To the Editor—Carbapenem-resistant Enterobacteriaceae (CRE) have become a global public health threat.Reference Duin and Doi 1 Although Klebsiella pneumoniae carbapenemase (KPC)–producing Klebsiella pneumoniae have been highlighted as the most prevalent CRE agent in most nosocomial infections, Enterobacter cloacae complex has been characterized as a second major pathogen in most surveillance studies presenting limited treatment options and high mortality.Reference Wilson, El Chakhtoura and Patel 2 , Reference Hargreaves, Shaw and Dobbins 3
The most common carbapenem-resistant associated mechanism is carbapenemase production. The bla KPC-2 gene occurs most predominantly in Brazil, whereas the bla KPC-3 and bla OXA-48 are most predominant in the United States.Reference Hargreaves, Shaw and Dobbins 3 – Reference Lyman, Walters, Lonsway, Rasheed, Limbago and Kallen 5 However, extended-spectrum β-lactamases (ESBLs), ampC β-lactamase overproduction, and decreased outer membrane protein expression combined with an active efflux pump may also result in a similar phenotype, particularly when ertapenem is used as a marker for carbapenem-resistance.Reference Pecora, Li and Allard 6
Enterobacter cloacae complex was the second most prevalent CRE following far behind KPC-producing K. pneumoniae, and the major discrepancies between them have been described in a previous study.Reference Perez 7 However, the impact of this phenotype on in vitro activity of other drugs has not been evaluated. Therefore, we conducted an analysis of E. cloacae complex isolates from inpatients to assess the impact of the “carbapenem-resistance profile,” using ertapenem (ETP) as a marker, on the in vitro potency of other 10 antimicrobial agents.
Enterobacter cloacae complex isolates were recovered from inpatients between January 1 and December 26, 2016, at a tertiary-care hospital in Porto Alegre, Southern Brazil. Bacterial identification was made using the MicroScan automated system (Beckman Coulter, Brea, CA). Testing for susceptibility to amikacin (AK), cefepime (FEP), ceftazidime (CAZ), ceftriaxone (CRO), ciprofloxacin (CIP), gentamicin (CN), meropenem (MEM), piperacillin/tazobactam (TZP) and sulfametoxazol/trimethoprim (SXT) was performed by disk diffusion. The polymyxin B minimum inhibitory concentrations (MICs) were determined by broth microdilution and were interpreted according to EUCAST break points for colistin (≤2 mg/L and >2 mg/L for susceptibility and resistance, respectively). 8 To attribute the resistance mechanism for the selected E. cloacae complex isolates confirmed to have reduced susceptibility to ETP, a synergistic test was applied using phenyl-boronic acid to detect KPC or using an enzymatic inhibition testing with clavulanic acid and cloxacillin to detect ESBLs and ampC enzymes, in that order, as reported elsewhere.Reference Perez 4
A total of 145 isolates, recovered from distinct clinical specimens, were identified as E. cloacae complex during the study period. This sample represents 4.8% (145 of 3028) of all Enterobacteriaceae isolates identified from inpatients in this period. Of these 145 isolates, 48 (33.1%) and 97 (66.9%) were resistant and susceptible to ETP, respectively. The antimicrobial susceptibility profiles presented by ETP-resistant and ETP-susceptible isolates are shown in Figure 1. Meropenem and AK were the most active agents among ETP-resistant (89.6% of susceptibility for both) and ETP-susceptible isolates (100% and 92.8% of susceptibility, respectively). High resistance rates to CAZ (100%; 48 of 48), to CRO (100%; 48 of 48), to TZP (81.2%; 39 of 48), to STX (79.2%; 19 of 24), to FEP (66.7%; 32 of 48), and to CIP (66.7%; 32 of 48) were observed among the ETP-resistant group (Figure 1). In contrast, resistance rates of only ~20% were found to the same agents among ETP-susceptible group. No MEM resistance was observed among these later.
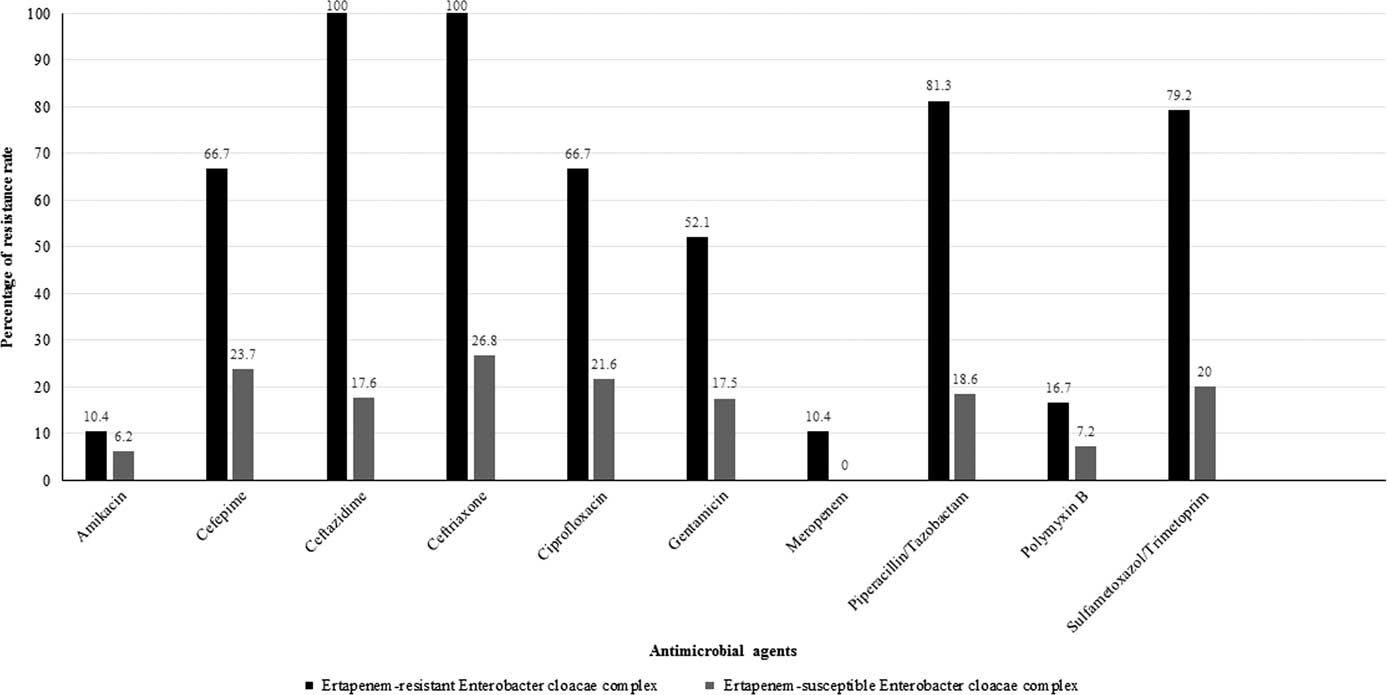
FIGURE 1 Antimicrobial resistance rates for the 145 Enterobacter cloacae complex isolates evaluated in this survey.
In this survey, no carbapenemase producer was found. All isolates were blue-carba test negative and/or gave negative results when EDTA or phenyl-boronic acid were applied. Notably, the inhibition of ampC enzymes using cloxacillin, among ETP-resistant isolates, was not able to bring antibiotics such as ETP, TZP and FEP up to the susceptibility level, which confirms an overlap of associated mechanisms (data not shown).
Meropenem resistance was observed only in 5 of 48 ETP-resistant isolates (10.4%). However, resistance to polymyxin B was observed in both groups: 16.7% (8 of 48) and 7.2% (7 of 97) of ETP-resistant and ETP-susceptible isolates.
Because therapeutics against CRE are scarce and the emergence of carbapenem-resistance mechanisms is a concern, all available options that still show some degree of susceptibility should be strictly monitored. Amikacin still showed a low resistance rate among E. cloacae complex; however, only in some specific site-related infections has their effectiveness been proven. An important matter, as previously reported, is polymyxin B resistance, which seems to be in an increasing trend, and is particularly associated with selection pressure related to higher use in clinical practice.Reference Perez 7 , Reference Rodrigues Perez and Dias 9
In this survey, E. cloacae complex was the second most prevalent CRE, with an extremely low rate compared to KPC-producing K. pneumoniae (9.8% vs 81.4%). However, E. cloacae complex may contain genotypes with epidemic potential associated with increasing rates of antimicrobial resistance, which justifies strict monitoring. As expected, β-lactam agents suffered the most reduction in their susceptibility rates. Furthermore, marked reductions were also observed for CIP, AK, CN, STX, and PMB (Figure 1).
In conclusion, special attention must be focused on the widespread resistance of KPC producers, which has important repercussions in Brazilian hospitals. However, little is known about the resistance (in particular, to polymyxin B) among Enterobacter cloacae complex isolates. Although this study did not include molecular characterization and emerging genotypes, measures of infection control and prevention of spreading are mandatory for this pathogen, especially when worrisome resistance (eg, to polymyxins) is detected.
ACKNOWLEDGMENTS
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: The author reports no conflicts of interest relevant to this article.





