1. Introduction
Homeostasis is the ability of an organism to maintain stable physico-chemical conditions that are compatible with cell metabolism despite fluctuations in the external environment. Plants are subject to permanent changes in the availability of water, nutrients and light energy as well as other parameters, such as temperature, which influence the entire metabolism. Plants are a semi-open system in which the concentrations of apoplastic fluid vary and depend on environmental parameters, in particular, the water potential of the soil and the concentration of dissolved substances, on the one hand, and on internal factors, such as stomatal conductance, on the other. Ion homeostasis is central to plant physiology as it regulates and maintains cell turgor, energises and controls nutrient flux (especially of sugars and amino acids) and is at the heart of numerous signalling pathways dependent on Ca2+ and H+. Each individual plant cell contributes to ion homeostasis by maintaining the concentrations of free ions in the syncytium and tissues that are optimal for growth and efficient ion-dependent signalling. Given the importance of ion homeostasis, understanding the mechanisms involved is crucial for improving crop yields and for developing strategies to cope with environmental stresses such as drought and nutrient deficiency.
Ion homeostasis involves the transport and sequestration or release into or out of various compartments such as apoplast, cytosol, vacuole, mitochondria, plastids, endocytic and exocytic membrane complexes. All these membranes have their own specific transporters and associated regulatory factors (regulatory proteins, voltage, pH, pCa, etc.) that form a highly complex system. Before approaching such complexity, a first step towards understanding the principles of ion homeostasis in plants can be a theoretical approach, looking at the simplest possible system and searching for emergent properties. Over the last four to five decades, our knowledge of plant membrane transport has improved considerably thanks to the development of new techniques and molecular genomic analyses (Anschütz et al., Reference Anschütz, Becker and Shabala2014; Blatt, Reference Blatt2024; Hedrich, Reference Hedrich2012; Jegla et al., Reference Jegla, Busey and Assmann2019; Stanton et al., Reference Stanton, Sanders, Krämer and Podar2022; Ward et al., Reference Ward, Mäser and Schroeder2009) and physiological data that enable such a theoretical approach. Nevertheless, membrane transport in plants is still widely analysed using more than 70 years old concepts. For instance, the classification of transporters as ‘high-affinity’ and ‘low-affinity’ transporters was very helpful in the 1950s and 1960s in identifying different transporters in vivo and distinguishing between them (Epstein et al., Reference Epstein, Rains and Elzam1963; Epstein & Hagen, Reference Epstein and Hagen1952). However, the categorisation into these two groups and the interpretation that transporters with ‘high affinity’ are active at low concentrations, while transporters with ‘low affinity’ take over at higher concentrations, led to unclear conclusions and even contradictions (for further details, please see Dreyer, Reference Dreyer2017; Dreyer & Michard, Reference Dreyer and Michard2020). It became evident that there is an urgent need for a new solid theoretical foundation that not only describes the observed phenomena, but can also clearly explain them based on first principles. One such approach resulted in the theory of homeostats (Dreyer, Reference Dreyer2021a), that is, transporter networks that act together and exhibit dynamic properties as a system that go beyond those of the isolated transporters (Dreyer et al., Reference Dreyer, Li, Riedelsberger, Hedrich, Konrad and Michard2022; Li et al., Reference Li, Grauschopf, Hedrich, Dreyer and Konrad2024). This unbiased, systemic approach combines thermodynamics with biophysics and translates biological phenomena into the language of mathematics, enabling the derivation of analytical solutions or computational simulations of specific situations.

Figure 1. The K homeostat. The K homeostat is a network of transporters that transport K+, in combination with the energising proton ATPase. It consists of H+ ATPases (pump), H+/K+ antiporters (H/Ka), H+/K+ symporters (H/Ks) and K+ channels (KC), which are embedded in a membrane that separates an internal compartment (inside) from an external compartment (outside). The system is determined by 12 parameters: Three of them (white) are determined by the geometry of the system: internal volume (Vol in), external volume (Vol out) and the membrane capacitance (C). Four of them (grey) describe the activity of the transporters: activity of the pump (act. pump), the antiporter (act. H/Ka), the symporter (act. H/Ks) and the K+ channel (act. KC). Two of them ([H+]in and [H+]out, light yellow) are partially influenced by buffer reactions and transport processes different from the K homeostat. The remaining three ([K+]in, [K+]out and V, yellow) depend on the setting of the K homeostat and are controlled by it.
To increase the understanding and accessibility of such an approach, this article intends to serve as a hands-on tutorial for modelling a homeostat using the example of the potassium (K) homeostat. The transporter network of the K homeostat is composed of K+ channels, H+/K+ symporters, H+/K+ antiporters and H+-ATPases establishing the necessary proton and voltage gradients that energise the different transport processes (Figure 1). The system, in which the K homeostat is embedded, is a membrane that separates two compartments (internal/inside and external/outside) and is characterised by the following parameters (Table 1): volumes of the internal and external compartment (Vol in, Vol out), proton and potassium concentrations in the internal and external compartment ([H+ ]in, [H+ ]out, [K+ ]in, [K+ ]out), the membrane capacitance and membrane voltage (C, V), as well as the activities of the transporters.
Table 1 Parameters of the K homeostat system

Note: The list of parameters determines the system of a K homeostat embedded in a membrane that separates two compartments. Appropriate definitions for the parameters marked with an asterisk (*) will be provided in the course of this study.
By having chosen the K homeostat, we follow a historical pattern, because research on K+ transport in plants has been a constant driving force in the past for progress in membrane transport and has influenced research on nutrient transport in general (Britto et al., Reference Britto, Coskun and Kronzucker2021; Dreyer, Reference Dreyer2021b). We start with explaining the mathematical description of transmembrane transport processes through the different transporters. Then we present the differential equations that describe the changes in concentrations and the membrane voltage due to the transport processes. Thereafter, we analyse the system in a steady state. In a follow-up study, we will consider deviations from the steady state condition in order to get an idea about the dynamic properties of the homeostat. The different steps can in principle be applied to any other homeostat, as recently demonstrated for the auxin homeostat in plants (Geisler & Dreyer, Reference Geisler and Dreyer2024).
2. Methods
2.1 Mathematical description of transport processes
A theoretical/mathematical description of membrane transport requires to quantify the transport processes involved. For this purpose, the biophysical knowledge on transport (Hille, Reference Hille2001) needs to be translated into the universal language of mathematics. Here, only basic knowledge on the membrane transport is often sufficient to obtain rather simple but very powerful equations. The art of mathematical modelling lies in the elimination of redundancies, which means that many physiological parameters can be combined into a few, non-redundant model parameters. As this manuscript will illustrate, such steps simplify both the equations and the computational handling of the model. However, in order to draw conclusions from the model calculations, the model output must be translated from mathematical language to biology. The challenge in understanding and the art of physiological interpretation lies particularly in the fact that independent physiological parameters in biology can be redundant in mathematics. Having this in mind, we may now tackle the question:
How can the net fluxes through a membrane transporter in a steady state be described?
Steady state corresponds to the state in which the system does not change any longer. Indeed, homeostatic conditions are steady state conditions because the system exhibits a certain stability and maintains its parameters constant. The homeostatic properties of a system can be analysed in a two-step process. First, the steady state is analysed and then, in the second step, the system is destabilised in order to investigate its resilience. In this study, we begin with the first step and will present the second in a follow-up paper.

Figure 2. Modelling of fluxes through a transporter in a steady state. (a) The flux curve of a transporter JX
(V) (grey line) is zero at
![]() $V={E}_X^{ss}$
(
$V={E}_X^{ss}$
(
![]() ${J}_X\left({E}_X^{ss}\right)=0$
, blue point). (b) In a steady state, flux and voltage are constant, and the curve is represented by the red point
${J}_X\left({E}_X^{ss}\right)=0$
, blue point). (b) In a steady state, flux and voltage are constant, and the curve is represented by the red point
![]() $\left({V}_{ss}|{J}_X^{ss}\right)$
. The abscissa of this point is Vss
, while the ordinate,
$\left({V}_{ss}|{J}_X^{ss}\right)$
. The abscissa of this point is Vss
, while the ordinate,
![]() ${J}_X^{ss}$
, can be expressed as m ⋅ (
${J}_X^{ss}$
, can be expressed as m ⋅ (
![]() ${V}_{ss}-{E}_X^{ss}$
) using triangulation (m = tan(α)). (c) Changes in the activity are mirrored by changes in the slope m and the angle α. An increase in the transporter activity (dashed grey line), for example, by a higher expression or activation by phosphorylation, results in a larger
${V}_{ss}-{E}_X^{ss}$
) using triangulation (m = tan(α)). (c) Changes in the activity are mirrored by changes in the slope m and the angle α. An increase in the transporter activity (dashed grey line), for example, by a higher expression or activation by phosphorylation, results in a larger
![]() ${J}_X^{ss}$
-value (purple point), which is represented by a larger angle (α1) and hence a larger slope (m1). A decrease in transporter activity (dotted grey line), on the other hand, results in a smaller
${J}_X^{ss}$
-value (purple point), which is represented by a larger angle (α1) and hence a larger slope (m1). A decrease in transporter activity (dotted grey line), on the other hand, results in a smaller
![]() ${J}_X^{ss}$
-value (pink point), smaller angle (α2) and smaller slope (m2). Thus, the slope m contains the regulatory features of the transporter.
${J}_X^{ss}$
-value (pink point), smaller angle (α2) and smaller slope (m2). Thus, the slope m contains the regulatory features of the transporter.
In the beginning, we consider passive or secondary active transporters, that is, membrane proteins that do not couple ATP hydrolysis to transport activity (pumps will be considered later). The flux through a passive transporter (JX
) depends on the electrochemical gradient (
![]() $\Delta \mu$
) of the transported solute across the membrane (Kedem & Katchalsky, Reference Kedem and Katchalsky1963). For a permeating substrate X, this gradient is given by:
$\Delta \mu$
) of the transported solute across the membrane (Kedem & Katchalsky, Reference Kedem and Katchalsky1963). For a permeating substrate X, this gradient is given by:
 $$\begin{align}{\Delta \mu}_X&={\mu}_X^{\mathrm{in}}-{\mu}_X^{\mathrm{out}}= RT\cdot \ln \left({\left[X\right]}_{\mathrm{in}}\right)+{z}_XF{\psi}_{\mathrm{in}}- RT\cdot \ln \left({\left[X\right]}_{\mathrm{out}}\right)\nonumber\\&\quad-{z}_XF{\psi}_{\mathrm{out}}=F\cdot \left({z}_XV-\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)\right),\end{align}$$
$$\begin{align}{\Delta \mu}_X&={\mu}_X^{\mathrm{in}}-{\mu}_X^{\mathrm{out}}= RT\cdot \ln \left({\left[X\right]}_{\mathrm{in}}\right)+{z}_XF{\psi}_{\mathrm{in}}- RT\cdot \ln \left({\left[X\right]}_{\mathrm{out}}\right)\nonumber\\&\quad-{z}_XF{\psi}_{\mathrm{out}}=F\cdot \left({z}_XV-\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)\right),\end{align}$$
with the gas constant R, the Faraday constant F, the absolute Temperature T, the internal and external electrical potentials
![]() ${\psi}_{\mathrm{in}}$
and
${\psi}_{\mathrm{in}}$
and
![]() ${\psi}_{\mathrm{out}}$
(
${\psi}_{\mathrm{out}}$
(
![]() ${\psi}_{\mathrm{in}}-{\psi}_{\mathrm{out}}=V$
, the membrane voltage), the valence zX
and the concentrations [X]in and [X]out, of the substrate X, respectively (Carpaneto et al., Reference Carpaneto, Geiger, Bamberg, Sauer, Fromm and Hedrich2005; Gerber et al., Reference Gerber, Fröhlich, Lichtenberg-Fraté, Shabala, Shabala and Klipp2016; Nour-Eldin et al., Reference Nour-Eldin, Andersen, Burow, Madsen, Jørgensen, Olsen, Dreyer, Hedrich, Geiger and Halkier2012). The net flux of X through the transporter (JX
) is a function of the gradient ΔμX
, that is, the concentrations and, if
${\psi}_{\mathrm{in}}-{\psi}_{\mathrm{out}}=V$
, the membrane voltage), the valence zX
and the concentrations [X]in and [X]out, of the substrate X, respectively (Carpaneto et al., Reference Carpaneto, Geiger, Bamberg, Sauer, Fromm and Hedrich2005; Gerber et al., Reference Gerber, Fröhlich, Lichtenberg-Fraté, Shabala, Shabala and Klipp2016; Nour-Eldin et al., Reference Nour-Eldin, Andersen, Burow, Madsen, Jørgensen, Olsen, Dreyer, Hedrich, Geiger and Halkier2012). The net flux of X through the transporter (JX
) is a function of the gradient ΔμX
, that is, the concentrations and, if
![]() ${z}_X\ne 0$
, also the membrane voltage. If the gradient is zero,
${z}_X\ne 0$
, also the membrane voltage. If the gradient is zero,
![]() $\Delta \mu =0$
, there is no net flux, that is, JX
= 0. Thus, if JX
depends on the membrane voltage, it is zero at
$\Delta \mu =0$
, there is no net flux, that is, JX
= 0. Thus, if JX
depends on the membrane voltage, it is zero at
![]() $V={E}_X$
, with
$V={E}_X$
, with
![]() ${E}_X=\frac{RT}{z_XF}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)$
, also known as the Nernst (or zero-flux) potential for X. Otherwise, it is zero if
${E}_X=\frac{RT}{z_XF}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)$
, also known as the Nernst (or zero-flux) potential for X. Otherwise, it is zero if
![]() ${\left[X\right]}_{\mathrm{out}}={\left[X\right]}_{\mathrm{in}}$
.
${\left[X\right]}_{\mathrm{out}}={\left[X\right]}_{\mathrm{in}}$
.
In the case of a secondary active co-transporter that transports nX molecules/ions of type X (valence zX ) along with nY molecules/ions of type Y (valence zY ), the electrochemical gradient is given by:
 $$\begin{align}&{\Delta \mu}_{XYs}={n}_x\cdot \Delta {\mu}_X+{n}_Y\cdot \Delta {\mu}_Y\\&\quad=F\cdot \left[\left({n}_X{z}_X+{n}_Y{z}_Y\right)\cdot V-{n}_X\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\kern-1.2pt\right)-{n}_Y\frac{RT}{F}\cdot \ln \left(\frac{{\left[Y\right]}_{\mathrm{out}}}{{\left[Y\right]}_{\mathrm{in}}}\kern-1.2pt\right)\kern-1.2pt\right],\nonumber\end{align}$$
$$\begin{align}&{\Delta \mu}_{XYs}={n}_x\cdot \Delta {\mu}_X+{n}_Y\cdot \Delta {\mu}_Y\\&\quad=F\cdot \left[\left({n}_X{z}_X+{n}_Y{z}_Y\right)\cdot V-{n}_X\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\kern-1.2pt\right)-{n}_Y\frac{RT}{F}\cdot \ln \left(\frac{{\left[Y\right]}_{\mathrm{out}}}{{\left[Y\right]}_{\mathrm{in}}}\kern-1.2pt\right)\kern-1.2pt\right],\nonumber\end{align}$$
for a symporter, and by
 $$\begin{align}&{\Delta \mu}_{XYa}={n}_x\cdot \Delta {\mu}_X-{n}_Y\cdot \Delta {\mu}_Y\\&\quad=F\cdot \left[\left({n}_X{z}_X-{n}_Y{z}_Y\right)\cdot V-{n}_X\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)+{n}_Y\frac{RT}{F}\cdot \ln \left(\frac{{\left[Y\right]}_{\mathrm{out}}}{{\left[Y\right]}_{\mathrm{in}}}\right)\right],\nonumber\end{align}$$
$$\begin{align}&{\Delta \mu}_{XYa}={n}_x\cdot \Delta {\mu}_X-{n}_Y\cdot \Delta {\mu}_Y\\&\quad=F\cdot \left[\left({n}_X{z}_X-{n}_Y{z}_Y\right)\cdot V-{n}_X\frac{RT}{F}\cdot \ln \left(\frac{{\left[X\right]}_{\mathrm{out}}}{{\left[X\right]}_{\mathrm{in}}}\right)+{n}_Y\frac{RT}{F}\cdot \ln \left(\frac{{\left[Y\right]}_{\mathrm{out}}}{{\left[Y\right]}_{\mathrm{in}}}\right)\right],\nonumber\end{align}$$
for an antiporter. In the case of symporters, the flux JXY
is zero at
![]() $V={E}_{XYs}$
, with
$V={E}_{XYs}$
, with
![]() ${E}_{XYs}=\frac{n_X}{n_X{z}_X+{n}_Y{z}_Y}\cdot {E}_X+\frac{n_Y}{n_X{z}_X+{n}_Y{z}_Y}\cdot {E}_Y$
. In the case of antiporters, it is zero at
${E}_{XYs}=\frac{n_X}{n_X{z}_X+{n}_Y{z}_Y}\cdot {E}_X+\frac{n_Y}{n_X{z}_X+{n}_Y{z}_Y}\cdot {E}_Y$
. In the case of antiporters, it is zero at
![]() $V={E}_{XYa}$
, with
$V={E}_{XYa}$
, with
![]() ${E}_{XYa}=\frac{n_X}{n_X{z}_X-{n}_Y{z}_Y}\cdot {E}_X-\frac{n_Y}{n_X{z}_X-{n}_Y{z}_Y}\cdot {E}_Y$
, if
${E}_{XYa}=\frac{n_X}{n_X{z}_X-{n}_Y{z}_Y}\cdot {E}_X-\frac{n_Y}{n_X{z}_X-{n}_Y{z}_Y}\cdot {E}_Y$
, if
![]() ${n}_X{z}_X-{n}_Y{z}_Y\ne 0$
; otherwise it is zero if
${n}_X{z}_X-{n}_Y{z}_Y\ne 0$
; otherwise it is zero if
![]() ${n}_X\cdot {E}_X={n}_Y\cdot {E}_Y$
.
${n}_X\cdot {E}_X={n}_Y\cdot {E}_Y$
.
As we will show now, this rudimentary information on Δμ = 0 and J = 0 can be used to mathematically describe the flux in the steady state even without further knowledge of the function JX
(V). The function JX
(V) describes a curve in a 2-dimensional space (Figure 2a). We know from the above consideration that JX
(EX
) = 0 (Figure 2, blue point). This point is strategic because at EX
the direction of the flux changes, for example, if JX
< 0 at V < EX
, then JX
> 0 at V > EX
(Figure 2, grey regions). In the steady state, both the membrane voltage and the flux through the transporter are constant. Thus, in this case, we need to describe just one point of the J
X(V) curve (Figure 2b, red point). For such a description two coordinates are sufficient: One is the membrane voltage in steady state, Vss
, and the other is the flux in steady state,
![]() ${J}_X^{ss}$
. Using triangulation,
${J}_X^{ss}$
. Using triangulation,
![]() ${J}_X^{ss}$
can be replaced by (Figure 2b, orange triangle)
${J}_X^{ss}$
can be replaced by (Figure 2b, orange triangle)
with slope m of magnitude
![]() $m=\tan \left(\alpha \right)$
. Flux values, JX
(V) have the unit s−1, while membrane voltage and EX
have the unit V. Therefore, the slope m has the unit V−1⋅s−1. We can consider it as a free parameter (ranging from zero to infinity), which allows us to reach any point on the vertical green line in Figure 2b. In this way, any JX
(V) curve is represented by
$m=\tan \left(\alpha \right)$
. Flux values, JX
(V) have the unit s−1, while membrane voltage and EX
have the unit V. Therefore, the slope m has the unit V−1⋅s−1. We can consider it as a free parameter (ranging from zero to infinity), which allows us to reach any point on the vertical green line in Figure 2b. In this way, any JX
(V) curve is represented by
![]() ${E}_X^{ss}$
, Vss
and the parameter m, even without knowing the exact shape of JX
(V). m combines several physiologically important parameters because a change in the activity of the transporter protein is reflected by a change of the slope m (i.e. the angle α). An increase in activity, for instance by enhanced expression of the transporter or its activation by posttranslational modifications, results in higher J-values (Figure 2c, dashed grey line, purple point). This is reflected by a larger angle (α1) and a larger slope (m1). Conversely, a reduction in activity (Figure 2c, dotted grey line, pink point) results in smaller J-values, a smaller angle (α2) and a smaller slope (m2). Thus, the parameter m gathers all regulatory features of the respective transporter. It can take on any value in the interval [0,∞). It should be noted, though, that m is generally not linear to biological parameters. The rather simple mathematical description of equation (2) therefore covers the entire range of possibilities without any constraints of approximations.
${E}_X^{ss}$
, Vss
and the parameter m, even without knowing the exact shape of JX
(V). m combines several physiologically important parameters because a change in the activity of the transporter protein is reflected by a change of the slope m (i.e. the angle α). An increase in activity, for instance by enhanced expression of the transporter or its activation by posttranslational modifications, results in higher J-values (Figure 2c, dashed grey line, purple point). This is reflected by a larger angle (α1) and a larger slope (m1). Conversely, a reduction in activity (Figure 2c, dotted grey line, pink point) results in smaller J-values, a smaller angle (α2) and a smaller slope (m2). Thus, the parameter m gathers all regulatory features of the respective transporter. It can take on any value in the interval [0,∞). It should be noted, though, that m is generally not linear to biological parameters. The rather simple mathematical description of equation (2) therefore covers the entire range of possibilities without any constraints of approximations.
To illustrate this approach in practical terms, we develop it in the following, step by step, for (i) K+ channels, (ii) H+/K+ symporters and (iii) H+/K+ antiporters. Finally, we will also introduce a suitable model for the (iv) H+-ATPase that primarily energises all transport processes.
(i) K+ channels
The electrochemical gradient (compare equation (1a)) that determines the flux across K+ channels is given in the case of highly selective K+ channels by:
 $$\begin{align}{\Delta \mu}_{\mathrm{KC}}={\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}= RT\cdot \ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)+F\cdot V,\end{align}$$
$$\begin{align}{\Delta \mu}_{\mathrm{KC}}={\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}= RT\cdot \ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)+F\cdot V,\end{align}$$
with the internal and external K+ concentrations [K+ ]in and [K+ ]out, respectively. This gradient is zero at the zero-flux potential for K+ channels, V = EK , which is the Nernst potential for potassium
 $$\begin{align}{E}_K=\frac{RT}{F}\cdot \ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right).\end{align}$$
$$\begin{align}{E}_K=\frac{RT}{F}\cdot \ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right).\end{align}$$
Thus, at
![]() $V={E}_K$
the function of the flux,
$V={E}_K$
the function of the flux,
![]() ${J}_{K,\mathrm{KC}}(V)$
(unit s−1), has the value
${J}_{K,\mathrm{KC}}(V)$
(unit s−1), has the value
![]() ${J}_{K,\mathrm{KC}}\left({E}_K\right)=0$
. Applying the same considerations that led to equation (2), the flux in steady state through the K+ channel can be described by:
${J}_{K,\mathrm{KC}}\left({E}_K\right)=0$
. Applying the same considerations that led to equation (2), the flux in steady state through the K+ channel can be described by:
The parameter m KC (unit V−1⋅s−1) determines the activity of the channels and comprises all potential regulatory features, such as functional protein expression, post-translational modifications and/or voltage dependence. However, it should be noted again that m KC is in most cases not linear to these biological parameters. The net K+ flux through the K+ channels causes a transmembrane electric current (unit A) given by:
with the elementary (positive) charge e0 (= 1.6 × 10−19 A⋅s).
(ii) H+/K+ symporters
The electrochemical gradient (compare equation (1b)) that determines the flux across a symporter with nS H+/1 K+ stoichiometry is given by:
 $$\begin{align}&{\Delta \mu}_{\mathrm{HKs}}={n}_s\cdot \left({\mu}_H^{\mathrm{in}}-{\mu}_H^{\mathrm{out}}\right)+\left({\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}\right)\\&\quad= RT\cdot \left[{n}_s\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{in}}}{{\left[{H}^{+}\right]}_{\mathrm{out}}}\right)+\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)\right]+F\cdot \left({n}_s+1\right)\cdot V,\nonumber\end{align}$$
$$\begin{align}&{\Delta \mu}_{\mathrm{HKs}}={n}_s\cdot \left({\mu}_H^{\mathrm{in}}-{\mu}_H^{\mathrm{out}}\right)+\left({\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}\right)\\&\quad= RT\cdot \left[{n}_s\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{in}}}{{\left[{H}^{+}\right]}_{\mathrm{out}}}\right)+\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)\right]+F\cdot \left({n}_s+1\right)\cdot V,\nonumber\end{align}$$
with the internal and external proton concentrations [H+ ]in and [H+ ]out, respectively. This gradient is zero at the zero-flux potential V = E HKs with
 $$\begin{align}&\left({n}_s+1\right)\cdot {E}_{\mathrm{HKs}}\\&\quad=\frac{RT}{F}\cdot \left[{n}_s\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{out}}}{{\left[{H}^{+}\right]}_{\mathrm{in}}}\right)+\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right)\right]={n}_s\cdot {E}_H+{E}_K,\nonumber\end{align}$$
$$\begin{align}&\left({n}_s+1\right)\cdot {E}_{\mathrm{HKs}}\\&\quad=\frac{RT}{F}\cdot \left[{n}_s\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{out}}}{{\left[{H}^{+}\right]}_{\mathrm{in}}}\right)+\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right)\right]={n}_s\cdot {E}_H+{E}_K,\nonumber\end{align}$$
and the Nernst potentials for potassium (EK ) and protons (EH ).
Hence, the net fluxes for K+ and protons, respectively, at V = E HKs are J K,HKs(E HKs) =0 and J H,HKs(E HKs) = ns ⋅ J K,HKs(E HKs) = 0. Applying the same considerations that led to equation (2), the fluxes in steady state can be described by:
Also here, the parameter m HKs gathers all regulatory features of the symporter. The transmembrane electric current (unit A) that is caused by the net H+ and K+ fluxes (unit s−1) through the H+/K+ symporters is given by:
(iii) H+/K+ antiporters
The electrochemical gradient (compare equation (1c)) that determines the flux across an antiporter with na H+/1 K+ stoichiometry is given by:
 $$\begin{align}&{\Delta \mu}_{\mathrm{HKa}}={n}_a\cdot \left({\mu}_H^{\mathrm{in}}-{\mu}_H^{\mathrm{out}}\right)-\left({\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}\right)\\&\quad= RT\cdot \left[{n}_a\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{in}}}{{\left[{H}^{+}\right]}_{\mathrm{out}}}\right)-\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)\right]+F\cdot \left({n}_a-1\right)\cdot V.\nonumber\end{align}$$
$$\begin{align}&{\Delta \mu}_{\mathrm{HKa}}={n}_a\cdot \left({\mu}_H^{\mathrm{in}}-{\mu}_H^{\mathrm{out}}\right)-\left({\mu}_K^{\mathrm{in}}-{\mu}_K^{\mathrm{out}}\right)\\&\quad= RT\cdot \left[{n}_a\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{in}}}{{\left[{H}^{+}\right]}_{\mathrm{out}}}\right)-\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{in}}}{{\left[{K}^{+}\right]}_{\mathrm{out}}}\right)\right]+F\cdot \left({n}_a-1\right)\cdot V.\nonumber\end{align}$$
This gradient is zero at E HKa with
 $$\begin{align}&\left({n}_a-1\right)\cdot {E}_{\mathrm{HKa}}\\&\quad=\frac{RT}{F}\cdot \left[{n}_a\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{out}}}{{\left[{H}^{+}\right]}_{\mathrm{in}}}\right)-\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right)\right]={n}_a\cdot {E}_H-{E}_K.\nonumber\end{align}$$
$$\begin{align}&\left({n}_a-1\right)\cdot {E}_{\mathrm{HKa}}\\&\quad=\frac{RT}{F}\cdot \left[{n}_a\cdot \ln \left(\frac{{\left[{H}^{+}\right]}_{\mathrm{out}}}{{\left[{H}^{+}\right]}_{\mathrm{in}}}\right)-\ln \left(\frac{{\left[{K}^{+}\right]}_{\mathrm{out}}}{{\left[{K}^{+}\right]}_{\mathrm{in}}}\right)\right]={n}_a\cdot {E}_H-{E}_K.\nonumber\end{align}$$
If we apply again the same considerations that led to equation (2), the net fluxes for K+ and H+ in steady state can be expressed as:
Here as well, the parameter m HKa combines all regulatory characteristics of the antiporter. The transmembrane electric current (unit A) that is caused by the net H+ and K+ fluxes (unit s−1) through the H+/K+ antiporters is given by:
It is zero, if na = 1. In this case, the antiport would be electroneutral and the flux would not depend on the membrane voltage, but would only be proportional to the difference EH − EK between the Nernst potentials of H+ and K+.
(iv) H+-ATPase
Finally, we consider the proton pump as an active membrane transporter. The derivation of a mathematical description of the pump current of H+-ATPases was presented in detail in the Supplementary Material by Reyer et al. (Reference Reyer, Häßler, Scherzer, Huang, Pedersen, Al-Rascheid, Bamberg, Palmgren, Dreyer, Nagel, Hedrich and Becker2020). The mechanistic model of a pump cycle (Dreyer, Reference Dreyer2017; Reyer et al., Reference Reyer, Häßler, Scherzer, Huang, Pedersen, Al-Rascheid, Bamberg, Palmgren, Dreyer, Nagel, Hedrich and Becker2020) results in a sigmoidal curve for pump currents (unit A) that describes very well the experimental findings (Lohse & Hedrich, Reference Lohse and Hedrich1992):
 $$\begin{align}{I}_{\mathrm{HATPase}}={I}_{\mathrm{Hmax}}\cdot \frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}={I}_{\mathrm{Hmax}}\cdot {i}_p(V),\end{align}$$
$$\begin{align}{I}_{\mathrm{HATPase}}={I}_{\mathrm{Hmax}}\cdot \frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}={I}_{\mathrm{Hmax}}\cdot {i}_p(V),\end{align}$$
with
![]() ${i}_p(V)=\frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}$
. The proton flux mediated by the H+-ATPase (J
HATPase; unit s−1) can be determined by dividing the current by the elementary (positive) charge e0
:
${i}_p(V)=\frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}$
. The proton flux mediated by the H+-ATPase (J
HATPase; unit s−1) can be determined by dividing the current by the elementary (positive) charge e0
:
 $$\begin{align}{J}_{\mathrm{HATPase}}=\frac{I_{\mathrm{HATPase}}}{e_0}&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot \frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}\nonumber\\&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot {i}_p(V).\end{align}$$
$$\begin{align}{J}_{\mathrm{HATPase}}=\frac{I_{\mathrm{HATPase}}}{e_0}&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot \frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left(V-{V}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot V+B\right)}+{e}^{-\left(D\cdot \frac{F}{RT}\cdot V+C\right)}}\nonumber\\&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot {i}_p(V).\end{align}$$
Here, the numerical values of B, C and D are of minor importance for the shape of the curve. Suitable values are B = 5.4, C = 2.5, D = 0.1 (Dreyer, Reference Dreyer2021a). Most important are the parameters I Hmax and V 0,pump. I Hmax determines the maximum pump current and is proportional to the number of active proton pumps in the membrane, while V 0,pump is the voltage at which the pump current is zero [ip (V 0,pump) = 0]. It depends in a complex manner on the cytosolic and apoplastic proton concentrations and the cytosolic ATP, ADP and Pi concentrations (Rienmüller et al., Reference Rienmüller, Dreyer, Schönknecht, Schulz, Schumacher, Nagy, Martinoia, Marten and Hedrich2012). For suitable simulations, in which the energy status of the cell does not change in the considered time interval, V 0,pump can be set and kept constant (e.g. V 0,pump = −200 mV). This value determines the most negative value that the membrane voltage can attain.
2.2 Mathematical description of changes in concentrations and voltage
The proton and K+ fluxes change the concentrations of H+ and K+ on both sides of the membrane. The combined net K+ efflux (JK
= J
K,KC + J
K,KHs + J
K,KHa; unit s−1) increases [K+
]out and decreases [K+
]in, while the combined H+ efflux (JH
= J
HATPase + J
H,KHs + J
H,KHa; unit s−1) increases [H+
]out and decreases [H+
]in. Additionally, the buffer capacities of the internal and external compartments can partially mitigate the changes in proton concentration, described by the general buffer reaction
![]() ${H}^{+}+{Buf}^{-}\rightleftarrows HBuf$
with an anionic base Buf-
and its conjugate acid, HBuf. All these changes are represented in mathematical terms by ordinary differential equations. The changes in K+ concentrations are governed by:
${H}^{+}+{Buf}^{-}\rightleftarrows HBuf$
with an anionic base Buf-
and its conjugate acid, HBuf. All these changes are represented in mathematical terms by ordinary differential equations. The changes in K+ concentrations are governed by:
 $$\begin{align}\frac{d{\left[{K}^{+}\right]}_{\mathrm{in}}}{dt}=\frac{-{J}_K}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot \left[{J}_{K,\mathrm{KC}}+{J}_{K,\mathrm{HKs}}+{J}_{K,\mathrm{HKa}}\right].\end{align}$$
$$\begin{align}\frac{d{\left[{K}^{+}\right]}_{\mathrm{in}}}{dt}=\frac{-{J}_K}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot \left[{J}_{K,\mathrm{KC}}+{J}_{K,\mathrm{HKs}}+{J}_{K,\mathrm{HKa}}\right].\end{align}$$
 $$\begin{align}&\frac{d{\left[{K}^{+}\right]}_{\mathrm{out}}}{dt}=\frac{J_K}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}=\frac{1}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}\cdot \left[{J}_{K,\mathrm{KC}}+{J}_{K,\mathrm{HKs}}+{J}_{K,\mathrm{HKa}}\right].\end{align}$$
$$\begin{align}&\frac{d{\left[{K}^{+}\right]}_{\mathrm{out}}}{dt}=\frac{J_K}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}=\frac{1}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}\cdot \left[{J}_{K,\mathrm{KC}}+{J}_{K,\mathrm{HKs}}+{J}_{K,\mathrm{HKa}}\right].\end{align}$$
Here NA is the Avogadro constant and Vol in and Vol out are the volumes of the compartments on the respective side of the membrane. Changes in proton concentrations are determined by:
 $$\begin{align}&\!\!\!\!\!\!\frac{d{\left[{H}^{+}\right]}_{\mathrm{in}}}{dt}=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot \big\{\left[{J}_{\mathrm{HATPase}}+{J}_{H,\mathrm{HKs}}+{J}_{H,\mathrm{HKa}}\right]\quad\\&\quad-{k}_{v,\mathrm{in}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{in}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{in}}-{K}_{s,\mathrm{in}}\cdot {\left[ HBuf\right]}_{\mathrm{in}}\right)\big\}.\nonumber\end{align}$$
$$\begin{align}&\!\!\!\!\!\!\frac{d{\left[{H}^{+}\right]}_{\mathrm{in}}}{dt}=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot \big\{\left[{J}_{\mathrm{HATPase}}+{J}_{H,\mathrm{HKs}}+{J}_{H,\mathrm{HKa}}\right]\quad\\&\quad-{k}_{v,\mathrm{in}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{in}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{in}}-{K}_{s,\mathrm{in}}\cdot {\left[ HBuf\right]}_{\mathrm{in}}\right)\big\}.\nonumber\end{align}$$
 $$\begin{align}&\!\!\!\!\!\!\frac{d{\left[{H}^{+}\right]}_{\mathrm{out}}}{dt}=\frac{1}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}\cdot \big\{\left[{J}_{\mathrm{HATPase}}+{J}_{H,\mathrm{HKs}}+{J}_{H,\mathrm{HKa}}\right]\quad\\&\quad-{k}_{v,\mathrm{out}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{out}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{out}}-{K}_{s,\mathrm{out}}\cdot {\left[ HBuf\right]}_{\mathrm{out}}\right)\big\}.\nonumber\end{align}$$
$$\begin{align}&\!\!\!\!\!\!\frac{d{\left[{H}^{+}\right]}_{\mathrm{out}}}{dt}=\frac{1}{{\mathrm{Vol}}_{\mathrm{out}}\cdot {N}_A}\cdot \big\{\left[{J}_{\mathrm{HATPase}}+{J}_{H,\mathrm{HKs}}+{J}_{H,\mathrm{HKa}}\right]\quad\\&\quad-{k}_{v,\mathrm{out}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{out}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{out}}-{K}_{s,\mathrm{out}}\cdot {\left[ HBuf\right]}_{\mathrm{out}}\right)\big\}.\nonumber\end{align}$$
Here,
![]() ${K}_{s,\mathrm{in}}={10}^{-{pK}_{s,\mathrm{in}}}$
and
${K}_{s,\mathrm{in}}={10}^{-{pK}_{s,\mathrm{in}}}$
and
![]() ${K}_{s,\mathrm{out}}={10}^{-{pK}_{s,\mathrm{out}}}$
indicate the dissociation constants of the buffer reactions that buffer pH
in and pH
out to pK
s,in and pK
s,out, respectively. The buffer concentrations, in turn, change according to:
${K}_{s,\mathrm{out}}={10}^{-{pK}_{s,\mathrm{out}}}$
indicate the dissociation constants of the buffer reactions that buffer pH
in and pH
out to pK
s,in and pK
s,out, respectively. The buffer concentrations, in turn, change according to:
 $$\begin{align}&\frac{d{\left[{Buf}^{-}\right]}_{\mathrm{in}}}{dt}=-\frac{d{\left[ HBuf\right]}_{\mathrm{in}}}{dt}\\&\quad=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot {k}_{v,\mathrm{in}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{in}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{in}}-{K}_{s,\mathrm{in}}\cdot {\left[ HBuf\right]}_{\mathrm{in}}\right).\nonumber\end{align}$$
$$\begin{align}&\frac{d{\left[{Buf}^{-}\right]}_{\mathrm{in}}}{dt}=-\frac{d{\left[ HBuf\right]}_{\mathrm{in}}}{dt}\\&\quad=\frac{-1}{{\mathrm{Vol}}_{\mathrm{in}}\cdot {N}_A}\cdot {k}_{v,\mathrm{in}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{in}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{in}}-{K}_{s,\mathrm{in}}\cdot {\left[ HBuf\right]}_{\mathrm{in}}\right).\nonumber\end{align}$$
 $$\begin{align}&\frac{d{\left[{Buf}^{-}\right]}_{\mathrm{out}}}{dt}=-\frac{d{\left[ HBuf\right]}_{\mathrm{out}}}{dt}\\&\quad=\frac{-1}{Vol_{\mathrm{out}}\cdot {N}_A}\cdot {k}_{v,\mathrm{out}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{out}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{out}}-{K}_{s,\mathrm{out}}\cdot {\left[ HBuf\right]}_{\mathrm{out}}\right).\nonumber\end{align}$$
$$\begin{align}&\frac{d{\left[{Buf}^{-}\right]}_{\mathrm{out}}}{dt}=-\frac{d{\left[ HBuf\right]}_{\mathrm{out}}}{dt}\\&\quad=\frac{-1}{Vol_{\mathrm{out}}\cdot {N}_A}\cdot {k}_{v,\mathrm{out}}\cdot \left({\left[{H}^{+}\right]}_{\mathrm{out}}\cdot {\left[{Buf}^{-}\right]}_{\mathrm{out}}-{K}_{s,\mathrm{out}}\cdot {\left[ HBuf\right]}_{\mathrm{out}}\right).\nonumber\end{align}$$
Compared to the transport reactions, the buffering reactions take place very quickly, which is reflected in large k v,in and k v,out values. Because of this difference in time scale, the parameters k v,in and k v,out do not need to be known in detail. In simulations, it is sufficient to set them 2–3 magnitudes larger than the other parameters (e.g. k v,[in,out] /(I max/e0 ) = 100 μM−2) to obtain almost instantaneous buffer reactions in comparison to the time-scale of transport.
The net fluxes usually cause a net charge transport across the membrane. This non-zero current provokes a change in the membrane voltage. The membrane is actually comparable to a dielectric in an electric capacitor that separates the charges in the aqueous interior from the aqueous exterior. Charge transport from one side to the other changes the membrane voltage according to:
with the membrane capacitance C (unit F).
2.3 Modelling the steady state with fixed transporters activities
At this point, we have translated Figure 1 into the language of mathematics. For each transporter, we expressed the fluxes and currents in equations (5), (6), (9)–(11) and (14)–(18) with a few parameters. The dynamic properties of the system are determined by equations (19)–(25). In the following, we will solve the differential equations for homeostatic conditions. If the system is considered in a steady state, neither the membrane voltage nor the concentrations change with time, which means that the left sides of equations (19)–(25) are zero. In this condition, the equations (19)–(25) simplify to two non-redundant equations taking also into account that IX = e0 ⋅JX :
All the other equations are either zero or linear combinations of the equations (26) and (27). In the next step, the
![]() ${J}_X^{ss}$
were replaced by the expressions deduced in equations (5), (9), (10), (14), (15) and (18) yielding:
${J}_X^{ss}$
were replaced by the expressions deduced in equations (5), (9), (10), (14), (15) and (18) yielding:
 $$\begin{align}0&={m}_{\mathrm{KC}}\cdot \left({V}_{ss}-{E}_K^{ss}\right)+{m}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad-{m}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
$$\begin{align}0&={m}_{\mathrm{KC}}\cdot \left({V}_{ss}-{E}_K^{ss}\right)+{m}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad-{m}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
 $$\begin{align}0&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot {i}_p\left({V}_{ss}\right)+{n}_s\cdot {m}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad+{n}_a\cdot {m}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
$$\begin{align}0&=\frac{I_{\mathrm{Hmax}}}{e_0}\cdot {i}_p\left({V}_{ss}\right)+{n}_s\cdot {m}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad+{n}_a\cdot {m}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
Although the mathematical parameters mX
can depend on relevant biological parameters such as the K+ and H+ concentrations or V (in a linear and non-linear way), they are constant in the steady state and are therefore fixed numbers with units. Then, we multiplied both equations with the factor
![]() ${e}_0/{I}_{\mathrm{Hmax}}$
and define
${e}_0/{I}_{\mathrm{Hmax}}$
and define
![]() ${{g}_X:= {m}_X\cdot {e}_0/{I}_{\mathrm{Hmax}}}$
(unit V−1). This operation eliminated a redundant parameter and reduced the set of four parameters (I
Hmax, m
KC, m
HKs, m
HKa) to a set of three (g
KC, g
KHs, g
KHa) representing the relative activity of the transporter proteins (relative to the maximal activity of the H+-ATPase).
${{g}_X:= {m}_X\cdot {e}_0/{I}_{\mathrm{Hmax}}}$
(unit V−1). This operation eliminated a redundant parameter and reduced the set of four parameters (I
Hmax, m
KC, m
HKs, m
HKa) to a set of three (g
KC, g
KHs, g
KHa) representing the relative activity of the transporter proteins (relative to the maximal activity of the H+-ATPase).
 $$\begin{align}0&={g}_{\mathrm{KC}}\cdot \left({V}_{ss}-{E}_K^{ss}\right)+{g}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad-{g}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
$$\begin{align}0&={g}_{\mathrm{KC}}\cdot \left({V}_{ss}-{E}_K^{ss}\right)+{g}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad-{g}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
 $$\begin{align}0&={i}_p\left({V}_{ss}\right)+{n}_s\cdot {g}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad+{n}_a\cdot {g}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
$$\begin{align}0&={i}_p\left({V}_{ss}\right)+{n}_s\cdot {g}_{\mathrm{HKs}}\cdot \left(\left({n}_s+1\right)\cdot {V}_{ss}-{n}_s\cdot {E}_H-{E}_K^{ss}\right)\\&\quad+{n}_a\cdot {g}_{\mathrm{HKa}}\cdot \left(\left({n}_a-1\right)\cdot {V}_{ss}-{n}_a\cdot {E}_H+{E}_K^{ss}\right).\nonumber\end{align}$$
Additionally, the equations (30) and (31) could be arranged in vector and matrix form:
with:
and:
 $$\begin{align}\overrightarrow{A}=\left(\begin{array}{c}{n}_s\cdot {g}_{\mathrm{HKs}}\cdot {E}_H-{n}_a\cdot {g}_{\mathrm{HKa}}\cdot {E}_H\\ {}{n}_s^2\cdot {g}_{\mathrm{HKs}}\cdot {E}_H+{n}_a^2\cdot {g}_{\mathrm{HKa}}\cdot {E}_H-{i}_p\left({V}_{ss}\right)\end{array}\right).\end{align}$$
$$\begin{align}\overrightarrow{A}=\left(\begin{array}{c}{n}_s\cdot {g}_{\mathrm{HKs}}\cdot {E}_H-{n}_a\cdot {g}_{\mathrm{HKa}}\cdot {E}_H\\ {}{n}_s^2\cdot {g}_{\mathrm{HKs}}\cdot {E}_H+{n}_a^2\cdot {g}_{\mathrm{HKa}}\cdot {E}_H-{i}_p\left({V}_{ss}\right)\end{array}\right).\end{align}$$
This might be considered as just a formalism and not needed for two equations. However, the conversion should illustrate that we have opened the powerful toolbox of linear algebra. More complex homeostats with more than two equations can be handled in the same way and the tools from linear algebra for solving this type of equation can be applied in the same manner. Equation (32) has exactly one solution for the
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pair that depends on the activities of the transporter proteins (gX
) and the proton gradient (EH
):
$\left({V}_{ss}|{E}_K^{ss}\right)$
pair that depends on the activities of the transporter proteins (gX
) and the proton gradient (EH
):
 $$\begin{align}{E}_K^{ss}={E}_H-{i}_p\left({V}_{ss}\right)\cdot \frac{g_{\mathrm{KC}}+\left({n}_s+1\right)\cdot {g}_{\mathrm{HKs}}-\left({n}_a-1\right)\cdot {g}_{\mathrm{HKa}}}{{\left({n}_s+{n}_a\right)}^2\cdot {g}_{\mathrm{HKs}}\cdot {g}_{\mathrm{HKa}}+{g}_{\mathrm{KC}}\cdot \left({n}_s^2\cdot {g}_{\mathrm{HKs}}+{n}_a^2\cdot {g}_{\mathrm{HKa}}\right)}\end{align}$$
$$\begin{align}{E}_K^{ss}={E}_H-{i}_p\left({V}_{ss}\right)\cdot \frac{g_{\mathrm{KC}}+\left({n}_s+1\right)\cdot {g}_{\mathrm{HKs}}-\left({n}_a-1\right)\cdot {g}_{\mathrm{HKa}}}{{\left({n}_s+{n}_a\right)}^2\cdot {g}_{\mathrm{HKs}}\cdot {g}_{\mathrm{HKa}}+{g}_{\mathrm{KC}}\cdot \left({n}_s^2\cdot {g}_{\mathrm{HKs}}+{n}_a^2\cdot {g}_{\mathrm{HKa}}\right)}\end{align}$$
Please note
-
(1) Equations (35) and (36) are not defined for cases in which two of the three parameters g KC, g HKs and g HKa are zero. In cases, in which only one gX is different from zero, the equations (30) and (31) result in:
g KC = g HKs = 0 (only active H+/K+ antiporters):
 ${V}_{ss}={V}_{0,\mathrm{pump}}$
,
${V}_{ss}={V}_{0,\mathrm{pump}}$
,
 ${E}_K^{ss}={n}_a\cdot {E}_H-\left({n}_a-1\right)\cdot {V}_{0,\mathrm{pump}}$
${E}_K^{ss}={n}_a\cdot {E}_H-\left({n}_a-1\right)\cdot {V}_{0,\mathrm{pump}}$
g KC = g HKa = 0 (only active H+/K+ symporters):
 ${V}_{ss}={V}_{0,\mathrm{pump}}$
,
${V}_{ss}={V}_{0,\mathrm{pump}}$
,
 ${E}_K^{ss}=\left({n}_s+1\right)\cdot {V}_{0,\mathrm{pump}}-{n}_s\cdot {E}_H$
${E}_K^{ss}=\left({n}_s+1\right)\cdot {V}_{0,\mathrm{pump}}-{n}_s\cdot {E}_H$
g HKs = g HKa = 0 (only active K+ channels):
 ${V}_{ss}={V}_{0,\mathrm{pump}}$
,
${V}_{ss}={V}_{0,\mathrm{pump}}$
,
 ${E}_K^{ss}={V}_{0,\mathrm{pump}}$
${E}_K^{ss}={V}_{0,\mathrm{pump}}$
-
(2) Equation (35); of type
 ${V}_{ss}-A+{i}_p\left({V}_{ss}\right)\cdot B=0$
, is an implicit solution. It cannot be explicitly solved analytically for Vss
because ip
(Vss
) is a function of Vss
. Nevertheless, mathematical tools also allow us to bypass this difficulty. The left-hand side of equation (35) before the equals sign is a strictly monotonically increasing function of Vss
, which means that the first derivative after VSS
is > 0. Or in other words, with increasing Vss
, the left-hand side of the equation also increases, starting from negative values at negative Vss
and reaching positive values at positive Vss
. The corresponding curve therefore cuts the zero axis only once. Thus, for given values of
${V}_{ss}-A+{i}_p\left({V}_{ss}\right)\cdot B=0$
, is an implicit solution. It cannot be explicitly solved analytically for Vss
because ip
(Vss
) is a function of Vss
. Nevertheless, mathematical tools also allow us to bypass this difficulty. The left-hand side of equation (35) before the equals sign is a strictly monotonically increasing function of Vss
, which means that the first derivative after VSS
is > 0. Or in other words, with increasing Vss
, the left-hand side of the equation also increases, starting from negative values at negative Vss
and reaching positive values at positive Vss
. The corresponding curve therefore cuts the zero axis only once. Thus, for given values of
 $A={E}_H$
and
$A={E}_H$
and
 $B=f\left({g}_{\mathrm{KC}},{g}_{\mathrm{HKs}},{g}_{\mathrm{HKa}}\right)$
there is exactly one Vss
that obeys this equation. The system is free from potential bifurcation. If EH
, g
KC, g
HKs and g
HKa are known, the value of Vss
can be determined numerically by root-finding algorithms, such as the Newton–Raphson method.
$B=f\left({g}_{\mathrm{KC}},{g}_{\mathrm{HKs}},{g}_{\mathrm{HKa}}\right)$
there is exactly one Vss
that obeys this equation. The system is free from potential bifurcation. If EH
, g
KC, g
HKs and g
HKa are known, the value of Vss
can be determined numerically by root-finding algorithms, such as the Newton–Raphson method.
3. Results
The presented thermodynamical and mathematical analysis of the transporter network has resulted in general solutions for
![]() ${V}_{ss}$
(equation (35)) and
${V}_{ss}$
(equation (35)) and
![]() ${E}_K^{ss}$
(equation (36)) in the steady state, that is, in homeostatic conditions. These values could be influenced by the cell via the proton gradient EH
and the parameters g
KC, g
HKs and g
HKa, that is, the (relative) activities of the different K+ transporters. Considering the fact that from a physiological point of view, the proton gradient is less flexible, the main setscrews to adjust
${E}_K^{ss}$
(equation (36)) in the steady state, that is, in homeostatic conditions. These values could be influenced by the cell via the proton gradient EH
and the parameters g
KC, g
HKs and g
HKa, that is, the (relative) activities of the different K+ transporters. Considering the fact that from a physiological point of view, the proton gradient is less flexible, the main setscrews to adjust
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
are the gX
values. These three mathematical parameters split in the biological reality into a manifold of physiological parameters, such as gene expression, protein turnover and post-translational regulation. Thus, one gX
value can represent several different biological realities.
$\left({V}_{ss}|{E}_K^{ss}\right)$
are the gX
values. These three mathematical parameters split in the biological reality into a manifold of physiological parameters, such as gene expression, protein turnover and post-translational regulation. Thus, one gX
value can represent several different biological realities.
To illustrate the consequences of the dependency of the homeostatic steady state on the parameters g
KC, g
HKs and g
HKa, the results for the case ns
= 1, na
= 1, V
0,pump = −200 mV, and EH
= +57.6 mV (pHin = 7, pHout = 6) are shown as an example. Below, we will show how different settings of these parameters influence the results. For each parameter gX
, we chose 0 and 19 logarithmically distributed values in a range between 10−6 mV−1 and 1 mV−1 (i.e. 0 mV−1, 1 × 10−6 mV−1, 2 × 10−6 mV−1, 5 × 10−6 mV−1, 1 × 10−5 mV−1, 2 × 10−5 mV−1, 5 × 10−5 mV−1, 1 × 10−4 mV−1, 2 × 10−4 mV−1, 5 × 10−4 mV−1, 1 × 10−3 mV−1, 2 × 10−3 mV−1, 5 × 10−3 mV−1, 1 × 10−2 mV−1, 2 × 10−2 mV−1, 5 × 10−2 mV−1, 1 × 10−1 mV−1, 2 × 10−1 mV−1, 5 × 10−1 mV−1, 1 mV−1). With respect to Figure 2b, this range covered a spectrum of the angle α between 0° and almost 90° and thus the entire range of possibilities. The resulting 20 × 20 × 20 table represented 8000 different (mathematical) realities of the system. For each of them, equation (35) was solved using the ‘Goal Seek’ routine in Excel by changing Vss
. This was done to show that no sophisticated computer programs are required for the analyses. With the resulting value for
![]() ${V}_{ss}$
, then the value for
${V}_{ss}$
, then the value for
![]() ${E}_K^{ss}$
was calculated using equation (36). The determined
${E}_K^{ss}$
was calculated using equation (36). The determined
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pair finally allowed us to calculate the different relative fluxes in steady state using equations (5), (9), (10), (14), (15) and (18) and the definition
$\left({V}_{ss}|{E}_K^{ss}\right)$
pair finally allowed us to calculate the different relative fluxes in steady state using equations (5), (9), (10), (14), (15) and (18) and the definition
![]() ${g}_X:= {m}_X\cdot {e}_0/{I}_{\mathrm{Hmax}}$
(see above):
${g}_X:= {m}_X\cdot {e}_0/{I}_{\mathrm{Hmax}}$
(see above):
 $$\begin{align}{J}_{\mathrm{HATPase}}^{ss, rel}={i}_p\left({V}_{ss}\right)=\frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left({V}_{ss}-{\mathrm{V}}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot {V}_{ss}+5.4\right)}+{e}^{-\left(0.1\cdot \frac{F}{RT}\cdot {V}_{ss}+2.5\right)}}.\end{align}$$
$$\begin{align}{J}_{\mathrm{HATPase}}^{ss, rel}={i}_p\left({V}_{ss}\right)=\frac{1-{e}^{-\left(\frac{F}{RT}\cdot \left({V}_{ss}-{\mathrm{V}}_{0,\mathrm{pump}}\right)\right)}}{1+{e}^{-\left(\frac{F}{RT}\cdot {V}_{ss}+5.4\right)}+{e}^{-\left(0.1\cdot \frac{F}{RT}\cdot {V}_{ss}+2.5\right)}}.\end{align}$$
3.1 K + and H + cycling in steady state
In the 8000 different cases, Vss
ranged between −200 mV (= V
0,pump) and +57.6 mV (= EH
), while
![]() ${E}_K^{ss}$
assumed values between −457.6 mV (= 2⋅ V
0,pump − EH
) and +57.6 mV (= EH
). Figure 3 illustrates the values of
${E}_K^{ss}$
assumed values between −457.6 mV (= 2⋅ V
0,pump − EH
) and +57.6 mV (= EH
). Figure 3 illustrates the values of
![]() ${V}_{ss}$
(a,d,g),
${V}_{ss}$
(a,d,g),
![]() ${E}_K^{ss}$
(b,e,h) and
${E}_K^{ss}$
(b,e,h) and
![]() ${E}_K^{ss}-{V}_{ss}$
(c,f,i) for several parameter sets of g
KC, g
HKs and g
HKa. The larger was the activity of the K+ channel (b<e<h), the less negative was the minimal
${E}_K^{ss}-{V}_{ss}$
(c,f,i) for several parameter sets of g
KC, g
HKs and g
HKa. The larger was the activity of the K+ channel (b<e<h), the less negative was the minimal
![]() ${E}_K^{ss}$
that could be achieved by highly active H+/K+ symporters. With hardly any active K+ channels (g
KC = 10−5 V−1) the symporter could (theoretically) accumulate a [K+]in that was almost 108-fold higher than [K+]out, which corresponded to
${E}_K^{ss}$
that could be achieved by highly active H+/K+ symporters. With hardly any active K+ channels (g
KC = 10−5 V−1) the symporter could (theoretically) accumulate a [K+]in that was almost 108-fold higher than [K+]out, which corresponded to
![]() ${E}_K^{ss}\approx -460\; mV$
(Figure 3b). In this condition, the membrane voltage was
${E}_K^{ss}\approx -460\; mV$
(Figure 3b). In this condition, the membrane voltage was
![]() ${V}_{ss}\approx -200\; mV$
(Figure 3a), that is, close to the limit of the pump V
0,pump. This means that all energy from the proton gradient and ATP-hydrolysis was used for K+ accumulation. These extreme values became more moderate with increasing channel activity. At 500-fold higher K+ channel activity (g
KC = 5 × 10−3 V−1),
${V}_{ss}\approx -200\; mV$
(Figure 3a), that is, close to the limit of the pump V
0,pump. This means that all energy from the proton gradient and ATP-hydrolysis was used for K+ accumulation. These extreme values became more moderate with increasing channel activity. At 500-fold higher K+ channel activity (g
KC = 5 × 10−3 V−1),
![]() ${E}_K^{ss}=-244\; mV$
was the most negative value at high g
HKs and low g
HKa (Figure 3e), which still corresponded to ~17.000-fold higher [K+]in than [K+]out. The reduction in the accumulation rate came along with an increased Vss
, in particular at higher symporter but also at higher antiporter activities (Figure 3d) pointing to an energy-dissipation process that increased with increasing K+ channel activity (Figure 3a,d,g). This was further corroborated when analysing the transmembrane fluxes (Supplementary Figure S1). Although the net fluxes of K+ and H+ were zero in steady state (equations (26)) and (27)), there was a transmembrane cycling of both ions. Permanent effluxes were compensated by permanent influxes. To illustrate the cycling, the H+ and K+ cycling flux amplitudes and the ratio between the two were displayed in Figure 4 relative to the maximally achievable proton pump-driven H+ efflux; that is, the maximum H+ efflux of the pump (I
Hmax/e0
, equation (18))) was normalised to 1. If the K+ channel activity was very low (g
KC = 10−5 V−1), the relative H+ efflux mediated by the pump ranged from 0 to ≈0.94 [=ip
(EH
)] with the highest value achieved with highly active sym- and antiporters (Figure 4a and Supplementary Figure S1A) at Vss
= EH
(Figure 3a). Under these conditions, the relative K+ cycling flux was half as large as the H+ flux (Figure 4b), indicating that two protons were looped for one K+ ion (Figure 4c). And indeed, one H+ was taken up together with one K+ by the H+/K+ symporter (Supplementary Figure S1B,E), while the other H+ was taken up by the H+/K+ antiporter releasing one K+ (Supplementary Figure S1C,F). The accumulated protons were released by the pump (Supplementary Figure S1A).
${E}_K^{ss}=-244\; mV$
was the most negative value at high g
HKs and low g
HKa (Figure 3e), which still corresponded to ~17.000-fold higher [K+]in than [K+]out. The reduction in the accumulation rate came along with an increased Vss
, in particular at higher symporter but also at higher antiporter activities (Figure 3d) pointing to an energy-dissipation process that increased with increasing K+ channel activity (Figure 3a,d,g). This was further corroborated when analysing the transmembrane fluxes (Supplementary Figure S1). Although the net fluxes of K+ and H+ were zero in steady state (equations (26)) and (27)), there was a transmembrane cycling of both ions. Permanent effluxes were compensated by permanent influxes. To illustrate the cycling, the H+ and K+ cycling flux amplitudes and the ratio between the two were displayed in Figure 4 relative to the maximally achievable proton pump-driven H+ efflux; that is, the maximum H+ efflux of the pump (I
Hmax/e0
, equation (18))) was normalised to 1. If the K+ channel activity was very low (g
KC = 10−5 V−1), the relative H+ efflux mediated by the pump ranged from 0 to ≈0.94 [=ip
(EH
)] with the highest value achieved with highly active sym- and antiporters (Figure 4a and Supplementary Figure S1A) at Vss
= EH
(Figure 3a). Under these conditions, the relative K+ cycling flux was half as large as the H+ flux (Figure 4b), indicating that two protons were looped for one K+ ion (Figure 4c). And indeed, one H+ was taken up together with one K+ by the H+/K+ symporter (Supplementary Figure S1B,E), while the other H+ was taken up by the H+/K+ antiporter releasing one K+ (Supplementary Figure S1C,F). The accumulated protons were released by the pump (Supplementary Figure S1A).

Figure 3. Membrane voltage and K+ gradient in homeostatic (steady state) conditions. The activities of K+ channels (g
KC), H+/K+ symporters (g
HKs) and H+/K+ antiporters (g
HKa) determine the membrane voltage (
![]() ${V}_{ss}$
, a,d,g) and the K+ gradient (
${V}_{ss}$
, a,d,g) and the K+ gradient (
![]() ${E}_K^{ss}$
, b,e,h) in steady state. The difference
${E}_K^{ss}$
, b,e,h) in steady state. The difference
![]() ${E}_K^{ss}-{V}_{ss}$
(c,f,i) is positive if the activity of antiporters is higher than that of symporters (
${E}_K^{ss}-{V}_{ss}$
(c,f,i) is positive if the activity of antiporters is higher than that of symporters (
![]() ${g}_{\mathrm{HKa}}>{g}_{\mathrm{HKs}}$
). It is negative if
${g}_{\mathrm{HKa}}>{g}_{\mathrm{HKs}}$
). It is negative if
![]() ${g}_{\mathrm{HKa}}<{g}_{\mathrm{HKs}}$
. Data were calculated for the case ns
= 1, na
= 1, V
0,pump = −200 mV, and EH
= +57.6 mV (ΔpH = 1). The magenta lines show the values in the absence of active H+/K+ antiporters (g
HKa = 0), whereas the cyan lines indicate the values in the absence of active H+/K+ symporters (g
HKs = 0).
${g}_{\mathrm{HKa}}<{g}_{\mathrm{HKs}}$
. Data were calculated for the case ns
= 1, na
= 1, V
0,pump = −200 mV, and EH
= +57.6 mV (ΔpH = 1). The magenta lines show the values in the absence of active H+/K+ antiporters (g
HKa = 0), whereas the cyan lines indicate the values in the absence of active H+/K+ symporters (g
HKs = 0).

Figure 4. H+ and K+ cycling in homeostatic (steady state) conditions. Although the net H+ and K+ fluxes are zero in homeostatic conditions, there are still continuous H+ and K+ effluxes that are compensated by influxes of the same magnitude. (a,d,g) Dependency of the H+ flux amplitude and (b,e,h) of the K+ flux amplitude on the activities of the K+ channels (g KC), H+/K+ symporters (g HKs) and H+/K+ antiporters (g HKa). The cycling fluxes are shown relative to the maximal H+ efflux that can be generated by the H+ ATPase (J Hmax = I Hmax/e0 ). (c,f,i) Ratio between H+ and K+ cycling fluxes as a measure for the H+/K+ cycling ratio. Data were calculated for the case ns = 1, na = 1, V 0,pump = −200 mV and EH = +57.6 mV (ΔpH = 1). The magenta lines show the values in the absence of active H+/K+ antiporters (g HKa = 0), whereas the cyan lines indicate the values in the absence of active H+/K+ symporters (g HKs = 0).
H+ and K+ cycling flux amplitudes became smaller with either decreasing symporter or decreasing antiporter activity (Figure 4a,b). This picture changed with increasing K+ channel activity. While the relative H+ and K+ fluxes remained unaffected by an increased channel activity at very high sym- and antiporter activity (compare the plateaus in the corners of Figure 4a,b,d,e,g,h), K+ channel activity had a strong effect when one of the H+/K+ transporters was less active. With less active antiporters and highly active symporters, increased K+ channel activity increased K+ cycling (compare Figure 4b,e,h, close to the magenta curve). The K+ efflux was mediated by the K+ channels (Supplementary Figure S1D,J,P), compensated by a larger K+ influx via the H+/K+ symporters (Supplementary Figure S1E,K,Q). The accompanying H+ influx (Supplementary Figure S1B,H,N) was neutralised by the H+ efflux via the pump (Supplementary Figure S1A,G,M) that also energised the combined H+/K+ cycling.
Likewise, K+ cycling also increased with increased K+ channel activity at highly active antiporters and less active symporters (compare Figure 4b,e,h, close to the cyan curve). However, this time, the efflux was not mediated by the K+ channels (Supplementary Figure S1D,J,P). They mediated K+ influx instead. The K+ efflux passed through the H+/K+ antiporters (Supplementary Figure S1F,L,R). When comparing the two situations, it turned out that in homeostatic steady state conditions, K+ channels served at the same membrane voltage, Vss , once as uptake and once as release channels depending on the H+/K+ transporter situation. In combination with H+/K+ symporters, they acted as K+ release channels, even at very negative voltages. In contrast, in combination with H+/K+ antiporters, they functioned as K+ uptake channels, even at positive voltages. These thermodynamically derived properties of K+ channels under homeostatic conditions apparently contradict the historically established separation of voltage-dependent K+ channels into hyperpolarisation-activated uptake (Kin) and depolarization-activated release (Kout) channels (Dreyer et al., Reference Dreyer, Sussmilch, Fukushima, Riadi, Becker, Schultz and Hedrich2021; Dreyer & Blatt, Reference Dreyer and Blatt2009; Dreyer & Uozumi, Reference Dreyer and Uozumi2011; Sharma et al., Reference Sharma, Dreyer and Riedelsberger2013). Thermodynamic considerations have now taught us that the nomenclature might be misguiding. Kin channels might also serve as K+ release channels to avoid over-accumulation of K+ by H+/K+ symporters, while Kout channels may also serve as K+ uptake channels under certain homeostatic conditions.
3.2 Conclusions on the cost efficiency of homeostasis
The considerations based on thermodynamic first principles indicated that homeostatic conditions can be achieved by combining two different K+ transporter types (K+ channels and H+/K+ symporters, Figure 5a, K+ channels and H+/K+ antiporters, Figure 5b, H+/K+ symporters and H+/K+ antiporters, Figure 5c) or all three K+ transporter types (Figure 5d). In all these cases, the homeostatic condition is inevitably accompanied by energy-consuming H+ and K+ loops across the membrane (Dreyer, Reference Dreyer2021a). In Figures 3 and 4 and Supplementary Figure S1 this was shown exemplarily for sym- and antiporters with a 1 H+:1 K+ stoichiometry. In the following, the more general case (ns
and na
) was considered to find out which values for ns
and na
make the most sense. The H+ and K+ cycles occurred because the homeostatic steady state was different from the equilibrium states of the different involved transporters. In the case of a transporter network with H+ pump, K+ channel and H+/K+ symporter (Figure 5a), for instance, the steady state membrane voltage, Vss
, is different from the equilibrium voltages of the pump (V
0,pump), the channel (
![]() ${E}_K^{ss}$
) or the symporter (
${E}_K^{ss}$
) or the symporter (
![]() ${E}_{HKs}=\left[{n}_s\cdot {E}_H+{E}_K^{ss}\right]/\left[{n}_s+1\right]$
). This means that the pump continuously pumps protons from the inside to the outside by consuming ATP. These protons return to the cell via the H+/K+ symporter and shuttle 1 K+ per ns
protons into the cell. The absorbed K+ is released again via the channel. These combined H+ and K+ cycles are yield-neutral in steady state, that is, they do not affect neither the concentrations nor the electrical charges at both sides of the membrane. The cost of the homeostatic condition for the cell is in this case ns
ATP per 1 looped K+. Most cost-efficient would therefore be ns
= 1. And indeed, even at this lowest coupling rate, the H+/K+ symporter is powerful enough to (theoretically) accumulate K+ under physiological voltage (V ≥ −200 mV) and pH (ΔpH = 1) conditions more than 105-fold in the interior compared to the exterior (
${E}_{HKs}=\left[{n}_s\cdot {E}_H+{E}_K^{ss}\right]/\left[{n}_s+1\right]$
). This means that the pump continuously pumps protons from the inside to the outside by consuming ATP. These protons return to the cell via the H+/K+ symporter and shuttle 1 K+ per ns
protons into the cell. The absorbed K+ is released again via the channel. These combined H+ and K+ cycles are yield-neutral in steady state, that is, they do not affect neither the concentrations nor the electrical charges at both sides of the membrane. The cost of the homeostatic condition for the cell is in this case ns
ATP per 1 looped K+. Most cost-efficient would therefore be ns
= 1. And indeed, even at this lowest coupling rate, the H+/K+ symporter is powerful enough to (theoretically) accumulate K+ under physiological voltage (V ≥ −200 mV) and pH (ΔpH = 1) conditions more than 105-fold in the interior compared to the exterior (
![]() ${E}_K^{ss}$
= −287.8 mV). Higher coupling rates (ns
> 1) would allow higher accumulation rates, but at the expense of higher ATP consumption. Considering the physiological conditions, the best cost-benefit ratio is therefore ns
= 1. The K+ transporter network shown in Figure 5a is very well suited for K+ accumulation, in particular when [K+
]out is small. However, it has the potential problem of K+ overaccumulation under certain conditions (moderate [K+
]out). The grey area in Figure 5a (lower panel) indicates the reachable range for Vss
and
${E}_K^{ss}$
= −287.8 mV). Higher coupling rates (ns
> 1) would allow higher accumulation rates, but at the expense of higher ATP consumption. Considering the physiological conditions, the best cost-benefit ratio is therefore ns
= 1. The K+ transporter network shown in Figure 5a is very well suited for K+ accumulation, in particular when [K+
]out is small. However, it has the potential problem of K+ overaccumulation under certain conditions (moderate [K+
]out). The grey area in Figure 5a (lower panel) indicates the reachable range for Vss
and
![]() ${E}_K^{ss}$
. This transporter network only allows steady state conditions with
${E}_K^{ss}$
. This transporter network only allows steady state conditions with
![]() ${E}_K^{ss}\le {V}_{ss}$
, which might imply rather high [K+
]in. This drawback could be eliminated by replacing the H+/K+ symporter with an H+/K+ antiporter (Figure 5b). The network of pump, channel and antiporter can establish homeostatic conditions with
${E}_K^{ss}\le {V}_{ss}$
, which might imply rather high [K+
]in. This drawback could be eliminated by replacing the H+/K+ symporter with an H+/K+ antiporter (Figure 5b). The network of pump, channel and antiporter can establish homeostatic conditions with
![]() ${V}_{ss}\le {E}_K^{ss}$
, and might therefore be suitable for moderate [K+
]out. In a steady state, the protons pumped out of the cell are reaccumulated by the antiporter, which releases K+, that is then reabsorbed by the channel. The cost of these H+ and K+ cycles is na
ATP per 1 looped K+. The na
value only affects the upper limit of
${V}_{ss}\le {E}_K^{ss}$
, and might therefore be suitable for moderate [K+
]out. In a steady state, the protons pumped out of the cell are reaccumulated by the antiporter, which releases K+, that is then reabsorbed by the channel. The cost of these H+ and K+ cycles is na
ATP per 1 looped K+. The na
value only affects the upper limit of
![]() ${E}_K^{ss}$
. For na
= 1,
${E}_K^{ss}$
. For na
= 1,
![]() ${E}_K^{ss}$
cannot be larger than EH
(Figure 5b, lower panel, grey area). Higher na
values would allow
${E}_K^{ss}$
cannot be larger than EH
(Figure 5b, lower panel, grey area). Higher na
values would allow
![]() ${E}_K^{ss}>{E}_H$
. However, this is physiologically not relevant because it would mean very low [K+
]in. Thus, H+/K+ antiporters with na
=1 are the most cost-efficient, that is, they function as electroneutral transporters. The limits of the two formerly considered networks can be overcome by combining the H+ pump with H+/K+ sym- and antiporters (Figure 5c). Here, the combined range of
${E}_K^{ss}>{E}_H$
. However, this is physiologically not relevant because it would mean very low [K+
]in. Thus, H+/K+ antiporters with na
=1 are the most cost-efficient, that is, they function as electroneutral transporters. The limits of the two formerly considered networks can be overcome by combining the H+ pump with H+/K+ sym- and antiporters (Figure 5c). Here, the combined range of
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pairs is in reach (light and dark grey areas). The drawback is, however, the increased cost of (ns
+na
) ATP per 1 looped K+, that is, 2 ATP per 1 cycled K+ in the most cost-efficient case. The cost can be reduced by including additionally K+ channels into the transporter network (Figure 5d). Without any compromise on the range of the reachable
$\left({V}_{ss}|{E}_K^{ss}\right)$
pairs is in reach (light and dark grey areas). The drawback is, however, the increased cost of (ns
+na
) ATP per 1 looped K+, that is, 2 ATP per 1 cycled K+ in the most cost-efficient case. The cost can be reduced by including additionally K+ channels into the transporter network (Figure 5d). Without any compromise on the range of the reachable
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pairs, 1 looped K+ costs here 1–2 ATP (in the most cost-efficient case ns
= na
= 1, Figure 4c,f,i).
$\left({V}_{ss}|{E}_K^{ss}\right)$
pairs, 1 looped K+ costs here 1–2 ATP (in the most cost-efficient case ns
= na
= 1, Figure 4c,f,i).

Figure 5. K homeostats in a steady state. The three different K+ transporter types (i) K+ channels (KC), (ii) H+/K+ symporters (H/Ks, stoichiometry ns
H+: 1 K+) and (iii) H+/K+ antiporters (H/Ka, stoichiometry na
H+: 1 K+) can be arranged to a K homeostat in four different combinations. In all combinations the transmembrane, yield-neutral, but energy-consuming cycling of H+ and K+ is a feature of the steady state condition. (a) A network of H+-pumps, K+ channels and H+/K+ symporters consumes ns
ATP per 1 looped K+, but allows only conditions for which
![]() ${E}_K^{ss}\le {V}_{ss}$
(dark grey area, shown for the most cost-efficient case ns
= 1). (b) A network of H+-pumps, K+ channels and H+/K+ antiporters consumes na
ATP per 1 looped K+, but allows only conditions for which
${E}_K^{ss}\le {V}_{ss}$
(dark grey area, shown for the most cost-efficient case ns
= 1). (b) A network of H+-pumps, K+ channels and H+/K+ antiporters consumes na
ATP per 1 looped K+, but allows only conditions for which
![]() ${E}_K^{ss}\ge {V}_{ss}$
(light grey area, shown for the most cost-efficient case na
= 1). (c) A network of H+-pumps, H+/K+ symporters and H+/K+ antiporters consumes (ns
+na
) ATP per 1 looped K+. It allows a broader range of
${E}_K^{ss}\ge {V}_{ss}$
(light grey area, shown for the most cost-efficient case na
= 1). (c) A network of H+-pumps, H+/K+ symporters and H+/K+ antiporters consumes (ns
+na
) ATP per 1 looped K+. It allows a broader range of
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pairs (combined grey areas, shown for the most cost-efficient case ns
= na
= 1). (d) A network of H+-pumps, K+ channels, H+/K+ symporters and H+/K+ antiporters allows the same broad range of
$\left({V}_{ss}|{E}_K^{ss}\right)$
pairs (combined grey areas, shown for the most cost-efficient case ns
= na
= 1). (d) A network of H+-pumps, K+ channels, H+/K+ symporters and H+/K+ antiporters allows the same broad range of
![]() $\left({V}_{ss}|{E}_K^{ss}\right)$
pairs as in (c) (combined grey areas, shown for the most cost-efficient case ns
= na
= 1). However, the ATP consumption is smaller and ranges between 1 and 2 ATP per 1 looped K+ (in the most cost-efficient case ns
= na
= 1). The dotted blue and red lines in the lower panels indicate roughly physiological upper and lower limits for
$\left({V}_{ss}|{E}_K^{ss}\right)$
pairs as in (c) (combined grey areas, shown for the most cost-efficient case ns
= na
= 1). However, the ATP consumption is smaller and ranges between 1 and 2 ATP per 1 looped K+ (in the most cost-efficient case ns
= na
= 1). The dotted blue and red lines in the lower panels indicate roughly physiological upper and lower limits for
![]() ${E}_K^{ss}$
at an endomembrane and the plasma membrane: +20 mV refers to [K+]out/[K+]in ≈ 2.3 (e.g. 267mM/120mM), −50 mV refers to [K+]out/[K+]in ≈ 0.13 (e.g. 16 mM/120 mM), while −250 mV refers to [K+]out/[K+]in ≈ 4.5 × 10−5 (5 μM/120 mM) (adapted from Dreyer, Reference Dreyer2021a).
${E}_K^{ss}$
at an endomembrane and the plasma membrane: +20 mV refers to [K+]out/[K+]in ≈ 2.3 (e.g. 267mM/120mM), −50 mV refers to [K+]out/[K+]in ≈ 0.13 (e.g. 16 mM/120 mM), while −250 mV refers to [K+]out/[K+]in ≈ 4.5 × 10−5 (5 μM/120 mM) (adapted from Dreyer, Reference Dreyer2021a).
3.3 Independence of the conclusions on the parameters V 0,pump and E H
The previous results were obtained for a fixed zero current voltage of the pump (V
0,pump = −200 mV) and a fixed pH gradient of ΔpH = 1 (EH
= 57.6 mV). To corroborate that different settings do not change the main conclusions, we have repeated the analysis with V
0,pump = −150 mV and EH
= 126.6 mV (pHin = 7.2, pHout = 5.0) as two examples (Supplementary Figure S2). The different V
0,pump and EH
values mainly affected the extreme values of
![]() ${V}_{ss}$
and
${V}_{ss}$
and
![]() ${E}_K^{ss}$
, but left the main trends unaffected. Likewise, the H+ and K+ cycling fluxes were barely distinguishable between V
0,pump = −200 mV (Supplementary Figure S1) and V
0,pump = −150 mV (Supplementary Figure S3). A less negative V
0,pump may represent energy consumption by other transport processes, for example, by an anion homeostat. The cycling fluxes in steady state are independent of EH
: If
${E}_K^{ss}$
, but left the main trends unaffected. Likewise, the H+ and K+ cycling fluxes were barely distinguishable between V
0,pump = −200 mV (Supplementary Figure S1) and V
0,pump = −150 mV (Supplementary Figure S3). A less negative V
0,pump may represent energy consumption by other transport processes, for example, by an anion homeostat. The cycling fluxes in steady state are independent of EH
: If
![]() ${V}_{ss}$
and
${V}_{ss}$
and
![]() ${E}_K^{ss}$
are replaced by the solutions of equations (35) and (36) in equations (37)–(42), all
${E}_K^{ss}$
are replaced by the solutions of equations (35) and (36) in equations (37)–(42), all
![]() ${J}_X^{ss}$
do not depend on EH
or a buffer concentration. This supports the notion that a buffer, which can only be available in a limited concentration, does not contribute to the maintenance of homeostasis.
${J}_X^{ss}$
do not depend on EH
or a buffer concentration. This supports the notion that a buffer, which can only be available in a limited concentration, does not contribute to the maintenance of homeostasis.
4. Discussion and conclusion
Cellular ion homeostasis is governed by fundamental physical and chemical laws. In this study, we used (i) the definition of the electrochemical potential, (ii) the basic consequence from this definition that net fluxes are zero if there is no gradient in the electrochemical potential, (iii) the chemical definition of concentrations and concentration changes, (iv) the physical properties of membranes to separate charges like a technical capacitor and we (v) assumed that the considered transporters function as perfect molecular machines as shown exemplarily for several of them (e.g. Carpaneto et al., Reference Carpaneto, Geiger, Bamberg, Sauer, Fromm and Hedrich2005). We described the different processes with mathematical equations and took advantage of the powerful toolboxes of differential equations and linear algebra. These basic considerations were sufficient to draw fundamental conclusions about the behaviour of K+ transporter networks responsible for setting homeostatic (steady state) conditions for potassium in a cellular or organelle environment:
-
(1) By setting the transporter activities (gX ), the cell can precisely adjust the steady state values for the membrane voltage Vss and the transmembrane K+ gradient
 ${E}_K^{ss}$
. A cell can change the parameters gX
by altering the activities through gene expression and post-translational modifications (e.g. phosphorylation), which points to the adjusting screws that integrate the homeostats into the cellular regulatory networks. It should be emphasised, however, that the gX
values usually do not follow the biological parameters in a linear manner.
${E}_K^{ss}$
. A cell can change the parameters gX
by altering the activities through gene expression and post-translational modifications (e.g. phosphorylation), which points to the adjusting screws that integrate the homeostats into the cellular regulatory networks. It should be emphasised, however, that the gX
values usually do not follow the biological parameters in a linear manner. -
(2) The cell cannot adjust [K+ ]in independent of [K+ ]out; it can only adjust
 ${E}_K^{ss}$
, which depends on both.
${E}_K^{ss}$
, which depends on both. -
(3) If the membrane has only one functional K+ transporter type, that is, all gX except one are zero, the steady state is independent of the activity of this transporter and cannot be adjusted. In other words: Homeostatic conditions involve always at least two different types of transporters.
-
(4) Transporter types are only different, if they are energised differently, that is, the driving gradient Δμ is different. For instance, voltage-dependent and voltage-independent K+ channels are not different; both are described by the same type of equations. The voltage dependence is covered by the g KC parameters, and several g KC values could be combined to one that represents all channels.
-
(5) A steady state condition of
 ${E}_K^{ss}<{V}_{ss}$
, is (indirect) proof of functional H+/K+ symporters in the membrane. The condition
${E}_K^{ss}<{V}_{ss}$
, is (indirect) proof of functional H+/K+ symporters in the membrane. The condition
 ${E}_K^{ss}<{V}_{ss}$
is only achievable with g
HKs > 0.
${E}_K^{ss}<{V}_{ss}$
is only achievable with g
HKs > 0. -
(6) Most cost-efficient are symporters of 1 H+: 1 K+ stoichiometry.
-
(7) A steady state condition of
 ${V}_{ss}<{E}_K^{ss}$
is (indirect) proof of functional H+/K+ antiporters in the membrane. The condition
${V}_{ss}<{E}_K^{ss}$
is (indirect) proof of functional H+/K+ antiporters in the membrane. The condition
 ${V}_{ss}<{E}_K^{ss}$
is only achievable with g
HKa > 0.
${V}_{ss}<{E}_K^{ss}$
is only achievable with g
HKa > 0. -
(8) Most cost-efficient are antiporters of 1 H+: 1 K+ stoichiometry, that is, they function as electroneutral antiporters.
-
(9) Hyperpolarisation-activated Kin channels may serve in homeostatic conditions as K+ release channels when acting together with H+/K+ symporters.
-
(10) Depolarization-activated Kout channels may serve in homeostatic conditions as K+ uptake channels when acting together with H+/K+ antiporters.
It might be surprising that all these far-reaching conclusions could be drawn solely from the knowledge on the thermodynamics of the different transport processes (Δμ). More knowledge about the transporters (e.g. KM-values or voltage-dependence) was not necessary. Actually, all these details are covered by the gX values. In this study, we set the gX values, without considering how they are set and determined the resulting steady state. This approach must be seen separate from the adjustments in a real cell. There, the setting of the gX values is the consequence of complex cellular processes that also involve feedback loops, such as the regulation of gX by membrane voltage. Nevertheless, once the homeostatic steady state has been established, the gX are also stable. This is actually the starting point of the thought experiments presented here.
The finding that the basic principle of homeostasis (steady state) does not depend on molecular details but shows a universal pattern that can be achieved in a huge variety of different nuances, indicates the enormous robustness of the biological system. It is therefore highly tolerant towards disturbances, such as many mutations in the transporter proteins and could be maintained even in dramatic evolutionary processes. It is thus not individual transporters but rather transporter networks (homeostats) that govern membrane transport and associated homeostatic processes in plant cells.
Conclusions (5–8) are of particular interest. The modelling of the K homeostat points to the existence of both, H+/K+ symporters and H+/K+ antiporters. (a)
![]() ${E}_K^{ss}<{V}_{ss}$
at steady state implies the participation of active H+/K+ symporters in the homeostat, while (b)
${E}_K^{ss}<{V}_{ss}$
at steady state implies the participation of active H+/K+ symporters in the homeostat, while (b)
![]() ${V}_{ss}<{E}_K^{ss}$
indicates the presence of active H+/K+ antiporters. A condition of V < EK
is not a temporary exception, but is observed quite frequently in plant cells (Koers et al., Reference Koers, Guzel-Deger, Marten and Roelfsema2011; Roelfsema et al., Reference Roelfsema, Steinmeyer, Staal and Hedrich2001, Reference Roelfsema, Hanstein, Felle and Hedrich2002; Thiel et al., Reference Thiel, MacRobbie and Blatt1992). It might be that these cells were not in a steady state but in an ongoing process of K+ accumulation. However, without H+/K+ antiporters, the steady state would always be
${V}_{ss}<{E}_K^{ss}$
indicates the presence of active H+/K+ antiporters. A condition of V < EK
is not a temporary exception, but is observed quite frequently in plant cells (Koers et al., Reference Koers, Guzel-Deger, Marten and Roelfsema2011; Roelfsema et al., Reference Roelfsema, Steinmeyer, Staal and Hedrich2001, Reference Roelfsema, Hanstein, Felle and Hedrich2002; Thiel et al., Reference Thiel, MacRobbie and Blatt1992). It might be that these cells were not in a steady state but in an ongoing process of K+ accumulation. However, without H+/K+ antiporters, the steady state would always be
![]() ${E}_K^{ss}\le {V}_{ss}$
, which could lead to rather large K+ gradients, especially if the external K+ concentration is in the mM range, as is often the case in tissues outside the roots. In such conditions, H+/K+ antiporters would serve as security valves against an overaccumulation of K+. Nevertheless, the role of this type of transporters is largely underrepresented in literature. In comparison to our very good knowledge about K+ channels and K+/H+ symporters, the information about K+/H+ antiporters in plants is rudimentary (see e.g. Isayenkov et al., Reference Isayenkov, Dabravolski, Pan and Shabala2020 for a recent review). The analyses presented in this study may explain why. Functionally, there was no difference between electroneutral (na
= 1) and electrogenic (na
> 1) K+/H+ antiporters. In the physiological range, both accomplished the same tasks. Nevertheless, systems with electrogenic K+/H+ antiporters consumed more energy than systems with electroneutral antiporters indicating that optimised organisms like plants would likely employ the electroneutral form. Electroneutral transporters, however, are hardly detectable in standard electrophysiological experiments and therefore extremely difficult to characterise. Thus, the mere fact that V < EK
is often observed in a plant cell strongly suggests the existence of electroneutral K+/H+ antiporters on the basis of the thermodynamic considerations in this study.
${E}_K^{ss}\le {V}_{ss}$
, which could lead to rather large K+ gradients, especially if the external K+ concentration is in the mM range, as is often the case in tissues outside the roots. In such conditions, H+/K+ antiporters would serve as security valves against an overaccumulation of K+. Nevertheless, the role of this type of transporters is largely underrepresented in literature. In comparison to our very good knowledge about K+ channels and K+/H+ symporters, the information about K+/H+ antiporters in plants is rudimentary (see e.g. Isayenkov et al., Reference Isayenkov, Dabravolski, Pan and Shabala2020 for a recent review). The analyses presented in this study may explain why. Functionally, there was no difference between electroneutral (na
= 1) and electrogenic (na
> 1) K+/H+ antiporters. In the physiological range, both accomplished the same tasks. Nevertheless, systems with electrogenic K+/H+ antiporters consumed more energy than systems with electroneutral antiporters indicating that optimised organisms like plants would likely employ the electroneutral form. Electroneutral transporters, however, are hardly detectable in standard electrophysiological experiments and therefore extremely difficult to characterise. Thus, the mere fact that V < EK
is often observed in a plant cell strongly suggests the existence of electroneutral K+/H+ antiporters on the basis of the thermodynamic considerations in this study.
It was surprising for us how many fundamental conclusions can be drawn in an airtight manner, without approximations, by applying basic thermodynamics and mathematics to plant biology. The discovery of homeostats enabled a new perspective on membrane transport in plants. And with the considerations presented here for K homeostats, we may have facilitated to adapt this concept to any type of homeostat as already carried out for anions (Dreyer et al., Reference Dreyer, Li, Riedelsberger, Hedrich, Konrad and Michard2022; Li et al., Reference Li, Grauschopf, Hedrich, Dreyer and Konrad2024), sugars (Dreyer, Reference Dreyer2021a) and auxins (Geisler & Dreyer, Reference Geisler and Dreyer2024), for instance. In a follow-up tutorial study, we will present the analyses of the dynamics of the K homeostat, that is, how the system reacts when it is deflected from the steady state. We will present the mathematical background that describes when the K homeostat is brought out of steady state (i) by a readjustment of transporter activities (tuning of gX values), (ii) by an external imbalance in the K+ gradient or (iii) by an electrical stimulus. Here, too, the detour via mathematics and computer simulations allows far-reaching conclusions to be drawn about the fundamental physiological properties of the transporter network.
Supplementary materials
The supplementary material for this article can be found at http://doi.org/10.1017/qpb.2024.8.
Competing interest
The authors declare none.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials. Raw data sets are available from the corresponding author, I.D., upon reasonable request.
Author contributions
I.D. conceived the study. All authors contributed to the design of the study. I.D. conducted the data analyses. N.H.-R., Y.B.-H., V.T.-C., S.Z.A.-M., E.D.-P., F.M.-Q., F.V.-V., O.A.-S. and M.E.R.-M. participated in data visualisation. I.D., J.R. and E.M. wrote the first draft of the manuscript. All authors contributed to the editing of the manuscript and approved its final version.
Funding statement
This work was supported by the Agencia Nacional de Investigación y Desarrollo de Chile (ANID), grant no. 21220432 to F.M.-Q., no. 21220419 to F.V.-V. and Anillo-ANID ATE220043 (the multidisciplinary centre for biotechnology and molecular biology for climate change adaptative in forest resources; CeBioClif) to I.D. and by Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (FONDECYT/Chile), grant no. 3190544 to M.E.R.-M., no. 1210920 to E.M. and no. 1220504 to I.D.




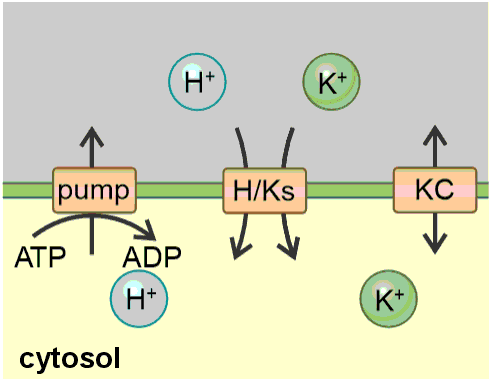
























Comments
Dear editor of Quantitative Plant Biology,
Thank you very much for your invitation to contribute to QPB. On behalf of all co-authors, please find enclosed our manuscript entitled “Homeostats – the hidden rulers of ion homeostasis in plants” for your kind consideration for publication in QPB.
Recent findings from modelling approaches provide evidence that it is not individual transporters but rather transporter networks that control membrane transport and associated homeostatic processes in plant cells. These networks are called homeostats to indicate their central physiological role. The modeling approaches published so far are not trivial and many aspects could not be treated in sufficient transparent detail. Therefore, we present in this manuscript a step-by-step description exemplarily of the modelling of the K homeostat in plants. The present manuscript could therefore serve as a blue-print for the modelling of other homeostats, and could be cited in future studies for technical aspects instead of repeating all explanations.
We would like to mention that the manuscript has been submitted in these days to the bioRxiv preprint server. The reason is that the first author has another manuscript on auxin homeostasis currently in revision in the journal New Phytologist and several questions of the reviewers will be answered by the current QPB-manuscript. It might be therefore appropriate to cite in the revised New Phytologist manuscript the new study with the DOI and the remark “submitted for peer-review to Quantitative Plant Biology”.
In the current manuscript we could handle (due to space limits) only the first part (steady state conditions) of the K homeostat. In order to provide a complete picture, we are currently working on the second part, in which we deal with the dynamic aspects of the homeostats. If possible, we would like to submit that manuscript also to QBP in order to have a complete theoretical framework. Both articles may fit into the Special Collection on Ion Homeostasis in Plants that is currently launched by the journal.
We are confident that our study will be appreciated by the community working on membrane transport in plants and look forward for a positive reply.
Sincerely Yours
Ingo Dreyer