Article contents
Copper–nickel oxide thin film library reactively co-sputtered from a metallic sectioned cathode
Published online by Cambridge University Press: 22 November 2013
Abstract
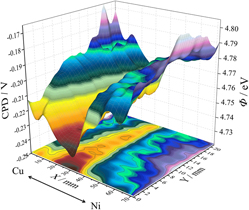
A Cu–Ni sectioned cathode made up of two hemicycles of each of the metals was used for reactive co-sputtering of a thin film combinatorial library of Cu–Ni oxides covering a total compositional spread of 63 at.%. The thickness profiling of the library showed a nonuniform film thickness with a maximum region shifted toward the Cu side of the cathode. The presence of CuO, Cu2O, NiO, and metallic Cu–Ni alloys was identified during the scanning x-ray diffraction investigations along the compositional spread. A distinct structural zone was defined between Cu–14 at.% Ni and Cu–19 at.% Ni, where the scanning electron microscopy investigations showed a higher surface porosity combined with smaller grain sizes. This zone corresponds to the maximum film thickness region and correlates well with the position of the maximum work function of the Cu–Ni oxide films as mapped using a scanning Kelvin probe. During local corrosion studies focused on Cu dissolution, an improved corrosion resistance was identified in the Ni rich side of the compositional spread.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 29 , Issue 1: Focus Issue: Synthesis of Nanostructured Functional Oxides , 14 January 2014 , pp. 148 - 157
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 11
- Cited by




