Dietary fish consumption contributes to a reduced risk of primary cardiac arrest( Reference Mozaffarian and Rimm 1 , Reference James, Sullivan and Metcalf 2 ), including primary prevention of sudden cardiac death( Reference Siscovick, Raudgunathan and Weinmann 3 ). Fish and fish oils (FO) are the primary sources of the n-3 long-chain PUFA (LC-PUFA) EPA and DHA. The myocardium of humans and animals preferentially incorporates DHA( Reference Metcalf, James and Gibson 4 , Reference Owen, Peter-Przyborowska and Hoy 5 ), even at a very low dose of FO supplementation( Reference Slee, McLennan and Owen 6 ). Increased myocardial DHA concentrations can modulate myocardial oxygen consumption( Reference Pepe and McLennan 7 ) and prevent ventricular fibrillation induced by ischaemia, reperfusion or programmed electrical stimulation( Reference Pepe and McLennan 7 – Reference Pepe and McLennan 9 ). The attributable advantage of n-3 LC-PUFA incorporation is a reduction in the resting heart rate (HR)( Reference Mozaffarian, Geelen and Brouwer 10 , Reference McLennan and Abeywardena 11 ). There is additional evidence that n-3 LC-PUFA improve cardiac function and reduce HR when myocardial oxygen demand is increased( Reference McLennan 12 ). FO supplementation has been found to reduce submaximal HR, underpinned by increased parasympathetic tone, in exercise-intolerant adults( Reference Ninio, Hill and Howe 13 ). A recent review supports the notion that increased dietary fish consumption can alter autonomic tone in favour of increased parasympathetic activity( Reference Xin, Wei and Li 14 ); however, studies are heavily weighted towards populations with compromised heart function. This is also true for HR recovery, whereby limited data have indicated that FO improved HR recovery at 1 min after the cessation of exercise in post-myocardial infarct males( Reference O'Keefe, Abuissa and Sastre 15 ), in whom the aetiology of the disease state is variable.
Studies carried out to date have shown that high-dose FO supplementation lowers HR during sustained endurance exercise in healthy, well-trained subjects( Reference Buckley, Burgess and Murphy 16 , Reference Peoples, McLennan and Howe 17 ) and improves exercise economy without compromising maximal aerobic capacity or peak HR( Reference Peoples, McLennan and Howe 17 ). However, supplemental dosages of FO in both human and animal studies are in the high intake ranges (6–8 g/d in humans( Reference McLennan 12 ) and 5–10 % of the overall fatty acid content of the diet as FO in rats( Reference Owen, Peter-Przyborowska and Hoy 5 )). Therefore, it remains unconfirmed whether, in line with the epidemiological evidence, cardiac function can be improved with intakes equivalent to two fishmeals per week for cardioprotection in individuals free from heart disease.
Slee et al. ( Reference Slee, McLennan and Owen 6 ) have demonstrated that in animals a dose of 1·25 % of overall diet as FO is above the critical threshold for n-3 LC-PUFA, particularly DHA, incorporation. Due to the hyperbolic nature of the relationship, dosages of EPA+DHA >1 % energy are above the requirements for optimising the membrane incorporation of n-3 LC-PUFA. This forms the primary basis for investigating the effects of a dietary achievable ‘low dose’ of DHA on the omega-3 index (DHA+EPA) and cardiac function. In humans, erythrocyte membranes are ideal for deriving the omega-3 index, as they are easy to obtain and have been accepted to reflect myocardial membrane n-3 LC-PUFA concentrations( Reference Metcalf, James and Gibson 4 , Reference Harris and von Schacky 18 ).
The present study was carried out in physically fit males to test the hypothesis that a dietary achievable ‘low dose’ of FO provided over 8 weeks could increase tissue n-3 content and improve cardiovascular function at rest and in response to intense physical exercise. The primary outcomes were the erythrocyte omega-3 index, indicative of increased DHA concentrations in the myocardium, HR during rest and exercise, and HR recovery after high-intensity cycling. HR variability was recorded as a secondary outcome.
Experimental methods
Subjects
A total of thirty-nine physically fit and healthy males aged between 18 and 40 years were recruited to participate in the study. Pre-screening and exclusion criteria were as follows: consumption of one or more fishmeals per week; consumption of dietary supplements containing n-3 PUFA( Reference James, Sullivan and Metcalf 2 ); ≤ 4 × 60 min moderate-to-vigorous physical activity training sessions per week as determined by the International Physical Activity Questionnaire 7 d physical activity recall survey( Reference Richardson, Ainsworth and Jacobs 19 ). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Research Ethics Committee of the University of Wollongong. All subjects gave written informed consent before participating in the study.
Study design
A double-blind, parallel study design was used in accordance with the recent guidelines( Reference Welch, Antoine and Berta 20 ). Before randomisation ( − 1 week), blood pressure at baseline sleeping, resting, submaximal cycling (at three levels: 80, 125 and 150 W – used to calculate age-predicted aerobic capacity) and resting conditions was measured and used to block groups. A venous blood sample (9 ml) was also collected at − 1 week for baseline analysis of erythrocyte membrane fatty acid composition. The subjects returned to the laboratory at 0 week and undertook the exercising and recovery cycling protocol. Subjects who successfully completed the cycling protocol were randomised to either the control or intervention group via a concealment process by a third-party senior researcher. The duration of the supplementation period was 8 weeks. The subjects returned to the laboratory after the supplementation period for repeat venous blood collection (9 ml), measurements of resting cardiovascular function, and the exercising and recovery cycling protocol (Fig. 1).
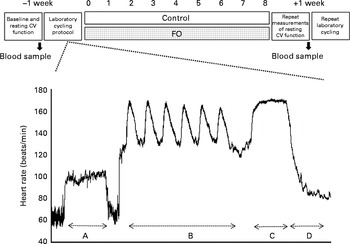
Fig. 1 Overview of the double-blind, parallel study design. Measurements were taken before and after 8 weeks of supplementation with soya bean oil (control) or fish oil (FO). The cycling and recovery protocol is expanded in the form of an example heart rate (beats/min) tracing taken from one subject using the Polar heart rate monitor. The protocol included the following: section A (10 min submaximal cycling at 125 W); section B (6 × 30 s Wingate cycling sprints/150 s recovery); section C (5 min work capacity trial); section D (supine recovery). CV, cardiovascular.
Supplementation
Randomised subjects consumed 2 × 1 g capsules/d. The control group was supplemented with soya bean oil (delivering 1004 mg/d of the n-6 PUFA linoleic acid, 400 mg/d of monounsaturated oleic acid (18 : 1n-9) and 150 mg/d of the n-3 PUFA linolenic acid (18 : 3n-3)). The intervention group was supplemented with n-3 LC-PUFA-rich, high-DHA, tuna FO capsules (kindly supplied by Nu-Mega), which delivered 140 mg of EPA and 560 mg of DHA/d. The relative fatty acid composition of the dietary supplements was quantified using GC (Table 2). To achieve double-blinding, unmarked coated capsules were prepared in blister packets by an independent third party, providing the daily consumption dosage (two capsules in each blister). Blister packs were assigned multiple colour codes, by a senior researcher, that were unknown to either the subjects or the experimenters. The experimenters were responsible for data collection, reduction and analysis before unblinding. Compliance with the supplementation programme was confirmed via a capsule count of returned blister packs and determination of n-3 incorporation into erythrocytes.
Determination of resting cardiovascular function
The resting HR of the subjects was measured in the laboratory 20 min before the cycling protocol. In addition, the subjects were supplied with a HR monitor to measure cardiac frequency under resting conditions at home. Cardiac function at home was measured from the time the subjects went to bed to the time they woke up. Each subject was instructed to lie quietly for the first 20 min in bed without going to sleep in order to measure his supine awake resting HR. This allowed analysis of the resting HR under four different conditions (laboratory, supine awake home, mean sleeping and minimum sleeping). The subjects were also supplied with an automated blood pressure monitor (Omron). Blood pressure was measured in triplicate in the morning and evening at home over three consecutive days according to the European Society of Hypertension Practice Guidelines for home blood pressure monitoring( Reference Parati, Stergiou and Asmar 21 ).
Exercising and recovery cycling protocol
The subjects completed 10 min of steady-state cycling (Fig. 1, section A) on a recumbent, electromagnetically braked cycle ergometer (Siemen-Elema 930; Siemens Elema AB). The cycle ergometer was set to a workload of 125 W and cadence was maintained at 60 ± 5 rpm. During submaximal cycling, systolic blood pressure (SBP) was measured every 2·5 min to derive the rate pressure product. The subjects then completed Wingate sprints (6 × 30 s/150 s active recovery (80 W); Fig. 1, section B). Upon completion of the sprints, after a 5 min active recovery (80 W) period, the subjects commenced the 5 min work capacity trial (Fig. 1, section C). The work capacity trial required the subjects to cycle as hard as possible during the entire 5 min. The sprints and the work capacity trial were done on an upright cycle ergometer (Wattbike). Upon completion of the cycling exercise, the subjects were allowed to recover for 10 min in a supine position under quiet conditions and dimmed lights (Fig. 1, section D). The protocol was adapted from a validated protocol for the evaluation of nutritional interventions in endurance cycling( Reference O'Hara, Thomas and Seims 22 ). The subjects consumed fluid at a dose of 20 ml/kg body weight and carbohydrates at a dose of 2 g/kg body weight before arriving for laboratory testing. Environmental room conditions of the exercise physiology laboratory were maintained at 18–20°C and 45–55 % relative humidity.
Measurements
Cardiac frequency was recorded using a polar HR monitor (Model CX800; Polar Electro Sport Tester). The RR (beat to beat) interval recordings from the HR monitors were downloaded to the Polar ProTrainer Software Program. The files were passed through an automatic filter set to low. The filter was used to remove artefacts and/or ectopic beats. The files were then exported into Kubios (Department of Physics of the University of Kuopio, Finland) for HR variability assessment. HR variability was assessed via time domain parameters including the mean NN (normalised beat to beat) interval, the standard deviation of NN intervals and the root mean square of differences of successive NN intervals. In addition, non-linear domain measures were assessed using Poincaré plots for quantitative beat-to-beat analysis of HR variability. This was done by calculating the SD1 (standard deviation of points perpendicular to the axis of line of identity), which is an index of instantaneous (vagal) beat-to-beat variation, and SD2 (standard deviation of points along the axis of line of identity), as a measure of sympathetic variability, along with the ratio of SD1:SD2, which represents the randomness in the HR variability time-series recording( Reference Camm, Malik and Bigger 23 ). Resting blood pressure (SBP/diastolic blood pressure) recorded at home over 3 d resulted in eighteen separate measurements. Individual means and standard deviations (for blood pressure variability) of SBP and diastolic blood pressure were calculated.
Fatty acid composition analysis
Incorporation of n-3 LC-PUFA into erythrocyte membranes was used as a biomarker for incorporation into myocardial membranes. At baseline and after supplementation, venous blood samples were collected into K2EDTA tubes and placed on ice until separation (3000 rpm, 4°C, 10 min) using a Hettich Universal 16R centrifuge. The erythrocyte layer and the plasma layer were stored in duplicate at − 80°C. The erythrocyte layer was thawed on ice, lysed in 10 ml of Tris buffer (10 mm, pH 7·4) and centrifuged at 4°C to separate erythrocyte membranes for fatty acid composition analysis. Membrane fatty acids were directly transesterified to produce methyl esters( Reference Lepage and Roy 24 ). The relative proportions of fatty acids incorporated into erythrocyte membranes were analysed by GC (Shimadzu GC-17A, 30 m long, 0·25 mm internal diameter capillary column). Fatty acids were identified by comparison with known standards and reported as relative percentage by weight.
Statistical analysis
Data are presented as means with their standard errors. Data were analysed using repeated-measures ANOVA with treatment and time as the main effects and treatment × time interaction. Tukey's test was used for post hoc comparisons of individual means (Statistix 10; Analytical Software). P values < 0·05 were considered to be significant.
Results
Subjects
After pre-screening and before randomisation, five subjects who self-identified as consuming more than two fishmeals per week or already taking FO supplements were excluded from the study( Reference James, Sullivan and Metcalf 2 ). In addition, four subjects were excluded because the pre-screening survey did not show evidence of ≥ 4 × 60 min moderate-to-vigorous physical activity training sessions per week. Prior to randomisation, one subject was excluded due to his intention of varying the training load during the trial and another subject was excluded because he was unable to complete the cycling protocol. Furthermore, two subjects withdrew from the study due to injury preventing them from continuing the training or undertaking the repeat post-treatment protocol, which resulted in twenty six subjects (control n 13; FO n 13) completing the dietary supplementation trial and post-testing protocol. Before supplementation, there were no significant differences between the control and FO groups (Table 1) with regard to age, anthropometric characteristics, cardiovascular health and cycling fitness defined as ‘trained’( Reference De Pauw, Roelands and Cheung 25 ). Fish consumption levels were equal and low in both groups, while physical activity levels were very high and did not change during the 8-week supplementation period (control 446 (sem 78) min/week; FO 468 (sem 58) min/week).
Table 1 Subject characteristic data collected in the laboratory environment, self-reported physical activity levels and fish consumption levels (Mean values with their standard errors)
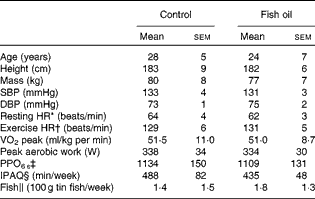
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; PPO, peak power output; IPAQ, International Physical Activity Questionnaire.
* Resting HR was measured in a seated position.
† Exercise HR was measured during 125 W steady-sate cycling.
‡ PPO6 s is the peak workload achieved in the maximal sprint cycling test over 6 s of duration.
§ IPAQ is the self-reported physical activity minutes in the previous 7 d.
∥ Fish consumption from self-reported intake of and reported as equivalent to 100 g tin portions.
Erythrocyte membrane fatty acid composition
There were no differences in erythrocyte membrane MUFA or PUFA composition at baseline or no significant changes in fatty acid composition after soya bean oil (control) supplementation (Table 2). After FO supplementation, the concentration of DHA (22 : 6n-3) in erythrocyte membranes increased significantly (P< 0·01), which underpinned the significant increase in the omega-3 index of the FO group (P< 0·01). Presented as an omega-3 index frequency distribution (within 0·5 % categories), the omega-3 index was shifted to the right from baseline in the FO group; however, no change was observed in the control group (Fig. 2). Neither the FO group nor the control group exhibited any significant change (P>0·05) in the composition of any other major fatty acid after supplementation, including linoleic acid (18 : 2n-6), which was the major component in the soya bean oil, and EPA (20 : 5n-3), which was the principal component in the FO supplement (Table 2).
Table 2 Relative percentage of major fatty acids in the dietary supplements and erythrocyte membranes (Mean values with their standard errors)

FO, fish oil; ND, not detected.
* Mean value was significantly different from that of the control group (P< 0·05).
† Mean value was significantly different from that of the control group before supplementation (P< 0·05).
‡‡‡ Mean value was significantly different from that of the control group after supplementation (P< 0·001).
§§§ Mean value was significantly different from that of the FO group before supplementation (P< 0·001).

Fig. 2 (a) Frequency distribution of the omega-3 index (EPA+DHA; 0·5 % category) at baseline (n 26) and (b) after 8 weeks of supplementation with either fish oil (FO; n 13, ![]() ) or control (n 13,
) or control (n 13, ![]() ).
). ![]() , FO/control.
, FO/control.
Resting cardiovascular function
There was no effect of FO supplementation on either resting blood pressure or blood pressure variability (Table 3), and this confirmed the excellent cardiovascular health of all the subjects. The mean resting HR recorded during the awake supine resting, average sleeping and minimum sleeping conditions were very low and did not differ between the groups at baseline (Table 3). The recorded baseline resting HR, however, varied significantly (up to 12 beats/min) from awake supine resting conditions to minimum overnight conditions (Table 3). Resting HR was not affected (P>0·05) by FO or soya bean oil supplementation under any of the recorded conditions (Table 3). There were no significant differences in HR variability recorded under supine resting conditions between the groups at baseline with respect to time or non-linear domain measures (Table 3). No significant changes were observed in the time domain parameters after FO supplementation. However, Poincaré analysis of the SD1:SD2 ratio recorded during the awake supine conditions demonstrated a decreasing trend in parasympathetic activity (SD1:SD2 ratio) in the FO group (Table 3). The average baseline SBP and diastolic blood pressure were in the healthy range and exhibited very little day-by-day variation when measured at home. There were no significant differences in blood pressure between the groups at baseline or after supplementation or no changes in any blood pressure measure within the groups after supplementation (Table 3).
Table 3 Heart rate, blood pressure and heart rate variability measured during resting conditions in the home environment (Mean values with their standard errors)

SD1, standard deviation of points perpendicular to the axis of line of identity; SD2, standard deviation of points along the axis of line of identity; NN, average time between consecutive NN intervals for recording period; SDNN, standard deviation between NN intervals; RMSSD, root mean square standard deviation between NN intervals.
* Decreasing trend in the SD1:SD2 ratio in the FO group (P= 0·18).
Exercise and recovery
Steady-state submaximal cycling
There were no significant differences between the groups with regard to baseline HR (control 117 (sem 6) beats/min; FO 120 (sem 5) beats/min), total beats over 5 min (control 586 (sem 5); FO 601 (sem 7)), SBP (control 159 (sem 3) mmHg; FO 156 (sem 3) mmHg) and the rate pressure product (control 18 592 (sem 969); FO 18 647 (sem 883)) before supplementation. No changes were observed in SBP (control 157 (sem 3) mmHg; FO 159 (sem 3)mmHg) and rate pressure product (control 17 949 (sem 850); FO 18 536 (sem 844)) after FO supplementation. However, the FO group exhibited a significant (P< 0·05) reduction ( − 22 (sem 6) beats) in total steady-state beats over 5 min cycling compared with the control group (+1 (sem 4) beats) (Fig. 3).
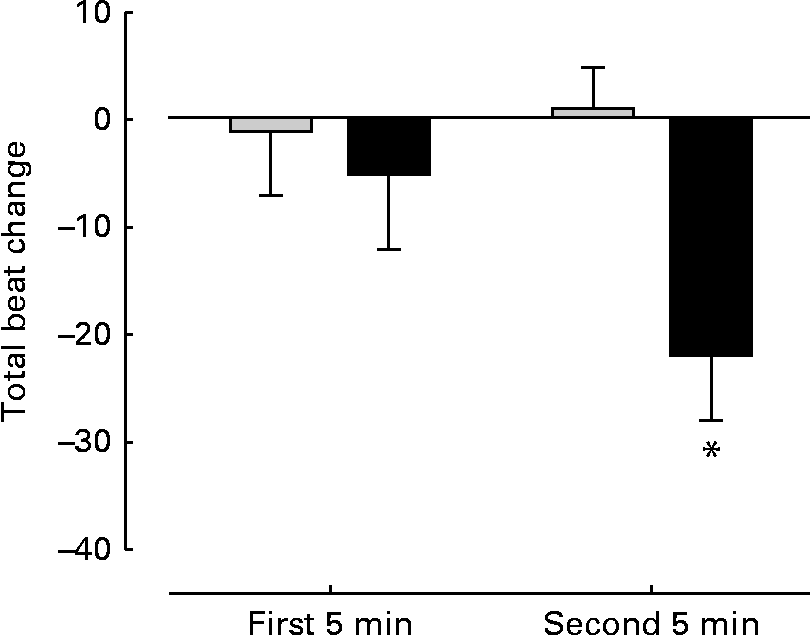
Fig. 3 Change in total heart beats during steady-state cycling. Measurements taken over the first and second 5 min of steady-state cycling. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (![]() ) after supplementation (P= 0·005).
) after supplementation (P= 0·005). ![]() , Fish oil.
, Fish oil.
Wingate supramaximal repeat-bout sprints and work capacity trial
At baseline, there were no significant differences in the peak HR during the six sprints; therefore, the peak HR during all the six sprints was averaged (control 174 (sem 1) beats/min; FO 176 (sem 1) beats/min). No changes were observed in the peak HR during the sprints (control 171 (sem 1) beats/min; FO 174 (sem 1) beats/min) after supplementation. In addition, at baseline, there were no significant differences in the peak HR measured in the final minute of the 5 min work capacity trial (control 176 (sem 1) beats/min; FO 177 (sem 1) beats/min) between the groups (P>0·05). No changes were observed in the peak HR during the final minute of the work capacity trial (control 178 (sem 1) beats/min; FO 177 (sem 1) beats/min) after supplementation. There were no significant differences between the peak HR achieved during the repeated Wingate sprints and that achieved during the work capacity trial, indicating that the subjects were exercising at their peak cardiovascular effort for each testing protocol.
Supine recovery phase
The FO and control groups commenced the recovery phase from the same peak HR (177 (sem 2) beats/min) as measured at the 5th minute of the work capacity trial. HR decreased rapidly in the first 90 s and then more slowly over the final 8 min of the recovery phase. There were no differences in HR (P>0·05) by the 10th minute of the recovery phase at baseline (control 91 (sem 3) beats/min; FO 88 (sem 2) beats/min) and after supplementation (control 89 (sem 2) beats/min; FO 89 (sem 2) beats/min). This resulted in no difference (P>0·05) being found in the average net HR between the groups. On average, the entire subject group dropped 88 beats/min ((average peak HR at the 5th minute) 177–(average 10th minute HR) 89) over the 10 min of the supine recovery phase. However, due to the non-linear nature of recovery HR, the recovery HR curves were further investigated using a single-phase logit model( Reference Stupnicki, Gabrys and Szmatlan-Gabrys 26 ), which calculates time to half recovery (t ½). At baseline, the calculated t ½ values differed from each other (control 62·3 (sem 2·3) s; FO 67·5 (sem 3·5) s; P= 0·2295). After supplementation, t ½ values were significantly reduced in the FO group ( − 8 s; 59·5 (sem 13) s, n 12) (P< 0·05), with no significant change being observed in the control group ( − 0·4 s; 61·9 (sem 2·4) s, n 13) (Fig. 4(a) and (b)). Yet, FO supplementation had no effect on non-linear HR variability in the 1st minute of the recovery phase including SD1 (control 3·6 (sem 0·3) ms; FO 3·6 (sem 0·2) ms), SD2 (control 44·7 (sem 6·2) ms; FO 48·1 (sem 6·1) ms) and SD1:SD2 ratio (control 0·088 (sem 0·010) ms; FO 0·091 (sem 0·015) ms).

Fig. 4 (a) Control logit heart rate (HR) and (b) fish oil (FO) logit HR v. log recovery time. Values are means for post-exercise (5 min work capacity trial) recovery in the control group (n 13) and the FO group (n 12), with their standard errors represented by vertical bars. The value of log time at logit = 0 corresponds to log half-recovery time (log t
½). Absolute time (s) of t
½ inset on graph. * Mean value was significantly different from that of the control group (P< 0·05). ![]() , Control pre-supplementation;
, Control pre-supplementation; ![]() , control post-supplementation;
, control post-supplementation; ![]() , FO pre-supplementation;
, FO pre-supplementation; ![]() , FO post-supplementation.
, FO post-supplementation.
Discussion
In the present study, ‘low-dose’ FO supplementation was found to increase the primary outcome measure, erythrocyte omega-3 index, indicative of the incorporation of DHA into the myocardium, in physically fit subjects free from CVD. Of physiological consequence, submaximal HR was reduced and post-exercise HR recovery was faster without compromising the peak HR. Resting HR, which was already very low due to the physically active nature of the study group, was not altered by FO supplementation, despite the vagal tone showing a decreasing trend, which would normally signal an increase in HR. Similarly, there was no measurable change in vagal tone contributing to the faster HR recovery after high-intensity cycling. These findings suggest a direct effect of FO on the heart, previously demonstrated in isolated rat hearts or atria( Reference Pepe and McLennan 7 , Reference McLennan 27 , Reference Laustiola, Salo and Metsa-Ketela 28 ) and in cardiac transplant patients, in whom vagal autonomic tone is absent( Reference Harris, Gonzales and Laney 29 ). This supports the premise that n-3 LC-PUFA exert intrinsic effects within the pacemaker region of the heart( Reference McLennan 12 ).
The mean baseline erythrocyte omega-3 index (total %EPA+DHA) was low ( < 5 %) in these subjects, in accordance with the low median or mean omega-3 index of the Australian population, which in turn is indicative of low fish or FO consumption levels( Reference Colquhoun, Ferreira Jardim and Udell 30 ). An omega-3 index < 5 % falls within the range associated with an elevated risk of cardiac disease( Reference Harris and von Schacky 18 ). The recruitment of subjects consuming low amounts of fish at baseline was highly successful, demonstrated by the frequency distribution of the entire cohort, and in addition meets the study design recommendations for demonstrating an increasing change in the omega-3 index( Reference James, Sullivan and Metcalf 2 , Reference von Schacky 31 ). Erythrocyte membranes are a reliable biomarker for the uptake of DHA into other membranes, particularly myocardial membranes( Reference Metcalf, James and Gibson 4 ). In the present study, a rightward shift was observed in the frequency distribution of the FO group, despite being supplemented at a low dose, supporting the claim of a DHA deficiency in the current Western diet( Reference Simopoulos 32 ) and the strong effect of dietary DHA supplementation. This also confirmed subjects’ compliance with the supplementation regimen( Reference Patterson, Metherel and Hanning 33 ). Importantly, incorporation of DHA into erythrocyte membranes significantly increased (with little effect on EPA) and primarily underpinned the increase in omega-3 index to 6·3 % in the FO group from the supplementation of only 4·9 g of EPA+DHA/week. The incorporation of DHA into erythrocytes lags behind its incorporation into myocardium( Reference Metcalf, James and Gibson 4 ) and may not have reached the maximum range in the 8-week supplementation period, making our measure a slight underestimate of the omega-3 index achievable with this low-dose supplementation. This is in agreement with the results of a recent dose–response study in human subjects in which supplementation of 600 mg EPA+DHA/d was found to elevate the omega-3 index to 6·8 % over 5 months in previously low fish-consuming subjects( Reference Flock, Skulas-Ray and Harris 34 ). As a 100 g serving of salmon contains approximately 2000 mg of EPA (approximately 550 mg)+DHA (approximately 1450 mg)( 35 ) and a typical salmon portion is 150–250 g, only one to two salmon meals per week would be needed to deliver the n-3 LC-PUFA equivalent to the supplemental dose used in the present study. Therefore, this supplemental dose could readily be achieved through the normal diet. Notably, soya bean oil (control supplement), containing α-linolenic acid, did not affect the omega-3 index. This supports the finding that minimal de novo synthesis of DHA in humans occurs from dietary α-linolenic acid( Reference Pawlosky, Hibbeln and Novotny 36 ). Furthermore, the control supplemental delivery of 1 g/d of n-6 linoleic acid produced no change in erythrocyte fatty acid composition, indicative of a high background n-6 PUFA dietary intake and already maximal membrane incorporation. The increased omega-3 index achieved with low-dose supplementation in the present study indicates that a modest increase in fish intake could readily modify membrane composition and translate into a reduced risk of CVD.
The present study supports the premise that the supplementation of n-3 LC-PUFA in the form of dietary FO lowers myocardial oxygen demand( Reference Pepe and McLennan 7 ) throughout the HR reserve, without affecting exercise tolerance. Specifically, the increased omega-3 index achieved with low-dose FO (2 g/d) supplementation in the physically fit males reduced the steady-state submaximal exercise HR. Previously, reductions in submaximal HR in humans have only been reported to be associated with the provision of therapeutic dosages of FO (6–8 g/d) in healthy groups( Reference Buckley, Burgess and Murphy 16 , Reference Peoples, McLennan and Howe 17 ) and patient groups( Reference O'Keefe, Abuissa and Sastre 15 , Reference Vacek, Harris and Haffey 37 ). The present study is the first to demonstrate a reduction in submaximal HR, in this case by 4·5 beats/min without compromising the peak HR or HR reserve using a dietary achievable dose. Thus, FO work in contrast to β-blockers, used extensively in slow HR treatment of hypertension and heart failure, but are associated with limited exercise tolerance( Reference Piña, Apstein and Balady 38 ) due to their property of reducing not only resting HR, but also peak HR and HR reserve.
Faster HR recovery as a result of FO supplementation has previously been observed in a myocardial infarction group with reduced ejection fraction( Reference O'Keefe, Abuissa and Sastre 15 ). HR recovery is related to the sinus node intrinsic firing rate, as well as changes in vagal (parasympathetic) and sympathetic autonomic tone( Reference Pierpont, Adabag and Yannopoulos 39 ). At baseline, all subjects exhibited HR recovery well in excess of 22 beats/min in the 1st minute after exercise, which is a validated prognostic cut-off point for an increased cardiovascular risk in cardiac patients( Reference Shetler, Marcus and Froelicher 40 ) and highlights an increased parasympathetic outflow( Reference Kannankeril, Le and Kadish 41 ) augmented by high physical activity behaviour( Reference Seals and Chase 42 , Reference Tulppo, Makikallio and Seppanen 43 ). Yet, in the present study, the significantly shorter HR recovery half-time after FO supplementation was not accompanied by a difference in vagal tone in the 1st minute of the recovery phase. Despite the great awareness of autonomic control of HR, in the case of transplanted hearts, there remains HR variability that cannot be attributed to sympathetic or parasympathetic nervous influences( Reference Bigger, Steinman and Rolnitzky 44 ). This variability in beat rate exists even in cardiomyocytes derived from human stem cells in culture( Reference Mandel, Weissman and Schick 45 ). Furthermore, when heart auto-arrhythmic cells incorporate high amounts of DHA, intrinsic beat rate is lower( Reference Verkerk, den Ruijter and Bourier 46 ), not dissimilar to the effect that physical activity has in lowering the intrinsic beat rate( Reference Lewis, Nylander and Gad 47 ), thus contributing to an efficient vagal tone output. Most importantly, incorporation of DHA exerts direct effects on heart physiology independent of vascular effects( Reference McLennan 12 ) and therefore supports the notion that intrinsic beat rate reduction may contribute to improved HR recovery.
FO supplementation has been reported to reduce resting HR in generally healthy but untrained humans( Reference Mozaffarian, Geelen and Brouwer 10 ), similar to the reductions in resting HR associated with regular physical activity( Reference Scheuer and Tipton 48 ). In the present study, FO supplementation was found to not reduce resting HR. However, the major lowering effects of FO have been observed in trials in subjects with a baseline HR ≥ 69 beats/min( Reference Christensen, Korup and Aaroe 49 ). In the present study, the resting HR of the subjects was already < 69 beats/min as expected in a trained group( Reference Tulppo, Makikallio and Seppanen 43 ), with a minimum HR of some subjects easily being < 50 beats/min. Furthermore, when measured under a variety of conditions from awake rest through to sleep, resting HR was found to vary by up to 15 beats/min, reflecting strong parasympathetic tone associated with training( Reference Nicolini, Ciulla and De Asmundis 50 ). In contrast, the decreasing trend observed in the corresponding SD1:SD2 ratio of the FO group after 8 weeks of supplementation was indicative of a reduced contribution of vagal tone to the bradycardia. Thus, the resting HR remained unchanged in these well-trained subjects, reflecting a direct influence of DHA on intrinsic beat rate( Reference McLennan 12 ), and was matched by a reciprocal reduction in vagal tone to maintain homeostasis. This supports the notion that DHA provided as dietary FO, even at a low dose, acts directly on the heart( Reference McLennan 12 ) and has the potential to act synergistically with exercise training to lower the intrinsic beat rate.
In conclusion, the results of the present study demonstrate that a low supplemental intake of FO probably increases myocardial incorporation of DHA, as measured indirectly through an increased erythrocyte omega-3 index. In subjects free from CVD, mean submaximal HR and HR recovery improved without compromising the peak HR. A relatively lower contribution of vagal tone to resting HR and HR recovery indicates a direct influence of n-3 DHA on cardiac intrinsic beat rate. The effectiveness of low-dose FO supplementation in a very fit and healthy subject population with reduced resting HR and strong HR recovery profiles already accentuated by exercise training demonstrates the potential translation through supplements or diet to diverse groups. These include patients with increased CVD risk as well as athletes and healthy ageing population groups, whereby a treat-to-target approach (10 % EPA+DHA)( Reference James, Sullivan and Metcalf 2 ) would address the extent of tissue incorporation and reciprocal physiological change such as improved cardiac function.
Acknowledgements
All authors substantially contributed to (1) the conception and design of the study and the acquisition of data or analysis and interpretation of data; (2) drafting of the article or critical revision of the article for important intellectual content; (3) final approval of the version to be published.
None of the authors has any conflicts of interest to declare.









