To the Editor—Intensive care units (ICUs) are one of the largest consumers of antimicrobials, with >70% of patients of receiving antimicrobials on a given day.Reference Vincent, Rello and Marshall1 With the continuing emergence of difficult to treat, costly, and deadly multidrug-resistant pathogens, antimicrobial stewardship strategies to reduce antimicrobial use are urgently needed.2, Reference Thorpe, Joski and Johnston3 Patients at the end of life are often cared for in ICUs and frequently receive antimicrobialsReference Thompson, Silveira, Vitale and Malani4 with uncertain benefits.Reference Reinbolt, Shenk, White and Navari5
We recently conducted a cluster randomized crossover trial of early palliative care consultation in our medical ICUs,Reference Ma, Chi and Buettner6 with the goal of determining the impact of early palliative care interventions on outcomes of medical ICU patients. The purpose of the current investigation was to determine whether palliative-care consultation in our medical ICU resulted in reduced antimicrobial use at the end of life.
Methods
The details of the trial have recently been described.Reference Ma, Chi and Buettner6 Briefly, the trial was a single-center cluster randomized crossover trial (August 2017–May 2018) at Barnes-Jewish Hospital (1,250 beds). The study included a 6-week washout period halfway through the study, followed by crossing over to intervention or usual care of the 2 medical ICUs. The Washington University School of Medicine Human Studies Committee approved this investigation, and the need for informed consent was waived (Institutional Review Board no. 201707067; ClinicalTrials.gov Identifier: NCT03263143). All patients admitted on weekdays that were ≥18 years of age and at high risk for morbidity and mortality based on predetermined palliative-care screening criteria could be enrolled as long as they did not meet exclusion criteria.Reference Ma, Chi and Buettner6 Patients in the intervention arm received palliative-care consultation within 48 hours of ICU admission, and the control arm received standard of care with palliative-care consultation at the discretion of the treating physicians.
Collected data included sociodemographic data; medical comorbidities; laboratory results; changes in resuscitation preferences; length of stay; duration and use of mechanical ventilation, vasopressors, and antimicrobials; place of discharge; mortality during the hospital stay; and antimicrobial prescriptions at discharge. Days of antimicrobial therapy were calculated by adding the total number of calendar days of each administered antimicrobial agent. Only antimicrobials with antibacterial properties were considered.
The primary outcome of the current investigation was the proportion of patients receiving antimicrobial prescriptions at hospital discharge among those who did and did not change their resuscitation preference. Our secondary outcomes were total duration of inpatient and outpatient antimicrobials. We compared discharge on antimicrobials between groups using the χ2 test and duration of inpatient antimicrobials using the Mann-Whitney U test. All statistical analyses were performed using SPSS version 25 software (IBM, Armonk, NY).
Results
In the original trial, 242 patients were enrolled, but only 199 were eligible for the primary outcome.Reference Ma, Chi and Buettner6 Of these patients, only 132 survived to hospital discharge and had not been on suppressive antimicrobials or active treatment for a previous infection at the time of hospital admission. Of 132 patients, 36 patients (27.3%) changed their code status to do not resuscitate/do not intubate. Of the patients that changed their code status, 6 (16.7%) were discharged on antibiotics compared to 36 patients (37.5%) that did not change their code status. For the 23 patients discharged alive to hospice, none was discharged on antimicrobials.
The proportion of patients discharged on antimicrobials was significantly lower among those having a change in code status (P = .022) (Table 1). Of the 132 patients, 116 (87.9%) either died within 30 days of hospital discharge or had at least 30 days of available follow-up data. From these 116 patients, there was no significant difference in the proportion discharged on antimicrobials between those that died within 30 days and those that did not (P = .14).
Table 1. Duration of Antimicrobials Among Patients Who Did and Did Not Change Their Code Status
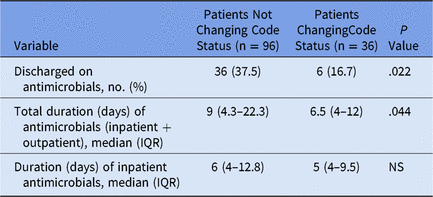
Note. IQR, interquartile range; NS, not significant.
Among the cohort of 132 patients, the median total duration of antimicrobial therapy (inpatient plus outpatient) was significantly different (P = .044) between the group that changed their code status and those that did not (Table 1). The median duration of inpatient antimicrobials was not statistically different between those that did and did not change their code status (Table 1). Patients discharged on hospice (n = 23) had significantly (P = .018) shorter durations of inpatient antimicrobial therapy (median, 5 days; interquartile range [IQR], 3–7) than patients not discharged to hospice (n = 109; median, 7 days; IQR, 4–12). When analyzing by randomization arm, we found no statistically significant difference in discharge on antimicrobial therapy (P = .14). However, patients in the control arm of the study were assigned to usual care, in which they could undergo palliative-care consultation.
Discussion
In a cohort of patients enrolled in a randomized controlled trial of early palliative-care consultation, patients who changed their code status were less likely to be discharged on antimicrobials and to receive overall shorter courses of antimicrobials. This difference was primarily driven by patients transitioning to hospice (n = 23) with 0 of 23 patients discharged on antimicrobials, a number substantially lower than in previous studies.Reference Thompson, Silveira, Vitale and Malani4
Palliative care consultants help clarify resuscitation preferences and discuss the risks and benefits of many different therapies for patients at the end of life; antimicrobials are among these. Antimicrobials in patients at the end of life are frequently used but with uncertain benefit.Reference Reinbolt, Shenk, White and Navari5, Reference Rosenberg, Albrecht and Fromme7 Our study suggests that early palliative-care consultation, even when not designed as an antimicrobial stewardship intervention, may nonetheless be effective in reducing antimicrobial consumption in patients at the end of life. Antimicrobial stewardship programs should consider engaging palliative-care providers in the development of end-of-life antimicrobial stewardship efforts.
Author ORCIDs
Jason Burnham, 0000-0002-4777-3006
Acknowledgments
Financial support
Dr. Kollef was supported by the Barnes-Jewish Hospital Foundation. This publication was made possible by the NIH-National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (grant no. UL1 TR002345, subaward KL2 TR002346). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Conflicts of interest
All authors have no conflicts of interest to report.





