Significant outcomes
-
Antibodies against myelin oligodendrocyte glycoprotein (MOG) are considered pathogenic and associated with MOG encephalomyelitis, which is a demyelinating central nervous system disease with an expanding clinical spectrum.
-
Therefore, two patients with psychosis and anti-MOG antibodies (detected via fixed cell-based and live cell-based assays), non-specific MRI and EEG alterations, and increased cerebrospinal fluid protein (in only one of these two patients) are presented.
-
The anti-MOG antibodies could be relevant, but due to moderate titres, they may have caused a rather ‘subtle’ clinical picture consisting of psychosis instead of ‘classical’ MOG encephalomyelitis.
Limitations
-
Neither case featured the established clinical symptoms or diagnostic findings associated with ‘classical’ MOG encephalomyelitis or definite autoimmune psychosis.
-
A false-positive assay was unlikely due to testing in two laboratories with different methodologies. However, the anti-MOG antibodies could have been 1) correct-positive but not clinically relevant, 2) correct-positive but not functional, or 3) coincidental.
-
To prove an association between psychosis and anti-MOG antibodies would require more clinical cases, and researchers must more fully study the biochemistry of the antibodies, especially in patients with psychosis.
Background
Schizophreniform psychoses are severe mental disorders that often result in significant impairment of the quality of life (Owen et al., Reference Owen, Sawa and Mortensen2016; Dziwota et al., Reference Dziwota, Stepulak, Włoszczak-Szubzda and Olajossy2018). Immunological causes have been identified in a small subset of patients, recently summarised as autoimmune psychosis (AP) (Pollak et al., Reference Pollak, Lennox, Müller, Benros, Prüss, Tebartz van Elst, Klein, Steiner, Frodl, Bogerts, Tian, Groc, Hasan, Baune, Endres, Haroon, Yolken, Benedetti, Halaris, Meyer, Stassen, Leboyer, Fuchs, Otto, Brown, Vincent, Najjar and Bechter2020). AP cases typically occur due to anti-neuronal antibodies against cell-surface antigens (e.g. against the N-methyl-D-aspartate receptor [NMDA-R]) but are also, albeit rarely, associated with antibodies against intracellular antigens (e.g. with anti-Hu antibodies) (Pollak et al., Reference Pollak, Lennox, Müller, Benros, Prüss, Tebartz van Elst, Klein, Steiner, Frodl, Bogerts, Tian, Groc, Hasan, Baune, Endres, Haroon, Yolken, Benedetti, Halaris, Meyer, Stassen, Leboyer, Fuchs, Otto, Brown, Vincent, Najjar and Bechter2020). However, the role of anti-myelin antibodies against MOG in this context remained unclear, and they are usually not examined in the routine workup of patients with psychosis (Endres et al., Reference Endres, Leypoldt, Bechter, Hasan, Steiner, Domschke, Wandinger, Falkai, Arolt, Stich, Rauer, Prüss and van Elst2020a; Reference Endres, Matysik, Feige, Venhoff, Schweizer, Michel, Meixensberger, Runge, Maier, Nickel, Bechter, Urbach, Domschke and Tebartz van Elst2020b; Pollak et al., Reference Pollak, Lennox, Müller, Benros, Prüss, Tebartz van Elst, Klein, Steiner, Frodl, Bogerts, Tian, Groc, Hasan, Baune, Endres, Haroon, Yolken, Benedetti, Halaris, Meyer, Stassen, Leboyer, Fuchs, Otto, Brown, Vincent, Najjar and Bechter2020). Anti-MOG antibodies were originally assumed to be involved in multiple sclerosis (MS) (Lalive et al., Reference Lalive, Menge, Delarasse, Della Gaspera, Pham-Dinh, Villoslada, von Büdingen and Genain2006). Recent studies have reported relevant associations between anti-MOG antibodies and optic neuritis, myelitis, brainstem encephalitis, and – predominantly in children – acute disseminated encephalomyelitis (ADEM)-like presentations (Mariotto et al., Reference Mariotto, Ferrari, Monaco, Benedetti, Schanda, Alberti, Farinazzo, Capra, Mancinelli, De Rossi, Bombardi, Zuliani, Zoccarato, Tanel, Bonora, Turatti, Calabrese, Polo, Pavone, Grazian, Sechi, Sechi, Urso, Delogu, Janes, Deotto, Cadaldini, Bianchi, Cantalupo, Reindl and Gajofatto2017; Waters et al., Reference Waters, Fadda, Woodhall, O’Mahony, Brown, Castro, Longoni, Irani, Sun, Yeh, Marrie, Arnold, Banwell and Bar-Or2020). Based on a growing number of research studies, anti-MOG antibody-associated disorders are now considered to constitute a separate disease entity from MS, and the term MOG encephalomyelitis has been established (Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). MOG encephalomyelitis is particularly characterised by symptoms of monophasic or recurrent acute optic neuritis, myelitis, encephalitis, brainstem encephalitis, or any combination of these syndromes. Recently, an expansion of the spectrum of symptomatology has been described, including disturbance of consciousness, behavioural changes, and epileptic seizures (Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). The median age at onset of MOG encephalomyelitis patients was 35 years, with 59% of them being female, and the disease course was mostly relapsing (50%) (Jarius et al., Reference Jarius, Pellkofer, Siebert, Korporal-Kuhnke, Hümmert, Ringelstein, Rommer, Ayzenberg, Ruprecht, Klotz, Asgari, Zrzavy, Höftberger, Tobia, Buttmann, Fechner, Schanda, Weber, Asseyer, Haas, Lechner, Kleiter, Aktas, Trebst, Rostasy, Reindl, Kümpfel, Paul and Wildemann2020). According to recommended indications for anti-MOG antibody testing, diagnostic examinations should show abnormalities in magnetic resonance imaging (MRI), cerebrospinal fluid (CSF), and electrophysiological investigations (Jarius et al., Reference Jarius, Ruprecht, Kleiter, Borisow, Asgari, Pitarokoili, Pache, Stich, Beume, Hümmert, Trebst, Ringelstein, Aktas, Winkelmann, Buttmann, Schwarz, Zimmermann, Brandt, Franciotta, Capobianco, Kuchling, Haas, Korporal-Kuhnke, Lillevang, Fechner, Schanda, Paul, Wildemann and Reindl2016a Reference Jarius, Ruprecht, Kleiter, Borisow, Asgari, Pitarokoili, Pache, Stich, Beume, Hümmert, Ringelstein, Trebst, Winkelmann, Schwarz, Buttmann, Zimmermann, Kuchling, Franciotta, Capobianco, Siebert, Lukas, Korporal-Kuhnke, Haas, Fechner, Brandt, Schanda, Aktas, Paul, Reindl and Wildemann2016b; Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). The anti-MOG immunoglobulin G (IgG) antibodies are mostly produced extrathecally, and serum titres are therefore higher than CSF levels, so serum detection is usually sufficient (Jarius et al., Reference Jarius, Ruprecht, Kleiter, Borisow, Asgari, Pitarokoili, Pache, Stich, Beume, Hümmert, Ringelstein, Trebst, Winkelmann, Schwarz, Buttmann, Zimmermann, Kuchling, Franciotta, Capobianco, Siebert, Lukas, Korporal-Kuhnke, Haas, Fechner, Brandt, Schanda, Aktas, Paul, Reindl and Wildemann2016b, Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). In 77% of cases, a brain MRI has shown abnormalities, mostly with infratentorial lesions (32%) or non-specific lesions (23%), while spinal cord MRI has shown pathologies in 58% of cases (Mariotto et al., Reference Mariotto, Ferrari, Monaco, Benedetti, Schanda, Alberti, Farinazzo, Capra, Mancinelli, De Rossi, Bombardi, Zuliani, Zoccarato, Tanel, Bonora, Turatti, Calabrese, Polo, Pavone, Grazian, Sechi, Sechi, Urso, Delogu, Janes, Deotto, Cadaldini, Bianchi, Cantalupo, Reindl and Gajofatto2017). In more than 50% of CSF samples, the white blood cell count has been elevated and a blood–CSF barrier dysfunction has been detected in 48% of the affected patients (Jarius et al., Reference Jarius, Pellkofer, Siebert, Korporal-Kuhnke, Hümmert, Ringelstein, Rommer, Ayzenberg, Ruprecht, Klotz, Asgari, Zrzavy, Höftberger, Tobia, Buttmann, Fechner, Schanda, Weber, Asseyer, Haas, Lechner, Kleiter, Aktas, Trebst, Rostasy, Reindl, Kümpfel, Paul and Wildemann2020). Electrophysiological investigations may reveal altered visually evoked potentials (VEPs) in patients with optic neuritis (Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). Treatment approaches in acute attacks suggest the use of high-dose corticosteroids and plasma exchange; for maintenance therapy azathioprine, mycophenolate mofetil, or rituximab are used (Whittam et al., Reference Whittam, Karthikeayan, Gibbons, Kneen, Chandratre, Ciccarelli, Hacohen, de Seze, Deiva, Hintzen, Wildemann, Jarius, Kleiter, Rostasy, Huppke, Hemmer, Paul, Aktas, Pröbstel, Arrambide, Tintore, Amato, Nosadini, Mancardi, Capobianco, Illes, Siva, Altintas, Akman-Demir, Pandit, Apiwattankul, Hor, Viswanathan, Qiu, Kim, Nakashima, Fujihara, Ramanathan, Dale, Boggild, Broadley, Lana-Peixoto, Sato, Tenembaum, Cabre, Wingerchuk, Weinshenker, Greenberg, Matiello, Klawiter, Bennett, Wallach, Kister, Banwell, Traboulsee, Pohl, Palace, Leite, Levy, Marignier, Solomon, Lim, Huda and Jacob2020).
The rationale of this article is to present and discuss a possible relationship between psychosis and anti-MOG antibodies based on two case reports.
Methods
A retrospective analysis of a cohort of lumbar-punctured patients from the Department of Psychiatry and Psychotherapy of the Medical Center of the University of Freiburg was approved by the local ethics committee (Faculty of Medicine, University of Freiburg, No. 396/18). The positive anti-MOG antibody finding in patient 2 has already been reported in earlier papers (Endres et al., Reference Endres, Matysik, Feige, Venhoff, Schweizer, Michel, Meixensberger, Runge, Maier, Nickel, Bechter, Urbach, Domschke and Tebartz van Elst2020b, Reference Endres, Meixensberger, Dersch, Feige, Stich, Venhoff, Matysik, Maier, Michel, Runge, Nickel, Urbach, Domschke, Prüss and Tebartz van Elst2020c), but written informed consent was obtained additionally from both patients for the preparation of this cumulative case report. Two thoroughly investigated cases of patients with schizophreniform psychosis and anti-MOG antibodies in serum can therefore be presented. Both patients were diagnosed following the principles of the Freiburg Diagnostic Protocol in Psychosis (Endres et al., Reference Endres, Matysik, Feige, Venhoff, Schweizer, Michel, Meixensberger, Runge, Maier, Nickel, Bechter, Urbach, Domschke and Tebartz van Elst2020b).
Results
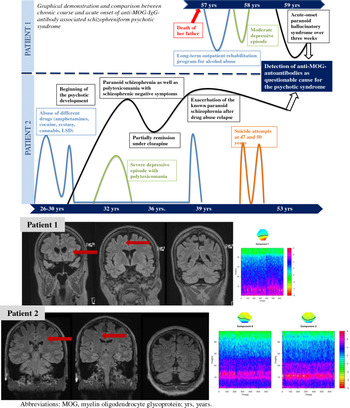
The clinical courses and findings of both patients are summarised in Fig. 1 and Table 1.

Fig. 1. Clinical course of two patients with anti-MOG autoantibody-associated schizophreniform psychosis. Patients 1 and 2 showed singular, non-specific white matter lesions (marked with arrow) in the magnetic resonance imaging. The white matter changes in patient 1 were somewhat more prominent, but still non-specific. In time–frequency analysis of selected independent components of the clinical electroencephalography sessions, patient 1 had right occipital intermittent rhythmic delta activity (component 7; peak at 6.6 Hz, distinct from 9 Hz alpha), patient 2 had a disorganised alpha activity (component 6; peaks at 9.8 and 13 Hz), which normalised after valproate treatment (component 2; peak at 11.3 Hz).
Table 1. Diagnostic findings in both patients (all altered findings are marked in bold). Serum was screened for anti-neuronal antibodies against intracellular antigens (Yo, Hu, CV2/CRMP5, Ri, Ma1/2, SOX1, Tr, Zic4, GAD65, amphiphysin) using an immunoblot (Ravo-Blot, Freiburg, Germany); serum and CSF samples were examined for anti-neuronal cell-surface antibodies (NMDA-R, LGI1, CASPR2, AMPA1/2-R, GABA-B-R, DPPX) by fixed cell-based biochip assays (Euroimmun assays, Lübeck, Germany); and serum was analysed for AQP4 and MOG antibodies using biochip assays (Euroimmun assays, Lübeck, Germany). A live cell-based assay for anti-MOG antibody detection was also performed (Labor Krone, Bad-Salzuflen, Bielefeld, Germany). EEGs were analysed using independent component analysis (ICA). MRIs and VEPs were investigated visually by experienced senior physicians.
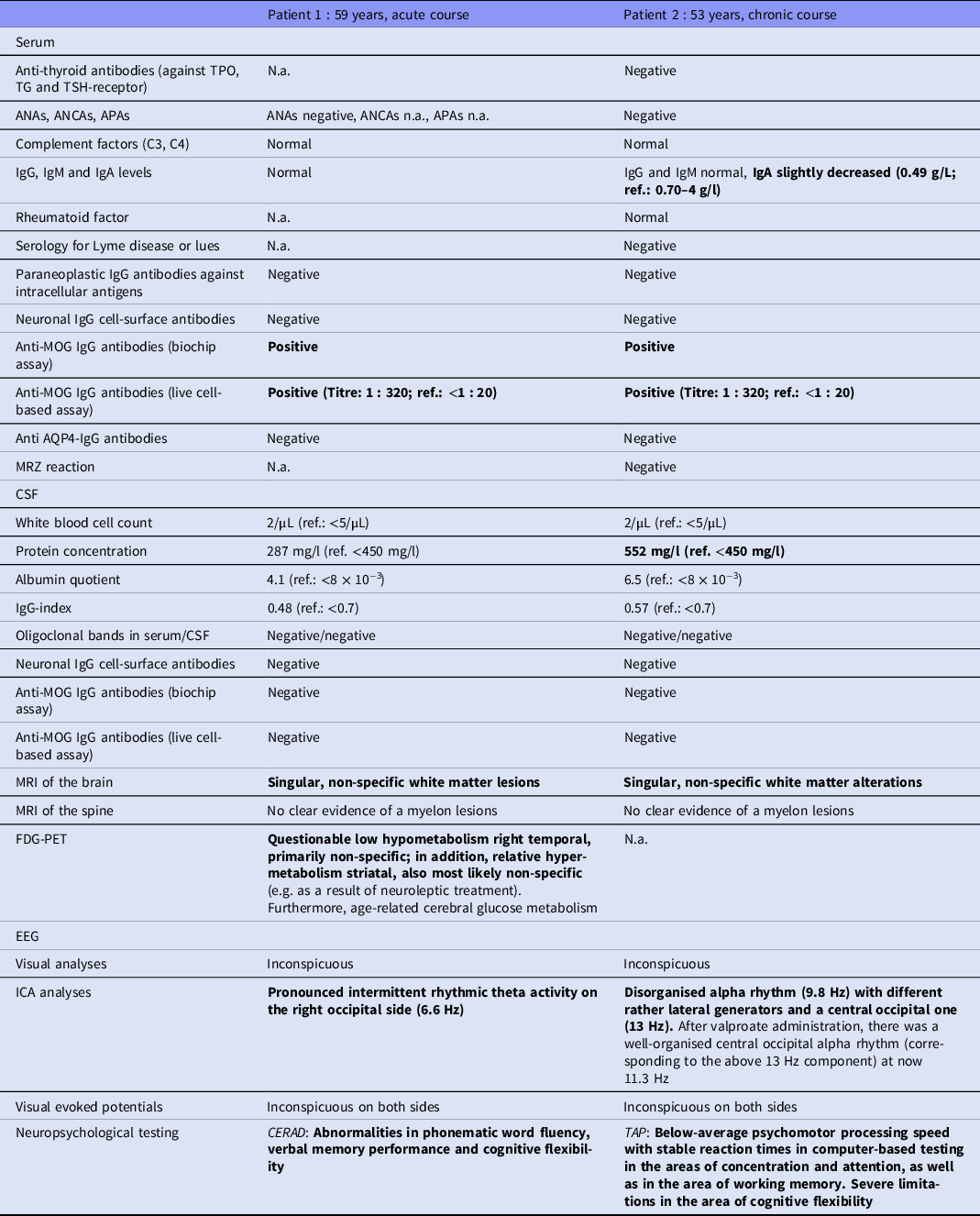
ANAs, antinuclear antibodies; ANCAs, anti-neutrophil cytoplasmic antibodies; APAs, antiphospholipid antibodies; AQP4, anti-aquaporin 4; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CSF, cerebrospinal fluid; EEG, electroencephalography; FDG-PET, [18F]fluorodeoxyglucose positron emission tomography; ICA, independent component analysis; Ig, immunoglobulin; MRI, magnetic resonance imaging; MRZ, antibody indices against measles, rubella, and varicella zoster virus; N.a., not available; ref., reference; TAP, test of attentional performances; TG, thyroglobulin; TPO, thyroid peroxidase; TSH, thyroid-stimulating hormone receptor.
Patient 1
A 59-year-old female patient presented with an acute-onset paranoid–hallucinatory syndrome lasting more than 3 weeks. She experienced paranoid thoughts about her neighbours and heard voices that were encouraging her to commit suicide. She was experiencing states of anxiety and felt threatened. Over the course of a year, she became increasingly anxious about the people surrounding her. Her father had died a year previously, and family members suspected that this might have triggered the psychotic episode. She had not experienced psychotic symptoms in the past, but had taken part in a long-term outpatient rehabilitation program for alcohol abuse at the age of 57.
Diagnostic findings
Neurological examination showed no pathological findings. Anti-MOG antibodies were repeatedly positive in serum using fixed biochip assays, but negative in CSF. Using live cell-based assays, an antibody titre of 1 : 320 (ref. < 1 : 20) was detected. CSF analysis showed no pathological findings. Due to a possible familial predisposition to dementia, dementia markers in the CSF were investigated at an external reference laboratory (University Hospital, Göttingen, Germany). The findings were unremarkable, except for elevated phospho-tau of 82 pg/ml (ref.: <61 pg/ml). MRI identified singular, non-specific white matter lesions. ICA of the EEG revealed a pronounced intermittent rhythmic theta activity (IRTA) on the right occipital side (6.6 Hz). The VEPs and sensory evoked potentials were inconspicuous.
Somatic illness and family history
The patient reported to have been smaller, paler, and more frequently sick than her two sisters. She suffered from Hashimoto’s thyroiditis but had no history of cancer or neurological disorders. Also, there was no known family history of autoimmune disorders. Her parents, however, did develop dementia in late life.
Treatment
The patient was treated with risperidone and olanzapine, leading to clinical improvement. However, in stressful situations, auditory hallucinations recurred. No immunotherapy was performed.
Patient 2
A 53-year-old male patient suffered from a chronic paranoid-hallucinatory syndrome with intermittent states of confusion. The current episode was characterised by thought insertion of contents of his own past, thought withdrawal, intrusive thoughts, and rumination. He repeatedly experienced states of confusion lasting from minutes to hours wandering around in a disoriented manner. In the past, he had suffered from abuse of multiple substances (amphetamine/cocaine/ecstasy/cannabis/LSD), beginning at the age of 26, and had developed psychotic episodes with intermittent exacerbations, chronification, and reduced functioning since the age of 31. Due to the psychotic symptoms, he was hospitalised several times and received a variety of psychotropic drugs.
Diagnostic findings
Neurological examination showed no pathological findings. Anti-MOG antibodies were repeatedly positive in serum using fixed cell-based assays, but negative in CSF. Live cell-based assays demonstrated titres of 1 : 320 (ref.: <1 : 20). Routine CSF analysis showed an increased protein concentration but otherwise normal findings. MRI analyses revealed no inflammatory lesions. The ICA analysis detected a strong disorganised alpha rhythm with multiple lateral generator areas at 9.8 Hz and one central occipital area at 13 Hz. The VEPs were inconspicuous on both sides.
Somatic illness and family history
The patient has no history of cancer, autoimmune, or neurological disorders, but his mother and grandfather both suffered from bowel cancer.
Treatment
During his most recent episode, the patient had received risperidone in addition to prescribed venlafaxine. This led to an improvement regarding psychotic symptoms. Following additional treatment with valproate, the intermittent confused states vanished, and the EEGs showed a well-organised occipital alpha rhythm, now at 11.3 Hz. Negative symptoms persisted. No immunotherapy was performed.
Discussion
Two cases of anti-MOG antibody-positive psychosis are presented in this article: Patient 1 had late-onset psychosis, non-specific white matter changes in the MRI, and EEG slowing. Patient 2 had a severe chronic course, non-specific MRI and EEG alterations, and an increased protein concentration in the CSF. In the further course of this article, we will review the relevant literature, put the two cases into perspective, and discuss the possible causal significance of the observed anti-MOG antibodies in these cases.
Current state of the literature
The literature to date is limited; a PubMed search on March 7, 2021, using the keywords ‘(MOG AND psychosis) OR (MOG AND schizop*)’ resulted in only 26 hits. A large serum survey of 1378 patients with schizophrenia showed a prevalence of anti-MOG antibodies of 1.1%, with IgG antibodies described for only one patient (0.07%; 4 patients had IgA and 10 had IgM antibodies). In comparison, in the healthy control group of 1694 people, only 0.5% showed seropositivity for anti-MOG antibodies, and one patient (0.06%) showed IgG seropositivity (six patients had IgM and two had IgA antibodies) (Dahm et al., Reference Dahm, Ott, Steiner, Stepniak, Teegen, Saschenbrecker, Hammer, Borowski, Begemann, Lemke, Rentzsch, Probst, Martens, Wienands, Spalletta, Weissenborn, Stöcker and Ehrenreich2014). The titre range was described as 1 : 10–1 : 320, but in the single patient and control subject with positive results for anti-MOG IgG antibodies there were no further reports of additional clinical investigations or other individual clinical data. In neurology, generally only anti-MOG IgG antibodies are investigated, since the relevance of isolated IgM or IgA antibodies remains unclear (Mariotto et al., Reference Mariotto, Ferrari, Monaco, Benedetti, Schanda, Alberti, Farinazzo, Capra, Mancinelli, De Rossi, Bombardi, Zuliani, Zoccarato, Tanel, Bonora, Turatti, Calabrese, Polo, Pavone, Grazian, Sechi, Sechi, Urso, Delogu, Janes, Deotto, Cadaldini, Bianchi, Cantalupo, Reindl and Gajofatto2017; Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018). A smaller case series of 76 schizophrenia patients and 34 controls merely investigating anti-MOG IgG antibodies, revealed that all the patients and controls were negative (Mantere et al., Reference Mantere, Saarela, Kieseppä, Raij, Mäntylä, Lindgren, Rikandi, Stoecker, Teegen and Suvisaari2018). In another study, Gerhards and colleagues investigated antibodies against another myelin protein, oligodendrocyte myelin glycoprotein (OMGP), in patients with different inflammatory neurological diseases (N = 429), non-inflammatory disease controls (N = 45), and healthy individuals (N = 114). They detected antibodies against OMGP only in patients with MS (in 2%) or ADEM (in 4%), with the exception of one patient with psychosis (Gerhards et al., Reference Gerhards, Pfeffer, Lorenz, Starost, Nowack, Thaler, Schlüter, Rübsamen, Macrini, Winklmeier, Mader, Bronge, Grönlund, Feederle, Hsia, Lichtenthaler, Merl-Pham, Hauck, Kuhlmann, Bauer, Beltran, Gerdes, Mezydlo, Bar-Or, Banwell, Khademi, Olsson, Hohlfeld, Lassmann, Kümpfel, Kawakami and Meinl2020). In the literature search, only one case report of a patient with psychosis and anti-MOG antibodies was found, but this patient also had comorbid anti-NMDA-R encephalitis (Zhou et al., Reference Zhou, Tan, Tan, Hu, Chen and Wang2018). The association of anti-NMDAR and anti-MOG antibodies is not new and has been reported in 4.0%–7.5% of patients with anti-NMDA-R encephalitis (Martinez-Hernandez et al., Reference Martinez-Hernandez, Guasp, García-Serra, Maudes, Ariño, Sepulveda, Armangué, Ramos, Ben-Hur, Iizuka, Saiz, Graus and Dalmau2020). In such cases, the psychotic symptoms can be well explained by anti-NMDA-R encephalitis, in the context of which paranoid–hallucinatory symptoms are often the presenting complaint (Graus et al., Reference Graus, Titulaer, Balu, Benseler, Bien, Cellucci, Cortese, Dale, Gelfand, Geschwind, Glaser, Honnorat, Höftberger, Iizuka, Irani, Lancaster, Leypoldt, Prüss, Rae-Grant, Reindl, Rosenfeld, Rostásy, Saiz, Venkatesan, Vincent, Wandinger, Waters and Dalmau2016; Dalmau et al., Reference Dalmau, Armangué, Planagumà, Radosevic, Mannara, Leypoldt, Geis, Lancaster, Titulaer, Rosenfeld and Graus2019). However, this does not explain the symptoms in the two cases reported here, as both were negative for anti-NMDA-R antibodies in serum and CSF.
Arguments for a causal role for anti-MOG antibodies
From a clinical perspective, the late-onset of disease in patient 1 was striking, but a second post-menopausal peak of incidence does also occur in females with idiopathic schizophrenia (Chen et al., Reference Chen, Selvendra, Stewart and Castle2018). The white matter alterations in MRI and the EEG slowing are non-specific findings which do neither exclude MOG encephalomyelitis nor prove it. In patient 2, the severe and chronic course and the atypical intermittent confused states are remarkable. Confusional states have previously been described in the context of MOG encephalomyelitis (Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018), and an increased protein concentration would be compatible with MOG encephalomyelitis (although it is a completely non-specific finding). It is also interesting that the patient benefited mainly from anticonvulsants and that there was normalisation of the EEG following this treatment. Speculatively, this could be indicative of a paraepileptic genesis that is secondary to an inflammatory process (Tebartz van Elst et al., Reference Tebartz van Elst, Stich and Endres2015). From a pathophysiological point of view, anti-MOG antibodies are considered pathogenic given their extracellular target (Spadaro et al., Reference Spadaro, Winklmeier, Beltrán, Macrini, Höftberger, Schuh, Thaler, Gerdes, Laurent, Gerhards, Brändle, Dornmair, Breithaupt, Krumbholz, Moser, Krishnamoorthy, Kamp, Jenne, Hohlfeld, Kümpfel, Lassmann, Kawakami and Meinl2018) and have been shown to cause experimental autoimmune encephalitis in animal models (Lalive et al., Reference Lalive, Molnarfi, Benkhoucha, Weber and Santiago-Raber2011). Clinical recommendations include testing in two different laboratories (as done here) to reduce false-positive cases. Therefore, a false-positive result is unlikely. Alterations in MOG genes have also been described as playing a role in the pathogenesis of schizophrenia. The expression of MOG, as well as the MOG gene, appears to be downregulated in schizophrenia (Tkachev et al., Reference Tkachev, Mimmack, Ryan, Wayland, Freeman, Jones, Starkey, Webster, Yolken and Bahn2003; Liu et al., Reference Liu, Qin, He, Yang, Chen, Zhou, Li, Gu, Xu, Feng, Sang, Hao, Zhang, Wang and He2005; Sokolov, Reference Sokolov2007). This gene is located on chromosome 6p21.3, which is considered to comprise a high-susceptibility area for schizophrenia within the major histocompatibility complex locus (Tkachev et al., Reference Tkachev, Mimmack, Ryan, Wayland, Freeman, Jones, Starkey, Webster, Yolken and Bahn2003). There is also evidence of impaired oligodendrocyte homeostasis in schizophrenia (Martins-de-Souza et al., Reference Martins-de-Souza, Gattaz, Schmitt, Rewerts, Marangoni, Novello, Maccarrone, Turck and Dias-Neto2009; Marui et al., Reference Marui, Torii, Iritani, Sekiguchi, Habuchi, Fujishiro, Oshima, Niizato, Hayashida, Masaki, Kira and Ozaki2018). A recent study has demonstrated that antibodies against a different myelin protein (OMGP) can be detected in patients with psychosis (Gerhards et al., Reference Gerhards, Pfeffer, Lorenz, Starost, Nowack, Thaler, Schlüter, Rübsamen, Macrini, Winklmeier, Mader, Bronge, Grönlund, Feederle, Hsia, Lichtenthaler, Merl-Pham, Hauck, Kuhlmann, Bauer, Beltran, Gerdes, Mezydlo, Bar-Or, Banwell, Khademi, Olsson, Hohlfeld, Lassmann, Kümpfel, Kawakami and Meinl2020). These findings may illustrate that dysregulation of oligodendrocyte homeostasis and function – as might be caused by the antibodies – could be associated with psychosis.
Arguments against a causal role for anti-MOG antibodies
From a clinical perspective, in patient 1, the CSF without inflammatory changes, the EEG, the MRI of the spine, and the VEPs argue against MOG encephalomyelitis, and the positive partial response to antipsychotics also suggests idiopathic schizophrenia, although a symptomatic effect of antipsychotics cannot be ruled out, even in organic processes (Endres et al., Reference Endres, Leypoldt, Bechter, Hasan, Steiner, Domschke, Wandinger, Falkai, Arolt, Stich, Rauer, Prüss and van Elst2020a). In addition, a schizophreniform presentation may also have developed as a consequence of early dementia given the positive family history of this disease and elevated phospho-tau in CSF. In patient 2, an unremarkable CSF – except for an elevated protein concentration – inconspicuous VEPs, and both cranial and spinal cord MRIs without inflammatory lesions all point against a causal role, with only a non-specifically altered EEG suggesting otherwise. The severity and chronic course could also be a sequel of the past multiple substance abuse and would be compatible with a chronic course of schizophrenia. The typical symptoms of MOG encephalomyelitis and inflammatory MRI or CSF findings are certainly not found in either patient. Although the MOG gene appears to play a potential role in the pathogenesis of schizophrenia in some studies, the exact pathomechanisms seem to be significantly more complex (Owen et al., Reference Owen, Sawa and Mortensen2016; Sekar et al., Reference Sekar, Bialas, de Rivera, Davis, Hammond, Kamitaki, Tooley, Presumey, Baum, Van Doren, Genovese, Rose, Handsaker, Daly, Carroll, Stevens and McCarroll2016; Huckins et al., Reference Huckins, Dobbyn, Ruderfer, Hoffman, Wang, Pardiñas, Rajagopal, Als, Nguyen, Girdhar, Boocock, Roussos, Fromer, Kramer, Domenici, Gamazon, Purcell, Demontis, Børglum, Walters, O’Donovan, Sullivan, Owen, Devlin, Sieberts, Cox, Im, Sklar and Stahl2019). Given these considerations, no immunotherapy was performed in either patient, so neither positive nor negative responses could be observed or discussed.
Conclusion
Based on our literature search, these are the first two detailed case presentations of patients with psychosis and anti-MOG antibodies (without comorbid anti-NMDA-R encephalitis). Both cases clearly do not meet the established clinical symptoms or diagnostic findings for classical MOG encephalomyelitis (Jarius et al., Reference Jarius, Paul, Aktas, Asgari, Dale, de Seze, Franciotta, Fujihara, Jacob, Kim, Kleiter, Kümpfel, Levy, Palace, Ruprecht, Saiz, Trebst, Weinshenker and Wildemann2018) or definite autoimmune psychosis (Pollak et al., Reference Pollak, Lennox, Müller, Benros, Prüss, Tebartz van Elst, Klein, Steiner, Frodl, Bogerts, Tian, Groc, Hasan, Baune, Endres, Haroon, Yolken, Benedetti, Halaris, Meyer, Stassen, Leboyer, Fuchs, Otto, Brown, Vincent, Najjar and Bechter2020), but the late-onset and EEG slowing in patient 1 and the striking intermittent confused states in patient 2 could still be indicative of subtle inflammatory pathomechanisms. A false-positive assay was unlikely due to testing in two laboratories with different methodologies; thus, 1) there is a possibility that the anti-MOG antibodies were correct-positive but not clinically relevant, 2) the antibodies could be correct-positive but not functional (e.g. caused by a different epitope than in classical MOG encephalomyelitis), 3) there is a possibility of coincidence (both patients could have asymptomatic myelin disease), and 4) the antibodies could be relevant, but due to moderate titres, they may have caused a ‘subtle clinical picture’ rather than a fulminant (it is possible that more pronounced symptoms only occur in the case of a second hit such as a more pronounced blood–CSF barrier disturbance). The authors consider the last possibility plausible (c.f. Spadaro et al., Reference Spadaro, Winklmeier, Beltrán, Macrini, Höftberger, Schuh, Thaler, Gerdes, Laurent, Gerhards, Brändle, Dornmair, Breithaupt, Krumbholz, Moser, Krishnamoorthy, Kamp, Jenne, Hohlfeld, Kümpfel, Lassmann, Kawakami and Meinl2018); however, to prove this, more clinical cases are needed, and the biochemistry (epitopes) of the antibodies must be better studied (especially the antibodies in patients with psychosis, distinct from patients with classical MOG encephalomyelitis). In perspective, the production of patient-specific monoclonal anti-MOG antibodies in the future may allow better investigation of causality (analogous to the situation with anti-NMDA-R or anti-LGI1 encephalitis) (Kreye et al., Reference Kreye, Wenke, Chayka, Leubner, Murugan, Maier, Jurek, Ly, Brandl, Rost, Stumpf, Schulz, Radbruch, Hauser, Pache, Meisel, Harms, Paul, Dirnagl, Garner, Schmitz, Wardemann and Prüss2016; Kornau et al., Reference Kornau, Kreye, Stumpf, Fukata, Parthier, Sammons, Imbrosci, Kurpjuweit, Kowski, Fukata, Prüss and Schmitz2020). If an association with atypical psychosis emerges, the spectrum of MOG encephalomyelitis may become broader than initially thought.
Acknowledgements
DE was supported by the Berta-Ottenstein-Programme for Advanced Clinician Scientists, Faculty of Medicine, University of Freiburg.
Authors’ contributions
KvZ and DE wrote the paper and performed the data search. The data search was supported by IM. BF performed the ICA analyses and EEG interpretation. BB performed the anti-MOG antibody biochip assays. HU performed MRI interpretation and analyses. MAS and AS performed the neuropsychological testing/ interpretation. MAS supported the interpretation regarding previous genetic studies. DE, MM, KR, DD, KN, BB, and LTvE treated the patients. KD critically revised the paper. HP performed neurological and neuroimmunological interpretation. All authors were critically involved in the theoretical discussion and composition of the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interests
BB: Received travel grants and/or training expenses from Bayer Vital GmbH, Ipsen Pharma GmbH, Norvartis, Biogen GmbH, and Genzyme, as well as lecture fees from Ipsen Pharma GmbH, Alexion Pharma GmbH, Merck, Sanofi Genzyme and Roche. KD: Steering Committee Neurosciences, Janssen. HU: Shareholder of the Veobrain. LTvE: Advisory boards, lectures, or travel grants within the last three years: Roche, Eli Lilly, Janssen-Cilag, Novartis, Shire, UCB, GSK, Servier, Janssen, and Cyberonics. All other authors declare no conflict of interest.
Ethics approval and consent to participate
A retrospective analysis of a cohort of lumbar-punctured patients from the Department of Psychiatry and Psychotherapy of the Medical Center of the University of Freiburg was approved by the local ethics committee (Faculty of Medicine, University of Freiburg, No. 396/18). Written informed consent was obtained additionally from both patients for the preparation of this cumulative case report.
Consent for publication
Both patients have given their signed written informed consent for both case reports, including the presented images, to be published.
Availability of data and material
All necessary data can be found in the paper.