INTRODUCTION
Clostridium difficile infections (CDI) are one of the most common healthcare-associated enteric infections and a frequent cause of morbidity and mortality in hospital settings. Predisposing factors for CDI are prior antibiotic use, age, length of hospital stay and severity of underlying disease [Reference Dubberke1, Reference McFarland, Surawicz and Stamm2]. Recent evidence shows that the attributable cost of CDI is high [Reference Vonberg3], and rising incidences of CDI have been reported in the USA and European countries [Reference Kuijper, Coignard and Tull4].
The question whether periods of high bed occupancy rates increase the risk for acquiring CDI remains unresolved and is the subject of the present study. A multivariate time-series analysis was applied to examine the influence of bed occupancy rates and length of hospital stay on the incidence of CDI in a tertiary care university hospital. The time-series analysis methodology has been demonstrated as being a suitable method for investigation of the hospital-wide correlation between incidences of multidrug-resistant organisms and bed occupancy rates [Reference Borg, Suda and Scicluna5].
METHODS
The monthly number of patients infected with C. difficile at University Medical Center Freiburg (UMCF) was recorded using isolation protocols to generate the incidence (CDI cases/1000 patient days) of CDI over a study period of 67 months (January 2003 to July 2008). All cases of CDI were diagnosed by C. difficile toxin detection from stool specimens and/or from cultivated C. difficile isolates.
Bed occupancy data were used to calculate series of hospital-wide monthly bed occupancy rates, average turnover intervals and the average length of stay. The bed occupancy rate was defined as the percentage of time that beds were occupied and was calculated according to the following formula:
Patient days were calculated as a real-time figure. Accordingly, a 12-h stay in hospital corresponds to 0·5 patient days. The number of available bed days is equal to the number of available beds multiplied by the number of days.
Turnover interval series were defined as the average length of time that a bed remained unoccupied between admissions and was calculated as follows:
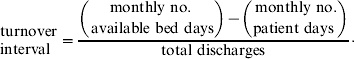
If the turnover interval equals 1, on average beds remain unoccupied for 1 day between discharge of a patient and admission of the next patient. The average length of stay was defined as the average length of time that a patient remained at the hospital.
The series of bed occupancy and length of stay were determined separately for general wards and intensive care units (ICUs). Unfortunately, we were not able to separate the monthly discharge levels of ICUs from those of general wards. Therefore, we determined the series of turnover intervals only once for the whole hospital. Finally, we were able to choose between five independent variables for integration into our time-series analysis.
Using an autoregressive integrated moving average (ARIMA) approach, a multivariate model was built to explain the incidence CDI. To obtain stationarity, first-order differencing was used for all input series. The model used was identified by determining the ARIMA model orders (p, d, q), according to the Box–Jenkins methodology. This includes an autoregressive term of order (lag) 1, first-order differencing of all input series and a moving average term of order (lag) 2. For the final model, we were able to incorporate the bed occupancy rate in general wards and the average length of stay in ICU settings. According to the augmented Dickey–Fuller test all the variables were stationary at the 1% level. We were able to reject the null hypothesis of serial correlation with the Breusch–Godfrey test; robust standard errors were calculated using the heteroskedasticity and autocorrelation consistent Newey–West estimator. The whole analysis was performed using EViews 5 (QMS, USA).
RESULTS
Our data show an upwards trend (P<0·001) in the incidence of CDI (mean 0·5 CDI cases/1000 patient days), based on regressions of the series on time. For this reason, first-order differencing was necessary to show that the incidence of CDI is stationary.
During the study period, the bed occupancy rate per month on general wards (mean 0·78, range 0·64–0·90) did not show a significant trend; neither did the average length of turnover intervals (mean 2·2 days, range 1·2–4·0). Interestingly, the bed occupancy rate (mean 0·81, range 0·64–0·92) increased in ICU settings during the study period (P<0·001), whereas the average length of stay in ICUs (mean 2·9 days, range 2·5–3·4) decreased (P=0·002). The average length of stay on general wards (mean 8·0 days, range 6·3–9·5) also decreased (P<0·001).
We were able to integrate two independent variables into our multivariate model to explain the temporal variations in the incidence of CDI (Table 1). According to the estimated coefficients, a temporal variation in bed occupancy rate on general wards was followed by a temporal variation in the same direction in the incidence of CDI. Equally, temporal variations in the average length of stay at the ICU level were followed by temporal variations in the incidence of CDI.
Table 1. Multivariate model explaining the monthly incidence of C. difficile infections (CDI) (R2=0·50)
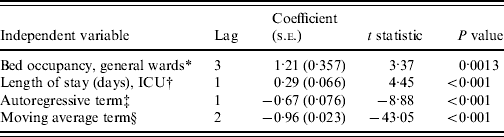
ICU, Intensive care unit.
* Percentage of time that beds are occupied in general wards (mean 0·78, range 0·64–0·90); 1=100%.
† Average length of stay in ICU (mean 2·9, range 2·5–3·4).
‡ The autoregressive term represents the past incidence of CDI.
§ The moving average term represents past disturbances in the incidence CDI.
The model included an autoregressive term of order (lag) 1 and a moving average term of order (lag) 2. An R 2 of 0·50 indicates that the model is able to explain up to 50% of the monthly variations in the incidence of CDI. See Supplementary Figure S1 (available online), for a graphical comparison of the monthly incidence of CDI and the weighted sum of lagged variables.
DISCUSSION
We found that bed occupancy rates on general wards and the average duration of patients' stay in the ICU significantly influences the incidence of CDI in hospital settings. It might appear contradictory that the length of ICU stay was associated with the incidence of CDI, although the length of ICU stay fell over the study period, while the incidence of CDI rose. However, it should be borne in mind that the study objective was not to correlate long-run trends, rather to analyse whether short-run temporal variations in the incidence of CDI were associated with short-run temporal variations in the average length of stay in ICU settings.
Another study showed similar results on the correlation between bed occupancy rates and the spread of MRSA in hospital settings [Reference Borg, Suda and Scicluna5]. A heavy nursing workload affects the standards to which hygiene measures can be implemented [Reference Bignardi and Askew6], and the workload that nurses are faced with is determined by actual patient numbers and the demands placed on the nursing staff. However, bed occupancy rates in these settings were far higher (up to 124% in St Luke's Hospital, Malta [Reference Borg, Suda and Scicluna5]) than they were at UMCF during the study period (up to 90% in general wards). Periods with a high bed occupancy rate may therefore be interpreted as relatively overcrowded. In contrast, a study in 2300 North American hospitals reported that hospitals with high CDI rates had longer lengths of hospital stay [Reference Ricciardi7].
The current study demonstrates that the incidence of CDI correlates with high bed occupancy rates on general wards and lengthier periods of ICU occupancy, whereas a recent study conducted at UMCF showed the incidence of CDI to be associated with antibiotic use [Reference Kaier8]. Use of third-generation cephalosporins, fluoroquinolones and/or macrolides was positively correlated with the hospital-wide incidence of CDI. Use of alcohol-based hand rub did not show any correlation with the incidence of CDI, which can be explained by the fact that C. difficile spores are likely to survive hand hygiene measures with alcohol-based antiseptics and routine disinfection of environmental surfaces.
Rising incidences of CDI have recently been reported in the USA and other European countries [Reference Kuijper, Coignard and Tull4]. This is presumably associated with the emergence and spread of new hypervirulent strains belonging to PCR ribotype 027. Outbreaks of this new strain have been identified in several European countries [Reference Debast9]. However, C. difficile 027 strain has not been found to be prevalent in Southern Germany, although an increase of C. difficile ribotype 001, which exhibits antibiotic resistance to erythromycin, ciprofloxacin, and moxifloxacin has been reported [Reference Borgmann10].
We obviously need to search for an alternative explanation for the recent increase in the incidence of CDI. Unfortunately, we could not distinguish between nosocomial cases of CDI and patients admitted with CDI. Hence, we were unable to analyse the influence of colonization pressure from outside the hospital by including the incidence of patients admitted with CDI as an independent variable. An interesting topic for further studies would also be to analyse correlations between the increasing proportions of C. difficile ribotype 001 and the hospital-wide use of fluoroquinolones.
However, bed occupancy rates on general wards and length of stay in ICUs might explain short-run fluctuations in the incidence of CDI.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
DECLARATION OF INTEREST
None.





