To adjust for oversampling individuals with a partner of non-Dutch nationality and/or multiple partners among controls invited to fill out the second questionnaire, a second weighing factor was introduced. Its value was calculated such that after weighing the frequency of those with a non-Dutch and/or multiple partners among those completing the second questionnaire matched this frequency among controls who completed the first questionnaire.
The effect of gender and sexual preference was examined in all adults (>17 years). The effect of other determinants was examined separately for three groups: adult women and heterosexual adult men, adult MSM, and children (participants aged <18 years).
Subsequently, we fitted multiple logistic regression models separately for these three groups to estimate effects of determinants adjusted for confounding. Prior to building the model, we classified potential determinants of infection hierarchically into groups ranging from distal to proximal to the outcome (HBV infection), as outlined in Figures 1 and 2. Distal determinants might have confounded more proximal ones, whilst the reverse is not the case. The building of the model consisted of as many steps as there were hierarchical levels. In the first model, only distal level determinants were added, so their effect could be estimated without inappropriately adjusting for the mediating effect of determinants situated at a more proximal level. In the next model, the determinants in the more proximal level were added, so that their effect could be estimated adjusting for the confounding effect of the distal determinants, but not for the mediating effect of determinants situated at a more proximal level [Reference Victora9]. Only determinants which had a P value of <0·1 in the univariate analysis were included, and these variables were kept in all subsequent models, irrespective of significance levels. Age group and gender were included in all models so that weights adjusting for these variables did not have to be taken into account. For adult women and heterosexual adult men the second weighing factor was taken into account in those models where variables ascertained by the second questionnaire were included.
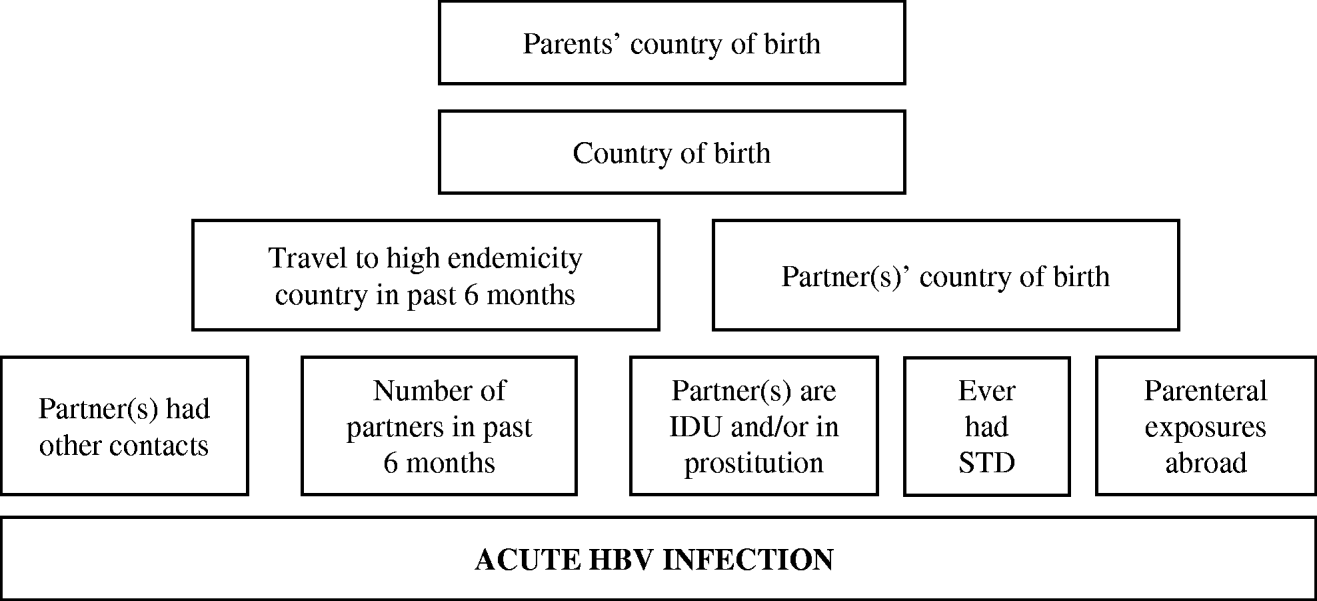
Fig. 1. Conceptual hierarchical framework for risk factors for acquisition of HBV infection among adult females and adult heterosexual men in The Netherlands.
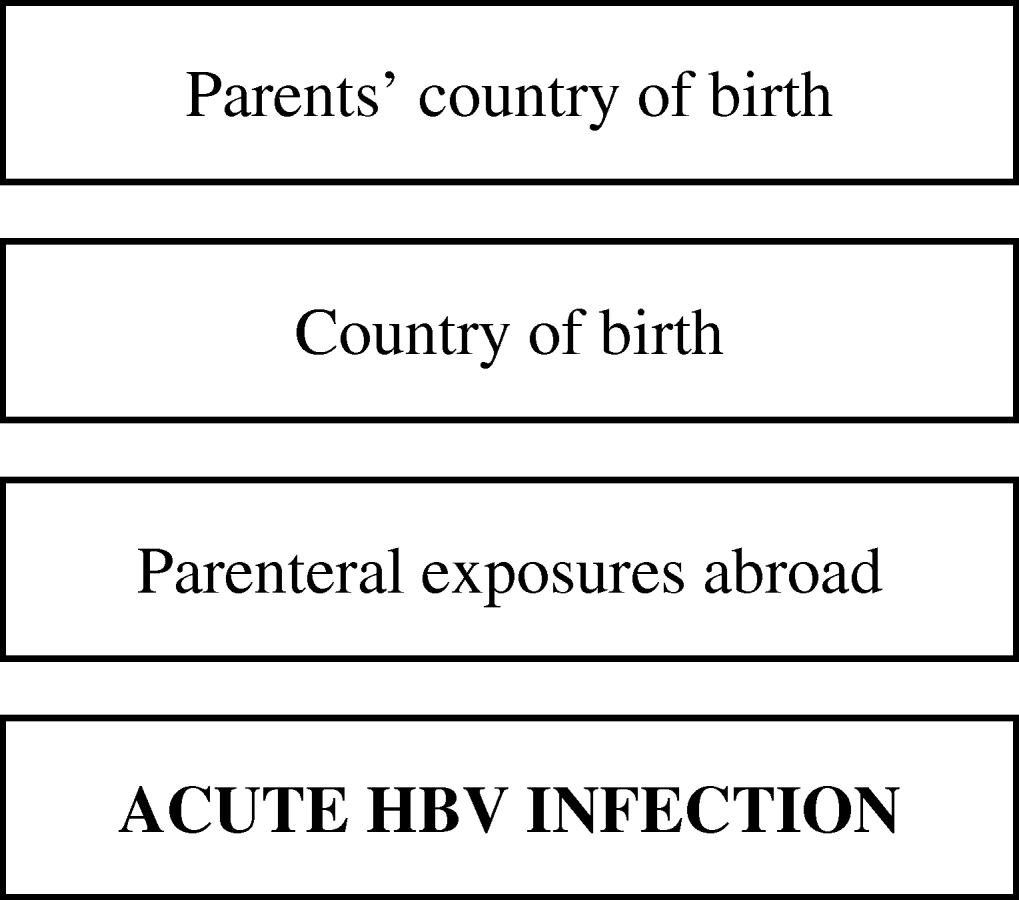
Fig. 2. Conceptual hierarchical framework for risk factors for acquisition of HBV infection among individuals aged <18 years in The Netherlands.
RESULTS
Response
Cases
During the study period, 289 notifications of acute HBV infection were received. Of these, 51 did not meet the case-definition. Of the 238 remaining patients, 120 (50%) agreed to participate by completing the questionnaire and being interviewed. For 20 patients a non-response form was received.
Controls
Of 7200 initial questionnaires sent, 4353 were returned (response rate 61%). Of these, 405 (9%) were excluded, since they were not at risk for HBV infection due to HBV immunization or infection in the past, leaving 3948 controls. Of these, 2554 were adults with at least one sexual contact in the 6 months prior to completing the questionnaire. Of these, 574 controls were sent the second questionnaire, of which 491 were returned (response rate, second questionnaire 86%). Of these, 186 were female (38%), 289 were males reporting heterosexual contact only (59%), and 16 were MSM (3%).
Overall analysis
The risk of acute HBV infection was increased in individuals aged >17 years compared to younger individuals, with the highest risk in those aged between 18 and 39 years (Table 1). The risk of HBV infection was increased among males compared to females, with particularly high risks among MSM (OR 145·6, 95% CI 77·5–273·8).
Table 1. Distribution of cases of acute HBV infection and controls with corresponding odds ratios (OR) and 95% confidence intervals (CI)
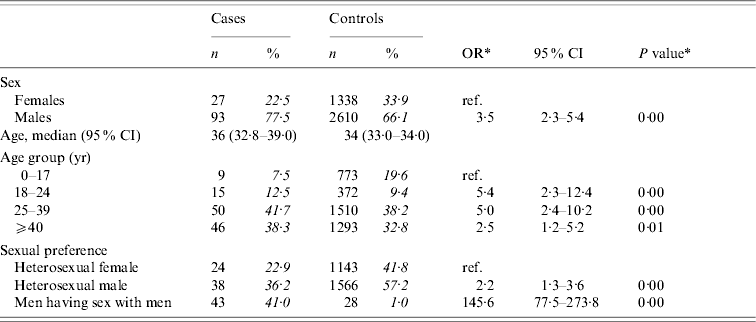
The analyses included 120 cases and 3948 controls. Percentages were calculated after excluding individuals with missing information.
* Odds ratios and P values were calculated by weighted analyses, taking into account oversampling of men and those aged 20–39 years among controls.
Subgroup analyses
Group 1: Adult women and heterosexual adult men
Univariate analyses
Table 2(a) presents crude ORs for exposures grouped as ‘demographic’, ‘parenteral’, and ‘sexual’ among adult men reporting only heterosexual contact and adult females. Significantly associated with the risk of acute HBV infection were: being male, being aged between 18 and 24 years, having at least one parent born in a medium or high endemicity country; being born in a medium or high endemicity country; having had any parenteral exposure in a medium or high endemicity country; having had more than one partner in the past 6 months; having a non-Dutch partner; having a partner who was involved in IDU or commercial sex work; having a partner who had other partners; and having had a sexually transmitted disease (STD) in the past 5 years. IDU or commercial sex work was not reported among cases.
Table 2(a). Cases of acute HBV infection in females aged >17 years, and in males aged >17 years reporting heterosexual contact only: distribution of demographic characteristics with corresponding odds ratios (OR) and 95% confidence intervals (CI)
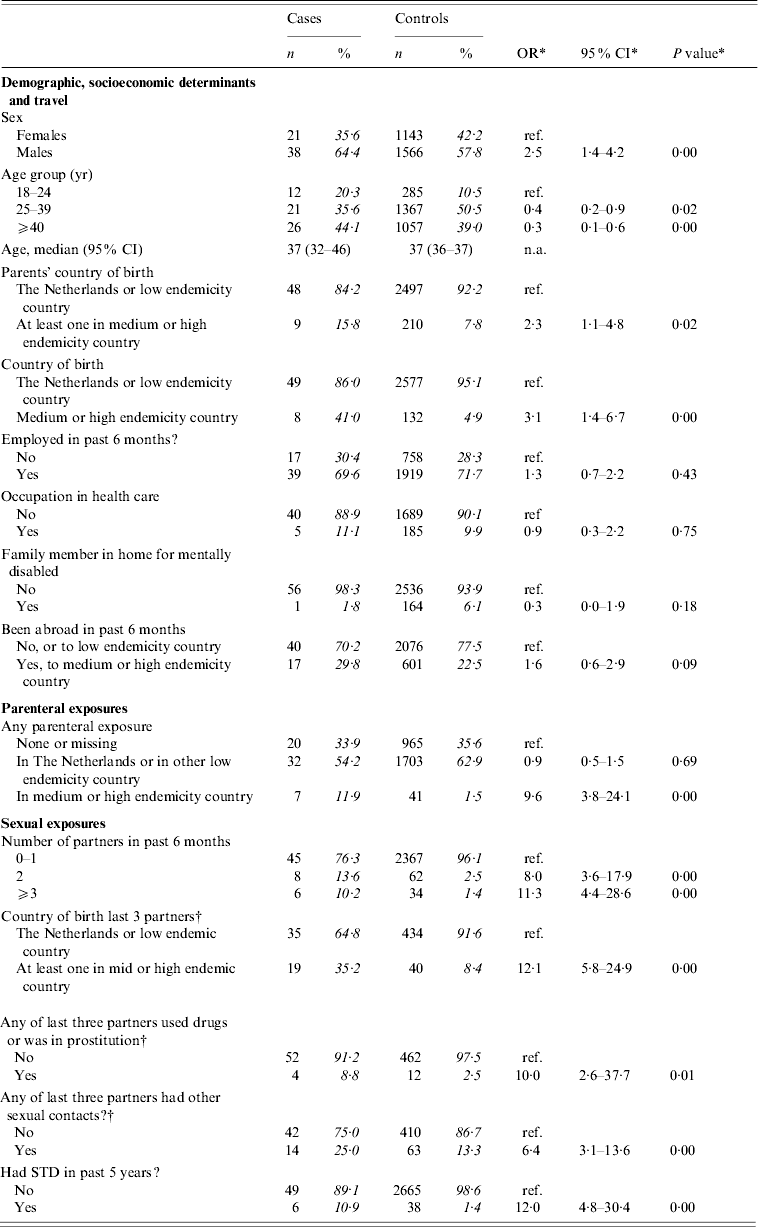
The analyses included 59 cases and 2709 controls. Percentages were calculated after exclusion of individuals with missing information.
* Odds ratios, confidence interval and P values were calculated by taking into account weights in order to adjust for oversampling of men and those aged 20–39 years among controls.
† Analyses included 474 controls. ORs were calculated by taking into account weights to adjust for oversampling among controls of men, those aged 20–39 years, those with a partner of non-Dutch nationality and/or with multiple partners.
Multivariate analyses
Figure 1 shows the conceptual hierarchical framework for adult female and heterosexual male cases, built with determinants which were associated (P<0·1) with the risk of acute HBV on univariate analysis. Table 2(b) lists the ORs resulting from the multivariate analyses. Model 1 indicated that descending from at least one parent born in a medium or high endemicity area for HBV is a significant risk factor for acquisition of HBV. However, the strength of this association decreases and becomes insignificant when ‘country of birth’ is added in a second model. In the third model, country of birth of partners and travel in the past 6 months were added. This showed that having a partner born in a medium or high endemicity country was a highly significant risk factor. Travel was not significant. In this model, the OR for country of birth decreased (from 3·6 to 0·7). On separate testing, it was found that this was largely explained by adding the country of birth of partners (results not shown). In the fourth model, the number of partners, risk behaviour of partners and parenteral exposures in medium or high endemicity countries was added. Only having two partners in the past 6 months was significant. In this model, the OR for having a partner from an endemic area fell to 8·9. On separate testing, this fall could largely be explained by adding the variables number of partners and having had an STD in the past (results not shown).
Table 2(b). Cases of acute HBV infection in females aged >17 years, and in males aged >17 years reporting heterosexual contact only: results of multivariate analyses
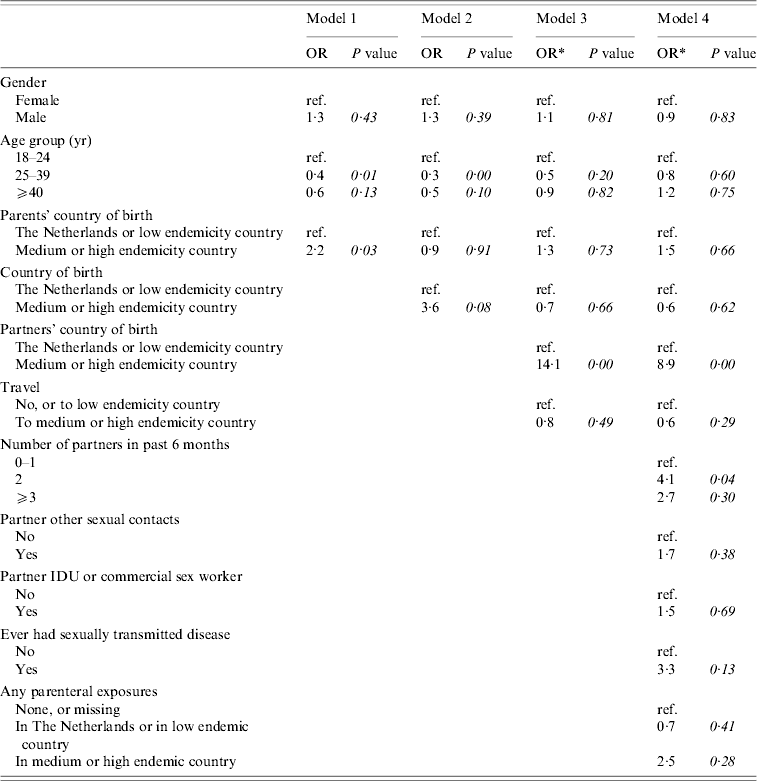
Models 1 and 2 included 57 cases and 2707 controls. Models 3 and 4 included 53 cases and 473 controls. Percentages were calculated after excluding individuals with missing information.
* Odds ratios are calculated after taking into account weights to adjust for oversampling of those with a partner of non-Dutch nationality and one or multiple partners among controls.
Group 2: MSM
Univariate analyses
Table 3 presents crude ORs for exposures grouped as ‘demographic’, ‘parenteral’, and ‘sexual’ among adult MSM. The only significant risk factor for acquisition of HBV was reporting to have had more than two partners in the past 6 months. No multivariate analysis was done.
Table 3. Cases of acute HBV infection in men who reported to have (had) sex with men (MSM): distribution of demographic characteristics with corresponding odds ratios (OR) and 95% confidence intervals (CI)
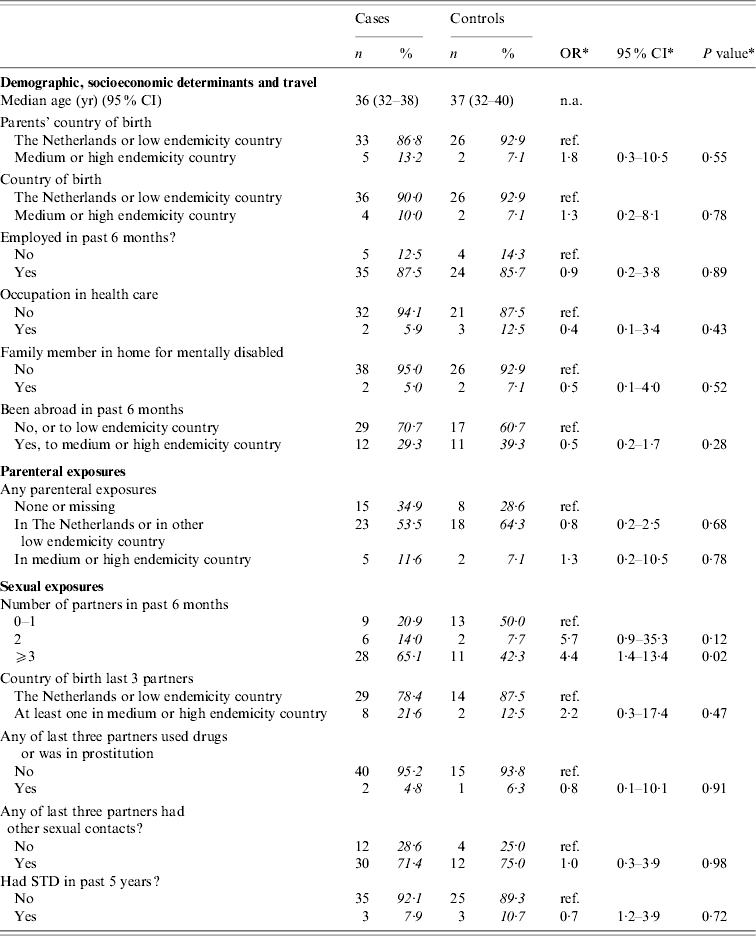
The analyses included 43 cases and 28 controls. Percentages were calculated after exclusion of individuals with missing information (phase 2 questions only available for 16 controls).
* Odds ratios, confidence interval and P values were calculated by taking into account weights in order to adjust for oversampling of men and those aged 20–39 years among controls.
Group 3: Children
Univariate analyses
There were nine cases and 773 controls aged <18 years of age. Three of the cases reported heterosexual contact as the most likely route of transmission. Information on sexual contact was not available for controls. Table 4(a) presents crude ORs for demographic and parenteral exposures for those aged <18 years. Having at least one parent born in a medium or high endemicity country, being born in such a country, and parenteral exposure in such a country were associated with infection. Travel to a medium or high endemicity country was no more frequent among cases than among controls.
Table 4(a). Cases of acute HBV infection in children: distribution of demographic characteristics with corresponding odds ratios (OR) and 95% confidence intervals (CI)
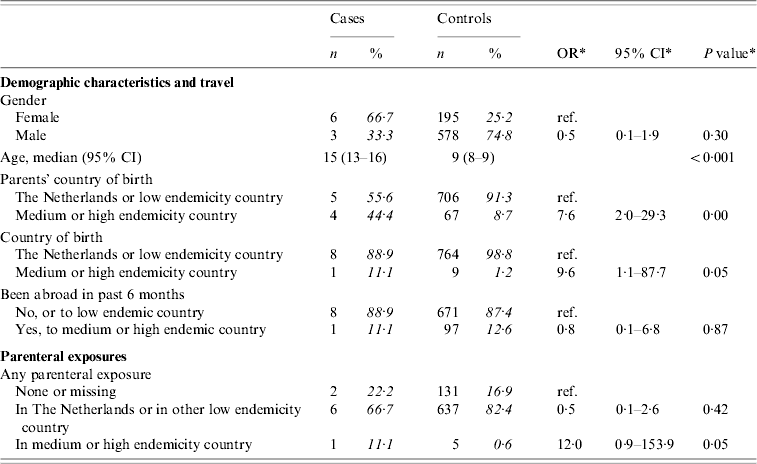
The analyses included nine cases and 773 controls. Percentages were calculated after exclusion of individuals with missing information.
* Odds ratios, confidence interval and P values were calculated by taking into account weights in order to adjust for oversampling of men and those aged 20–39 years among controls.
Multivariate analyses
Figure 2 shows the conceptual hierarchical framework for participants aged <18 years, built with determinants which were associated with the risk of acquisition of HBV on univariate analysis (P<0·1). Table 4(b) lists the ORs resulting from the multivariate analyses. Model 1, including ‘age’, ‘gender’ and ‘parents’ country of birth’, showed that having one or both parents born in a medium or high endemicity country significantly increased the risk of acquisition of HBV. The second model added ‘country of birth’, which was no longer significant. A subsequent model added parenteral exposures abroad. This made the OR for ‘parents’ country of birth’ decrease, suggesting that part of the effect of the country of birth of the parents is explained by an increased frequency of parenteral exposures in medium or high endemicity countries in children with parents born in a HBV-endemic country compared to those with parents born in a non-HBV endemic country.
Table 4(b). Cases of acute HBV infection in children: results of multivariate analyses
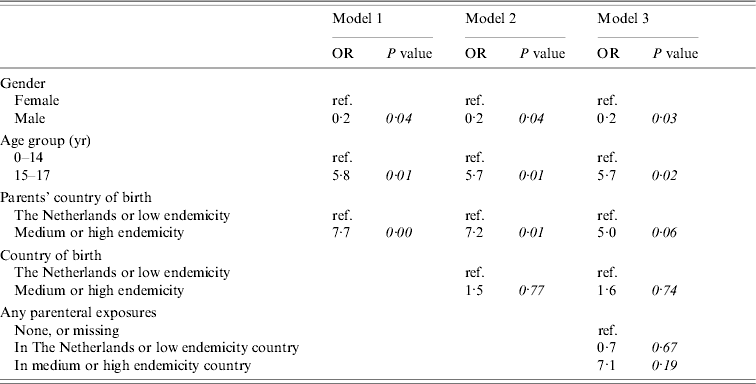
Models 1, 2 and 3 included nine cases and 773 controls.
DISCUSSION
To describe transmission of HBV, case-series are of limited value. However, in countries such as The Netherlands where the incidence of HBV infection is very low, analytical studies are difficult to perform due to the extreme range in prevalence of risk factors for transmission. Our study is the first reported population-based case-control study on risk factors for acute HBV infection in a country with a very low incidence. By including over 30 controls per case, and oversampling of known risk groups among controls, we attempted to get precise estimates of risks associated with both frequent and infrequent determinants.
The response rate among cases was 50%, and those responding may not represent all cases of acute HBV infection. However, the age and sex distribution did not differ between responders and non-responders [Reference Veldhuijzen, Smits and van de Laar10].
We conclude that the most important route of transmission of HBV in The Netherlands is through male homosexual contact: this was reported for over half of male cases in our study, and was strongly associated with infection. The number of partners in the past 6 months was the only risk factor identified among MSM. In particular, among MSM, import of HBV through partners from HBV endemic countries does not seem to play a role. This suggests that HBV transmission is sustained among MSM in The Netherlands, which is consistent with the results of a recent mathematical model of HBV transmission in The Netherlands [Reference Kretzschmar11]. This model also predicted that heterosexual transmission without import of new cases is not sufficient for ongoing transmission of HBV in the Dutch population [Reference Kretzschmar11]. Our data is also consistent with this: we found that the most important risk factor among heterosexual adults for acquisition of HBV is to have a partner born in a medium or high endemicity country.
Using a hierarchical method of analysis allowed us to estimate risks of HBV infection associated with having parents born abroad and being born abroad without controlling for mediating factors such as travel abroad. Due to temporal associations these mediating factors can not confound the relation between (parents') country of birth and HBV infection, and therefore should not be controlled for. Similarly, whereas the country of birth of parents can be a confounder in assessing the risk associated with country of birth, the reciprocal is not the case.
The second advantage of using a hierarchical method of analysis is that it allowed us to demonstrate that the increased risk of HBV infection among heterosexual adults associated with having parents born in a medium or highly endemic country, and being born in such a country, is explained largely through the increased likelihood of these individuals to have a partner born in an endemic country. This strongly suggests that the main route of transmission in Dutch heterosexual adults is through sexual rather than, for example, household contact.
None of our cases reported IDU or commercial sex work, suggesting that direct transmission through these routes is infrequent in The Netherlands. An alternative explanation for this observation is that IDUs and commercial sex workers may have been less likely to take part in the study, and, if taking part, may have been reluctant to admit to it. However, the high HBV seroprevalence among IDUs in The Netherlands (past infection 35–67%, carriers 4–7%) indicates low levels of susceptibility [Reference Veldhuijzen8], which is consistent with our findings. Recently published results of molecular analyses of cases of acute HBV in Amsterdam suggests that the IDU cluster disappeared after 1998 [Reference van Houdt12]. Having a partner who was involved in IDU and/or commercial sex work was a significant risk factor on univariate analysis among heterosexuals, suggesting that transmission through contact with IDUs and/or commercial sex workers is occurring.
We had few cases among children, since the majority of HBV infections in children remain asymptomatic and therefore were not included. The most important risk factor among children was to have (one or two) parents born in a medium or high endemicity country, and part of this risk was explained by a higher frequency of parenteral exposures in medium or high endemicity areas. Since there was only one case in a child with any parenteral exposures abroad, further exploration of this exposure was not possible. Some of the risk remained after including parenteral exposures abroad in the model, and excluding the childhood cases reported to have occurred through sexual contact, suggesting that other routes of transmission remain in childhood.
Implications for control of HBV infection through vaccination
In The Netherlands, at the time of our study, HBV immunization was offered to health-care workers, individuals with certain chronic diseases, contacts of HBV carriers and babies born to infected mothers (identified by universal antenatal screening). Subsequent to our study, from autumn 2002 onwards, additional target groups have been identified, including MSM, IDUs, heterosexuals attending STI clinics and commercial sex workers. Furthermore, from March 2003 onwards, all children born to one or two parents born in intermediate or high endemicity countries, as well as all asylum seekers aged <18 years, are included in the target groups for immunization [13, Reference Waldhober and Heijnen14]. We found that MSM are at high risk of contracting HBV, and contribute to over one third of all cases. This suggests that the current Dutch programme which provides free vaccine for all MSM is appropriate [Reference Waldhober and Heijnen14]. Preventing infections in MSM has the potential to be highly effective since it would introduce a herd-immunity effect [Reference Kretzschmar11]. Our study suggests that all MSM (irrespective of additional risk factors such as casual partnerships) are at increased risk of HBV infection. Indeed, having had an STD in the past 5 years was not a significant risk factor for HBV infection among MSM. This implies that delivering vaccine only through municipal health services and STD clinics may not be sufficient, and that active outreach may be necessary. At the moment only some regions in The Netherlands provide this [Reference Waldhober and Heijnen14].
Our study identified children born to parents born in medium or high endemicity countries as a high-risk group for HBV infection. However, for adult heterosexuals with parents born abroad, this risk largely arises as a result of the increased likelihood of having partnerships with non-Dutch nationals: of the nine adult heterosexual cases whose parents were born abroad, seven had a partner born abroad and six were born abroad themselves. When sexual mixing with non-Dutch nationals becomes less determined by (parent's) country of birth, the effectiveness of targeting vaccination based on the latter will decrease. Part of the increased risk of having a partner born in an endemic country was explained by sexual risk behaviour. Vaccination targeted at those with sexual risk behaviour might therefore prevent some cases among those with a foreign partner.
Among heterosexual adults, only commercial sex workers and those attending an STD clinic are offered HBV vaccine free of charge. In our case-series, complete implementation of this policy would have prevented only 11% (6/55) of cases in heterosexuals. Immunization of all heterosexuals with partners of non-Dutch nationality would have prevented 36% (20/55) of cases in heterosexuals. The effectiveness of such a programme would, however, depend on whether vaccination can be given early enough to prevent transmission. Screening of individuals born in medium and high endemicity areas, and subsequent immunization of contacts of identified carriers, may be a more effective strategy to prevent heterosexual transmission of HBV. In addition, this may allow early treatment of carriers, helping to reduce the occurrence of sequelae. However, further information is necessary on feasibility, ethical issues and cost-effectiveness of such screening programmes.
ACKNOWLEDGEMENTS
This study was funded by the Dutch Ministry of Health, Welfare and Sport. The authors thank the participating municipal health services, and Anita Watzeels and Karien van Rozendaal for their help in the general organization of the study.
DECLARATION OF INTEREST
None.












