INTRODUCTION
Tuberculosis (TB) is a major public health problem in prisons of countries of high and intermediate endemicity such as Brazil [Reference Baussano1], a situation attributed to several causes: living conditions in overcrowded, poorly ventilated and poorly illuminated cells, limited prison health services and difficult access to these services. Frequent history of TB treatment and incarceration, high HIV prevalence and drug use further aggravate the situation [Reference Larouzé, Sánchez and Diuana2].
In Rio de Janeiro State, TB incidence rate in the prison system during the year previous to our study was 2686/100 000 with a 73% cure rate [3]. This incidence rate was 33 times higher compared to that of the general population of the state [4]. X-ray surveys showed active TB prevalences ranging from 4·6% to 8·6% in detainees from three different prison units [Reference Sánchez5, Reference Sánchez6] and a 2·7% prevalence in detainees entering the prison system from police remand centres [Reference Sánchez7].
TB incidence, prevalence and risk factors have been extensively investigated in inmates using conventional epidemiological methods [Reference Sánchez5–Reference Abebe10]. However, combined with these methods, molecular approaches provide more in-depth information on the dynamics of Mycobacterium tuberculosis (MTB) transmission as it allows the identification of cases presumably related to recent infections [Reference Glynn11]. This approach has proven particularly informative when applied to highly endemic prisons by demonstrating the intensive circulation of strains sharing the same genotypic profile and patterns of transmission [Reference Chaves12–Reference Rasolofo-Razanamparany14].
Previous molecular epidemiological studies of TB in prisons have been performed on cases identified mostly through passive detection of inmates. In contrast, nested in a follow-up study aimed at evaluating the impact of X-ray screenings on incidence of TB, this 13-month prospective study was conducted on TB cases identified by both passive and active detection in newly admitted and already incarcerated inmates.
METHODS
Background
In the Rio de Janeiro (RJ) State prison system (around 20 000 inmates distributed in 37 prison units), the TB control programme is based on the recommendations of the National TB Control Programme for the general population [15]: passive detection based on passive detection and supervised treatment. TB patients are treated and followed up in their own prison unit. Those who need special attention (e.g. resistant cases and HIV or diabetes-associated cases) are hospitalized in the Sanatorio Penal.
Study setting and population
The study was performed in a medium security prison unit for inmates aged >18 years with a mean duration of incarceration of 4 years (maximum 8 years) and with a high turnover (around 60% annually). Newly admitted inmates are transferred from remand centres after being sentenced or from other prisons. During the year prior to the present study, TB incidence rate in that prison was 8185/100 000, one of the highest in RJ prisons units [3].
The characteristics of this unit, which hosted 1418 inmates at the beginning of our study, are similar to those of most other prisons of RJ state and Brazil. As seen in Figure 1, it comprises, on a single level, two independent blocks, organized similarly: block A (n=785 inmates) with 12 cells and block B (n=633) with nine cells. Each cell hosts between 60 and 75 inmates. In each block, cells open onto a corridor where inmates stay during daytime whereas, during the night, they are locked in their cells. Changes from one block to another and, within the same block, from one cell to another are not infrequent, but records of these changes are not available. Corridors and cells have poor ventilation and illumination. Within cells, bunks are located on two levels.
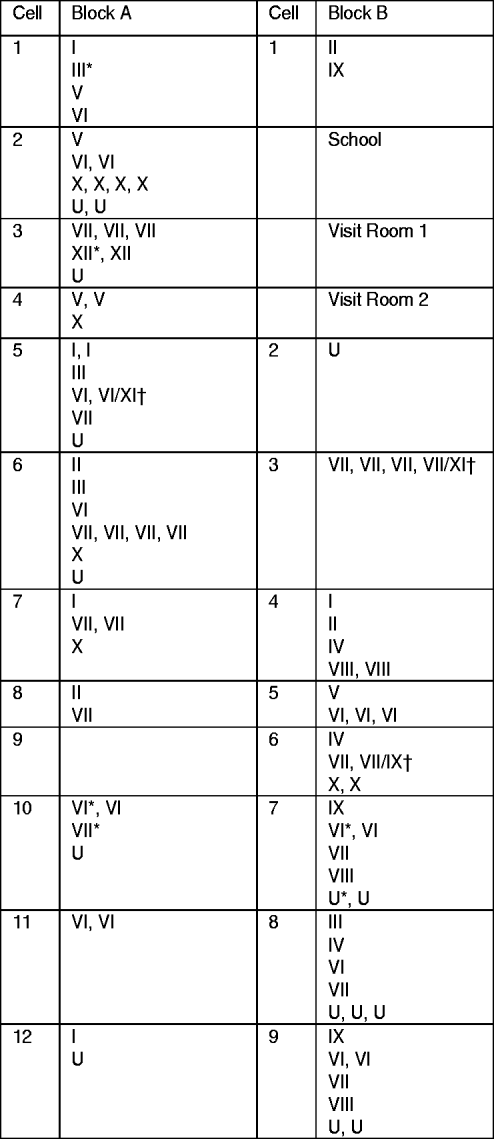
Fig. 1. Distribution of clustered and non-clustered tuberculosis cases by blocks and cells at the time of first examination in a highly endemic prison. Cases in clusters are indicated by the cluster number (from I to XII); non-clustered cases are indicated by the letter U. † Cases infected by strains belonging to two different clusters. * Cases diagnosed in inmates entering the prison.
Twice a week, inmates from each block spend 4 hours in an open courtyard. Additional contacts between inmates from the two blocks occur in the refectory, the dispensary, the prison school attended by around 30% of the detainees, and during religious services. In addition, twice a week, inmates from each block receive visits from families and friends in the courtyard.
Case detection
During our 13-month prospective study (June 2005 to July 2006), TB cases were identified according to one of the three following methods: (1) X-ray screening at entry to the prison performed within 1 week after admission; (2) X-ray cross-sectional mass screening performed at the beginning of the study and again 1 year later; (3) passive detection.
Information regarding the inmates was obtained during a face-to-face interview using a standardized questionnaire concerning sociodemographic, penal (history of incarceration and information on actual incarceration) and clinical characteristics. Additional information on place and time of previous incarceration(s) was obtained from the RJ State prison system records.
The following algorithms (Fig. 2) were used to identify our study population:
-
(1) X-ray screenings were performed in a systematic manner, independently of the presence of symptoms, and all inmates presenting any X-ray pleural, mediastinal and/or pulmonary abnormality underwent bacteriological examinations (sputum Ziehl–Neelsen smear microscopic examination and culture on Lowenstein–Jensen slant) performed in the laboratory of the Sanatorio Penal.
-
(2) All inmates attending the prison dispensary during the study period with cough of ⩾3 weeks duration or other symptoms suggestive of TB had a sputum smear microscopic examination and an X-ray. Sputum culture was performed if inmates were AFB smear-positive and/or had any X-ray abnormality.
Cases with an MTB-positive culture were included in the phenotypic and genotypic analyses. TB cases were tested for HIV antibodies according to recommendations of the Brazilian Ministry of Health [16]. Susceptibility to Rifampicin (RFM), Isoniazid (INH), Streptomycin (SM) and Ethambutol (EMB) was evaluated by the standard proportion method [Reference Canetti17] at the Professor Helio Fraga National Reference Center, ENSP, Fiocruz, MS.

Fig. 2. Diagnostic algorithms of culture-positive tuberculosis identified through X-ray screenings and passive detection in a highly endemic prison.
Genotyping
Genotyping of MTB isolates was performed by IS6110-based restriction fragment-length polymorphism (RFLP) according to a standardized method described previously [Reference van Embden18] with minor modification [Reference Saad19] at the Cellular Microbiology Laboratory, Fiocruz, MS. The DNA fingerprint patterns were analysed by computer software BioNumerics 1·50 (Applied Maths NV, Belgium) with a similarity matrix and dendogram constructed using the Dice coefficient and UPGMA algorithm with a position tolerance of 0·72%. These patterns were visually verified for consistency. Cluster pattern strains were defined as those strains having at least 90% similarity. Isolates with fewer than six IS6110 copy numbers were analysed using the spoligotyping method described by Kamerbeek et al. [Reference Kamerbeek20].
Laboratory cross-contamination was ruled out by verification in the laboratory register that strains with an identical fingerprint pattern had not been processed the same day.
Data analysis
χ2 and Fisher's exact tests were used to compare proportional variables. The Student's t test was used for analysing continuous variables. Values of P<0·05 were considered indicative of significance. In addition, the time–space pattern of TB occurrence in subjects harbouring strains belonging to a same cluster was determined in an attempt to reconstitute chains of transmission.
Ethical approval
Institutional ethical approval was obtained from the Ethics Committee for Human Research of the Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, Brazil.
RESULTS
A total of 3372 X-rays were performed for screening, a figure which represents 97·6% of the total number of X-rays expected to be performed based on the nominal lists of detainees provided by the prison administration. All eligible inmates underwent bacteriological examinations. Out of the 127 culture-positive cases diagnosed during the study period, 94/127 (74·0%) were available for genotyping including 7/14 (50·0%) cases diagnosed in newly admitted inmates, 34/48 (70·8%) during the first mass screening, 24/27 (88·9%) during the second mass screening and 29/38 (76·3%) cases identified through passive detection.
Drug susceptibility of MTB strains was tested in 89/94 (94·7%) cases. One case was resistant to RFM and INH (multidrug resistant; MDR) and five others were resistant only to SM (n=3) or INH (n=2). Another case, initially resistant to INH, was identified as being MDR 6 months later. Out of the 94 genotyped cases, 61 (64·9%) were tested for HIV, and one (1·6%) was HIV-seropositive. The comparison of the 94 genotyped cases vs. the 33 cases not genotyped did not show significant differences for sociodemographic, penal and clinical variables (data not shown).
Of the 94 genotyped cases, 79 (84·0%) were infected by strains belonging to one of the 12 clusters identified (2–21 strains each) numbered I–XII; two main clusters (VI, 18 cases; VII, 21 cases) represented 48·1% of the clustered cases. Out of the 94 genotyped cases, five (5·3%) hosted strains with fewer than six IS6110 hybridizing bands. One remained as an orphan pattern. The four others belonged to cluster III which, after strain spoligotyping, was divided into two spoligoclusters with two cases each.
Strains belonging to two different clusters were identified in three patients. The first two patients, whose infection was considered as polyclonal, were identified from the 27 TB cases whose strains were isolated from two sputum samples collected within a maximum of 15 days (Table 1). From the third patient, an INH-resistant strain belonging to cluster VII was isolated during the first mass screening and, 6 months later, a MDR strain belonging to cluster IX. All three cases were HIV-seronegative.
Table 1. Characteristics of multiple Mycobacterium tuberculosis isolates from the same patients with different genotype detected in a highly endemic prison population

* Interval between the two strain isolations.
† Time elapsed between previous and actual active TB episodes.
‡ Cure based on clinical, bacteriological and X-ray criteria.
Differences in sociodemographic, penal and clinical characteristics of clustered and non-clustered cases were not of substantial magnitude except for history of TB treatment, which was twice as frequent in clustered cases, without statistical significance (Table 2).
Table 2. Characteristics at the time of first examination of clustered and non-clustered tuberculosis cases in a highly endemic prison
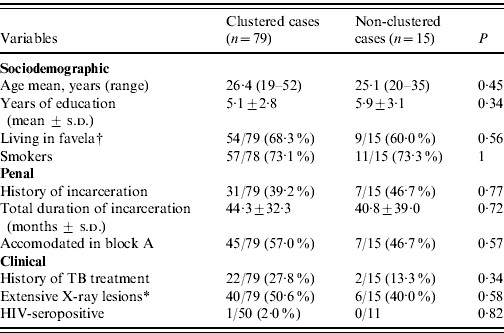
s.d., Standard deviation.
* Cases with bilateral or excavated X-ray lesions.
† An informal settlement without normal urbanization.
Of the 79 clustered cases, 22 (27·8%) had a history of TB of which seven were identified in the first screening (n=6) or at entry into the prison unit (n=1). The 15/22 (68·2%) remaining cases, identified through passive detection and during the second mass screening, had no evidence of active TB on clinical, radiological or bacteriological grounds when they were screened during the first mass screening or at prison entry, i.e. before the actual diagnosis of TB.
The proportions of clustered cases in patients who had TB strains genotyped were not substantially different (P=0·39) in cases identified by screening at admission (71·4%, 5/7), compared to cases identified in the first mass screening (85·7%, 30/35), in the second mass screening (78·3%, 18/23) or through passive detection (89·7%, 26/29).
The percentage of clustered cases was not significantly different according to the block where inmates were kept: 80·8% (45/52) in block A vs. 78·6% (33/42) in block B (P=0·46). With the exception of cluster XII (two cases) observed only in block A and cluster VIII (four cases), cluster IX (four cases) and cluster IV (three cases) in block B, cases belonging to the same cluster were distributed in both blocks (Fig. 1). In only two cells did all TB cases belong to the same cluster: cell A11 with two cases of cluster VI and cell B6 with four cases of cluster VII, including the case who successively hosted two different strains belonging to clusters VII and XI.
Given the difficulty in determining precisely the time of onset of a disease as insidious as TB, based on the patient's perception of symptoms, the data shown in Figure 3 were based on the date of the initial clinical and X-ray examination. The 29 cases identified through passive detection as well as the 24 cases identified in the second mass screening had no evidence of active TB during the first mass screening or screening at entry.

Fig. 3. Temporal distribution of tuberculosis (TB) cases at the time of first examination according to cluster in a highly endemic prison. Each circle or square represents a TB case. For each cluster, cases are distributed according to date of first examination. Open squares or circles represent cases identified in inmates at entry; shaded squares or circles represent cases identified in already incarcerated inmates during the first or second mass screening or, independently of mass screenings, through passive detection. Squares represent cases with a history of TB treatment; circles represent cases without history of TB treatment. 1 Indicates cases resistant only to INH; 2 indicates multi-drug resistant. The three cases from which strains belonging to different clusters were isolated are indicated by a line between cases.
Out of the five clustered cases identified in inmates entering the prisons, none was the first of a cluster. All of them had previously been incarcerated for 11–18 months in the same prison while at least one of the cases belonged to the same cluster. All INH-resistant and MDR cases identified during the study belonged to the same cluster (cluster IX) with the exception of the above-mentioned case with two strains successively isolated (the first strain, INH resistant, belonged to cluster VII and the second, MDR, to cluster IX).
DISCUSSION
The two main findings of our study in a prison unit where inmates were screened at entry, at the beginning of the study and again 1 year later are: (1) a high percentage (84%) of clustered cases, principally belonging to two main genotypes; (2) a large proportion (68%) of clustered strains in cases identified through passive detection and at the second mass screening.
Compared to previous molecular epidemiological studies performed in a prison setting which were based on passive detection [Reference Chaves12] with active detection limited to newly admitted inmates [Reference March13, Reference Rasolofo-Razanamparany14], our approach associating passive and active detection allows a more precise study of the MTB strain circulation. It is possible that some cases with limited X-ray lesions may have missed being diagnosed resulting in an underestimation of the real magnitude of the problem, but we emphasize that, as recommended by den Boon et al. [Reference Boon21], the criteria used to select subjects eligible for bacteriological investigations was the presence of ‘any radiological abnormality’ and not ‘presence of abnormality suggestive of TB’. Thus, X-ray was not used as a diagnostic tool but as a tool used for identification of suspected TB cases, an approach which limits the difficulties of radiological interpretation.
Our data are consistent with the fact that a majority of cases diagnosed in the prison during mass screenings and through passive detection (around 71·3% after excluding the presumed index case of each cluster) are due to recent infections by strains circulating in the prison in concomitant micro-epidemics and suggest that the strains belonging to the two main clusters may be more transmissible than others. This indicates that recent intra-institutional transmissions of TB (exogenous infection) and not reactivation of latent infections contributes substantially to the high TB endemicity. These observations are consistent with the many close contacts between inmates living in the two blocks of the prison in a confined, overcrowded, poorly ventilated and poorly illuminated environment. This explains why, with few exceptions, strains belonging to a given cluster are spread throughout the prison rather than only in a given block or cell. In this context of major overcrowding, collecting individual information on contacts between inmates in order to reconstitute transmission chains would not be feasible.
Previous studies in highly endemic prisons showed that a history of TB treatment is frequent among inmates (25·5% overall in our study population) and is a risk factor associated with TB in such settings [Reference Sánchez5–Reference Jittimanee9]. Cases with a history of TB are commonly considered as recurrent cases most often due to poor treatment compliance. The results of our study suggest that the majority of these cases are, in fact, due to recent infections (exogenous infections) acquired within the prison and are consistent with the results of a study performed in the general population of a high TB incidence area in South Africa [Reference Verver22]. In our study, this fact was also evidenced by the absence of clinical, radiological and bacteriological evidence of active TB during the first mass screening or screening of inmates at entry who were later diagnosed as TB cases through passive detection or during the second mass screening. These observations may have important implications in the management of subjects with successive episodes of active TB (e.g. the drug susceptibility of MTB strains may differ from one episode to another).
The high rate of clustering previously observed in population- and hospital-based studies [Reference Small23, Reference Alland24] and the occurrence of an outbreak in a housing facility for HIV-infected persons [Reference Daley25] were explained in part by the fact that HIV-associated immunosuppression accelerates the transition from TB infection to active TB in subjects exposed to MTB strains. Our study provided evidence that a high rate of clustering and a rapid progression of infection may also be observed in populations where TB is highly endemic, independently of HIV infection.
Several observations in the present study strongly suggest that the circulation of MTB strains should be considered not only at the scale of the prison investigated but at the scale of the entire RJ State prison system: (1) as shown in Figure 3, out of seven TB cases identified in newly admitted inmates, five belonged to one of the clusters and none was the first of a cluster; (2) the percentage of clustered strains was similar among strains identified at entry, during the mass screenings or through passive detection; (3) clustered cases identified in newly admitted inmates had been in contact with at least one of the cases belonging to the same cluster during a previous incarceration. During this period, they may have infected each other or have been infected from a common source with the same MTB strain. This important MTB strain circulation at the scale of the prison system could be related to the high turnover of inmates and the high TB endemicity in the remand centres and other prisons for men in the system.
The comparison of clustered vs. non-clustered cases for sociodemographics, penal and clinical parameters did not show significant differences for any of the variables investigated, but the statistical power of our analysis was limited due to the small number of non-clustered cases.
In this population exposed to many different MTB strains, we detected only two polyclonal infections, but our study was not designed to evaluate the frequency of this occurrence as only 27 TB patients had strains isolated from two successive sputum samples and genotyped. This procedure is necessary to accurately detect polyclonal infections as emphasized by Shamputa et al. [Reference Shamputa26] who, in a highly endemic Georgian prison, estimated the frequency of such infections to be 13%.
The MDR and the two INH-resistant cases identified belonged to a same cluster (IX) demonstrating the potential role of the prison ambiance in amplifying the diffusion of a resistant strain through micro-epidemics. The isolation from a HIV-seronegative patient of an INH-resistant strain belonging to cluster VII and, 6 months later, of an MDR strain belonging to cluster IX is most likely related to an exogenous infection from the other MDR case belonging to cluster IX who was incarcerated in the same block and was diagnosed with TB 3 months earlier. Successive infections by different MTB strains have been previously observed in HIV-infected inmates [Reference Chaves27].
The relatively low frequency of drug-resistant cases in our study population is in apparent contrast with the high frequency of past TB treatment. This may be explained by the fact that most cases with a history of TB treatment are not recurrent cases but are due to recent exogenous infections.
To control TB in prisons, conventional measures should remain the priority [Reference Sánchez5–Reference Sánchez7] including: (1) educational measures aimed at improving the awareness of inmates, guards and prison health workers in regard to TB for earlier detection of cases through passive detection; (2) implementation of active detection (screening at 12-month intervals and its feasibility considered); (3) improvement of treatment supervision in order to obtain better cure rates. However, the fact that a large proportion of cases observed in our study, even in subjects with a history of TB, were presumably due to recent exogenous infections suggests that the efficacy of any TB control strategy is likely to be limited if it is not associated with decreasing the overcrowding and implementing environmental measures aimed at limiting the extensive circulation of strains within the prison system (e.g. improvement of ventilation and natural illumination, use of ultraviolet lamps) [Reference Larouzé, Sánchez and Diuana2, Reference Noeske28]. As resources allocated to prisons and interest for inmates' wellbeing are limited, these measures should be simple, based as often as possible on low cost for the implementation of measures and maintenance of equipment to improve ventilation and illumination. These measures should be urgently integrated into any TB control programme in prisons and may impact on other communicable diseases as well. They should be applied to the construction of new prison units and the rehabilitation of old ones without necessarily incurring major costs, but are likely to have an important effect on the incidence of TB, not only for the benefit of inmates, but also for the professionals working within the prisons, the families of inmates and the communities where the inmates will reintegrate after being freed. Hence, the Brazilian Ministries of Health (National TB programme) and Justice together with the TB Brazilian Global Fund initiated in 2010 a programme aimed at implementing, in partnership with architects, these environmental measures in the prisons of Brazil [Reference Sánchez, Diuana and Larouzé29].
ACKNOWLEDGEMENTS
The authors thank Mahinda Siriwardana for his editorial assistance and the Professor Helio Fraga National Reference Centre, ENSP, FIOCRUZ, for its technical and logistical contributions. This study was supported by funds from the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Conselho Nacional de Pesquisa – Ministério de Ciências e Tecnologia do Brasil (CNPQ), the Fundação Oswaldo Cruz (FIOCRUZ) and the International Cooperation Programme FIOCRUZ – Institut National de la Santé et de la Recherche Médicale, France.
DECLARATION OF INTEREST
None.







