Introduction
Honey bee (Apis mellifera Linnaeus) (Hymenoptera: Apidae) royal jelly is a nutrient-rich substance produced and secreted by the hypopharyngeal and mandibular glands of nurse bees that has strong epigenetic influence on the polyphenism of caste development. Honey bee larvae fed royal jelly ad libitum will develop into queens, resulting in a caste with remarkable life history differences compared to other caste members, including larger bodies, longer lifespan, and the ability to lay fertilised eggs (Haydak Reference Haydak1943; Ramanathan et al. Reference Ramanathan, Nair and Sugunan2018; Slater et al. Reference Slater, Yocum and Bowsher2020). Over the past decade, the life history–altering effects of royal jelly have been discovered to be interspecific, eliciting changes in body size, feeding, longevity, development time, and fertility in the fruit fly, Drosophila melanogaster Meigen (Diptera: Drosophilidae) (Gardner Reference Gardner1948; Kamakura Reference Kamakura2011; Kayashima et al. Reference Kayashima, Yamanashi, Sato, Kumazawa and Yamakawa-Kobayashi2012; Shorter et al. Reference Shorter, Geisz, Özsoy, Magwire, Carbone and Mackay2015; Arruda et al. Reference Arruda, Gelineau, De Pina, Hatzidis, Nascimento, Hicks and Seggio2017), the nematode, Caenorhabditis elegans Maupas (Rhabditida: Rhabditidae) (Honda et al. Reference Honda, Fujita, Maruyama, Araki, Ichihara and Sato2011, Reference Honda, Araki, Hata, Ichihara, Ito, Tanaka and Honda2015; Detienne et al. Reference Detienne, De Haes, Ernst, Schoofs and Temmerman2014), the domestic silk moth, Bombyx mori Linnaeus (Lepidoptera: Bombycidae) (Hayashiya et al. Reference Hayashiya, Kato and Hamamura1965; Nguku et al. Reference Nguku, Muli and Raina2007; Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016), and the two-spotted field cricket, Gryllus bimaculatus DeGeer (Orthoptera: Gryllidae) (Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016).
Royal jelly is composed of water (60–70%), proteins (8–18%), carbohydrates (7–18%), fatty acids and lipids (3–8%), and small amounts of vitamins and minerals (Wytrychowski et al. Reference Wytrychowski, Chenavas, Daniele, Casabianca, Batteau, Guibert and Brion2013; Kunugi and Ali Reference Kunugi and Ali2019). Royal jelly’s variation in nutrient content depends in part on which honey bee species produced it and their geographical location, the timing of harvest, botanical source, storage conditions, pesticide exposure, and production methods (Sano et al. Reference Sano, Kunikata, Kohno, Iwaki, Ikeda and Kurimoto2004; Zheng et al. Reference Zheng, Hu and Dietemann2011; Wytrychowski et al. Reference Wytrychowski, Chenavas, Daniele, Casabianca, Batteau, Guibert and Brion2013; Kunugi and Ali Reference Kunugi and Ali2019; Al-Kahtani and Taha Reference Al-Kahtani and Taha2020; Chaves et al. Reference Chaves, Faita, Ferreira, Poltronieri and Nodari2020; Mokaya et al. Reference Mokaya, Njeru and Lattorff2020; Qi et al. Reference Qi, Ma, Wang, Zhang, Hao and Li2020; Ma et al. Reference Ma, Zhang, Feng, Fang, Hu and Han2021; Milone et al. Reference Milone, Chakrabarti, Sagili and Tarpy2021). More than 80% of the protein in royal jelly is in the form of nine major royal jelly proteins (Klaudiny et al. Reference Klaudiny, Hanes, Kulifajová, Albert and Šimúth1994; Albert and Klaudiny Reference Albert and Klaudiny2004; Xin et al. Reference Xin, Chen, Chen, Xiao, Parnell and Zhao2016; Kunugi and Ali Reference Kunugi and Ali2019). These major royal jelly proteins drive the specific physiological and life history changes that occur during honey bee queen development (Schmitzová et al. Reference Schmitzová, Klaudiny, Albert, Schröder, Schreckengost and Hanes1998). Dietary protein is important for providing amino acids required for growth and body-size regulation (Harrison et al. Reference Harrison, Raubenheimer, Simpson, Godin and Bertram2014; Han and Dingemanse Reference Han and Dingemanse2017; Reifer et al. Reference Reifer, Harrison and Bertram2018), and royal jelly contains 17 free amino acids (Silici et al. Reference Silici, Ekmekcioglu, Eraslan and Demirtas2009; Ahmad et al. Reference Ahmad, Campos, Fratini, Altaye and Li2020), six of which are essential for insects (lysine, valine, threonine, phenylalanine, leucine, and isoleucine; Cohen Reference Cohen2015). Protein is also important for insect egg production (Joern and Behmer Reference Joern and Behmer1997; Harrison et al. Reference Harrison, Raubenheimer, Simpson, Godin and Bertram2014; Roeder and Behmer Reference Roeder and Behmer2014). Although protein more strongly influences growth than carbohydrates do when diets are provided ad libitum (McDonald et al. Reference McDonald, Edwards, Greenhalgh, Morgan, Sinclair and Wilkinson2011; Harrison et al. Reference Harrison, Raubenheimer, Simpson, Godin and Bertram2014), the high sugar content – and thus high carbohydrate content – of royal jelly could still affect growth. Sugars are a feeding stimulant that increases the quantity of food consumed (Buttstedt et al. Reference Buttstedt, Ihling, Pietzsch and Moritz2016; Kunugi and Ali Reference Kunugi and Ali2019) and thus play an indirect role in body size because individuals who consume more generally tend to grow larger (Gutiérrez et al. Reference Gutiérrez, Fresch, Ott, Brockmeyer and Scherber2020).
Insects are commonly used as model systems in nutritional ecology due to their short generation times, ease of rearing, and high reproductive output (Gutiérrez et al. Reference Gutiérrez, Fresch, Ott, Brockmeyer and Scherber2020). These same life history traits make insect species amenable to mass rearing, including being farmed for food and feed. Insects have been consumed around the world for centuries, but insect-farming and mass-rearing facilities are relatively new to Europe and North America. Recently, North America has seen a burgeoning interest in entomophagy (Schrader et al. Reference Schrader, Oonincx and Ferreira2016), with 35 new Canadian insect-protein producers and companies offering insect-containing food products in the last decade (Natural Products Canada 2022). Crickets are economically important to these protein-producing companies because these adult insects are more desirable to consumers than larval-stage insects such as mealworms and black soldier fly larvae (Dossey et al. Reference Dossey, Tatum and McGill2016; Reverberi Reference Reverberi2020).
Advancements in diet technology and formulation could potentially reduce the costs associated with farming insects. Diet can have strong life history influences in insects (e.g., dietary protein and carbohydrates – Harrison et al. Reference Harrison, Raubenheimer, Simpson, Godin and Bertram2014; Cammack and Tomberlin Reference Cammack and Tomberlin2017; Reifer et al. Reference Reifer, Harrison and Bertram2018; Kim et al. Reference Kim, Park, Kim, Song, Roh and Kim2021; salts – Welti et al. Reference Welti, Sanders, de Beurs and Kaspari2019; Peterson et al. Reference Peterson, Welti and Kaspari2021; lipids – Blaul and Ruther Reference Blaul and Ruther2011; Krabbe et al. Reference Krabbe, Arnan, Lannes, Bergstedt, Larsen, Pedersen and Shik2019). Changes in diet formulations and the use of dietary supplements therefore have the potential to enhance yields of insect farms without additional labour. Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) discovered that feeding nymphs royal jelly resulted in enlarged body sizes of both male and female crickets, Gryllus bimaculatus (Orthoptera: Gryllidae); this effect was dose dependent and optimised at a 15% royal jelly diet. These results suggest that royal jelly, or its component major royal jelly proteins, is a dietary supplement that could enhance cricket farm yields. However, because the effects of dietary royal jelly differ among invertebrate orders and species (Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016), the dietary requirements for optimal fitness of mass-reared insect species regularly used by the industry may differ from those for Gryllus bimaculatus, a species not used in this industry. The time is ripe to translate the effects of royal jelly to increasing the yield of a mass-reared insect model regularly used by industry. We therefore determined the effects of royal jelly on male and female growth rates and adult body sizes of a mass-reared insect to determine whether royal jelly could be used to grow farmed insects bigger and faster.
We ran two experiments using a commercially reared cricket species, Gryllodes sigillatus Walker (Orthoptera: Gryllidae), as our model organism. In experiment 1, we asked what minimum amount of royal jelly supplement is required to elicit a significant morphological response. We then proceeded with a more thorough investigation (experiment 2) to determine the individual-level life history responses of G. sigillatus to royal jelly over time. We hypothesised that royal jelly would enhance growth in both males and females. Following Miyashita et al.’s (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) findings on Gryllus bimaculatus, we predicted that both male and female G. sigillatus would grow larger when fed a control diet that included a 15% royal jelly supplement compared with males and females fed a control diet without royal jelly supplement.
Materials and methods
Cricket rearing
Gryllodes sigillatus eggs were hatched in a medium of peat moss inside an incubator (56 × 56 × 99 cm, Precision Scientific Instruments Corp., New Delhi, India) maintained at ∼31.5–33 °C and 60% relative humidity. A 14:10-hour light:dark cycle was maintained using a single LED light strip installed along the length of the incubator interior. These conditions were conserved for the duration of both experiments. Every other day, the peat moss and eggs were moistened with water to prevent desiccation and gently stirred to prevent mould growth. The eggs were monitored twice daily for emergence. Upon emergence, only those crickets that emerged within a 12-hour time frame were used for experiments. Newly emerged cricket nymphs were each placed into individual rearing containers made from 96.1-mL plastic condiment cups with aerated lids. Twelve rearing containers were evenly spaced apart in a 4 × 3 grid on plastic cafeteria trays. The trays were assigned a new position inside the incubator every four days, and no tray was assigned the same position for the duration of the experiment. Diet treatments were randomly allocated for each tray. Each cricket was provided with a 14-mm-wide polyethylene push-in cap for a food dish and a 0.75-uL polymerase chain reaction tube (lid removed) for a water vial, which was stoppered with 38-mm-wide saturated dental cotton wick (Healifty, Guangdong Province, China). Food and water were provided ad libitum and replaced every 3–4 days. After five weeks, all crickets were euthanised by freezing.
The standard diet (Earth’s Harvest Organic Cricket Grower; Earth’s Harvest, Oxford Mills, Ontario, Canada) consisted of a mixture of 40.4% corn, 27.9% soybean meal, 15.0% fishmeal, and 1.70% vitamin and mineral mix (NutriMix Elite Grower DDG; Nutri+, Sunnyvale, California, United States of America). Fresh 100% pure royal jelly (Planet Bee Honey Farm, Vernon, British Columbia, Canada) was mixed with the standard diet to make treatment diets consisting of 10, 15, 20, and 30% w/w royal jelly. Diets were stored in a refrigerator at 4 °C.
Measurements
Live cricket individuals were placed into clear plastic Ziploc bags and photographed dorsally using a Dino-Lite Edge 3.0 Digital Microscope (Dunwell Tech, Inc., Torrance, California, United States of America) and DinoCapture 2.0 software (AnMo Electronics Corp., New Taipei City, Taiwan). The camera was calibrated for measurements before photographing specimens, and the subsequent photos were supplemented with a scale bar to accurately perform digital measurements. Head width (maximal distance between the outer edges of the eyes) and pronotum width (maximal distance across the coronal width of the pronotum) and length (maximal distance down the sagittal length of the pronotum) were measured every seven days using ImageJ, version 1.48 software (National Institutes of Health, Bethesda, Maryland, United States of America). Eggs were photographed using the same equipment and methods and were manually counted in ImageJ using the Cell Counter plugin (De Vos, University of Sheffield, Sheffield, United Kingdom).
Experiment 1: dose–response feeding curve
To determine the dose-dependent responses of G. sigillatus body size and body mass to royal jelly supplements and the probability of reaching adulthood when fed a royal jelly supplement, we reared G. sigillatus nymphs from hatch to adulthood on standard diets with a range of 0–30% royal jelly. Two separate trials were conducted. Upon emergence, cricket nymphs were weighed, photographed, measured, and then placed into individual containers. For trial 1, diets consisting of 0%, 10%, 15%, and 20% royal jelly were fed to 25 crickets per diet (N = 100). In trial 2, diets consisting of 0%, 10%, 20%, and 30% royal jelly were fed to 48 crickets per diet (N = 192), for a total of 292 crickets in experiment 1 collected across the two trials. Body size and weight measurements were performed 35 days after hatch.
Experiment 2: 15% royal jelly supplement
We tested how adding a 15% royal jelly supplement to the standard diet affected the growth and egg production of G. sigillatus. This treatment reflects the dose used by Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) and reflects the maximum mass and abdomen responses observed in experiment 1 (Fig. 1). Two separate trials were conducted, and in each trial, 96 crickets were fed either the standard diet with no royal jelly supplement (control; N = 48) or the standard diet with a 15% royal jelly supplement (N = 48), for a total of 192 crickets in experiment 2. Body mass was measured weekly starting seven days after hatch. Body size and mass measurements were performed weekly for 35 days after hatch, and the crickets were then frozen. We performed post hoc measurements on abdomen length (maximal distance down the sagittal length of the abdomen) and the number of eggs produced per cricket (N = 15 standard diet; N = 28 15% royal jelly). Abdomens were opened with a ventral longitudinal incision and a smaller perpendicular horizontal incision near the anal opening. The digestive tract was removed, and all eggs were flushed out of the abdominal cavity with water. An image of the eggs was captured, and the number of eggs was counted manually.

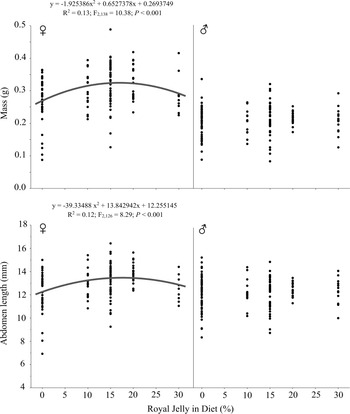
Fig. 1. Royal jelly dose–response regressions of Gryllodes sigillatus (N = 169, 73, 121, 73, and 48 for the 0%, 10%, 15%, 20%, and 30% royal jelly diets, respectively). Female mass and abdomen length are explained by significant quadratic relationships.
Data analysis
All statistical analyses were conducted using JMP Pro, version 15.0.0 (SAS Institute, Cary, North Carolina, United States of America). Normality of the data was confirmed through residual analyses and Shapiro–Wilk goodness-of-fit tests for each variable (P > 0.05). We used the false discovery rate B–Y method (FDRBY) to adjust alpha for each statistical test (Benjamini and Yekutieli Reference Benjamini and Yekutieli2001). “Trial” was included as a random effect in all analyses and was never statistically significant (P > 0.05).
Experiment 1: dose–response feeding curve
Data from experiments 1 (0%, 10%, 15%, 20%, and 30% royal jelly supplements) and 2 (0% and 15% royal jelly supplements) were pooled to increase sample size, for a total of 484 crickets (N = 169, 73, 121, 73, and 48 for the 0, 10, 15, 20, and 30% royal jelly diets, respectively). In total, 304 crickets survived, and 280 reached adulthood (see Supplementary material, Table S1 for survival distribution). Crickets that did not reach adulthood by the end of the experiment were removed from the analyses. We used logistic regression to explore how royal jelly supplements affected the probability that individual crickets would reach adulthood. The categorical dependent variable was whether the individual was a juvenile or an adult at 35 days after imaginal moult, and the independent variable was the percentage of royal jelly (w/w) in the diet.
To determine whether royal jelly concentration impacted adult mass and body size, we used linear mixed models. If significant linear and quadratic regressions were found for a variable, sample-size adjusted Akaike information criterion values (AICc) were used to select the best fit. Diet (0%, 10%, 15%, 20%, and 30% royal jelly) and sex (female or male) were included as fixed effects, and experiment trial (experiment 1: trials 1 and 2; experiment 2: trials 3 and 4) was included as a random effect.
Experiment 2: 15% royal jelly supplement
A total of 161 of 192 crickets reached adulthood in experiment 2. Crickets that did not reach adulthood by the end of the experiment were removed from the analysis. Of the surviving 161 crickets, 45 males and 33 females were fed the standard diet and 36 males and 47 females were fed the royal jelly diet. A chi-squared test was performed to determine if these ratios significantly differed from those expected. Principal component analysis was used to extract orthogonal vectors from head width, pronotum width, and pronotum length at adulthood to quantify adult body size into a single measurement. The first principal component explained 92.12% of the variation in size (eigenvalue = 2.76); all size measures loaded equally on the first principal component. We used a linear mixed model to determine whether diet treatment influenced mass, body size (first principal component), head width, pronotum width, pronotum length, and abdomen length. In all analyses, diet (standard diet only – the control – or standard diet with 15% royal jelly supplement), age (days since hatch), sex (male or female), and all possible interactions were included as fixed effects. Given the repeated aspects of the experiment, both cricket identification number and trial were included as random effects. We used a linear model to determine whether royal jelly affected day-of-eclosion adult mass, body size (first principal component), head width, pronotum width, pronotum length, and abdomen length. Diet, sex, and the interaction between diet and sex were included as fixed effects, and trial was included as a random effect. A separate linear model was used to determine whether royal jelly affected the number of eggs at adulthood, with diet as the only effect in the model because only females produced eggs and eggs were dissected only in trial 1. Pairwise comparisons for the linear model were developed using Tukey’s honestly significant difference test.
Results
Dose–response feeding curve
The odds ratio of crickets reaching adulthood after 35 days was 19.2 higher for crickets fed royal jelly (X 2 = 14.27, df = 1, P < 0.001, R 2 = 0.12). Significant linear and quadratic relationships were found between royal jelly concentration and female mass and abdomen length (Table 1; Fig. 1), and the quadratic relationships both fit better than the linear relationships, based on the lower AICc values. The quadratic relationships between royal jelly and female mass and abdomen length predicted maximised responses when fed 16.95% and 17.60% royal jelly, respectively. Summary statistics from the linear mixed model are provided in Supplementary Material, Table S2.
Table 1. Summary statistics from the linear mixed model of 35-day-old adult Gryllodes sigillatus measurements from experiment 1: dose–response feeding curve.

Terms in bold are statistically significant.
15% Royal jelly supplement
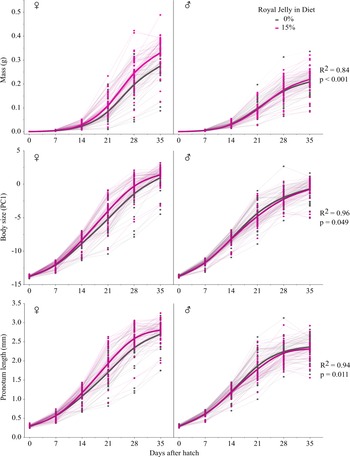
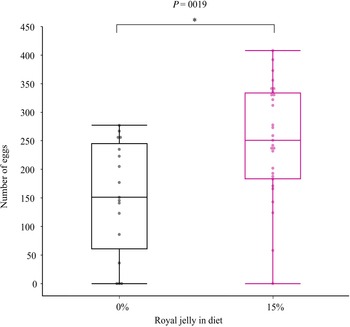
The ratio of crickets that survived and developed into males and females was significantly different than expected (X 2 = 3.89, df = 1, P = 0.049). However, this result was made less clear after pooling the 0% and 15% data across the two experiments and running the same chi-squared test, with no difference found between expected and observed ratios (X 2 = 2.34, df = 1, P = 0.13). The 15% royal jelly supplement significantly affected G. sigillatus mass (P < 0.001) and pronotum length (P = 0.025), but males and females were affected differently (Table 2). Females grew heavier (P = 0.031) when fed a royal jelly supplement, but the royal jelly supplement did not significantly affect overall body size as encapsulated by the first principal component (P = 0.07; Table 2). Over time, females increased mass, grew larger bodies (first principal component), and grew longer pronotums more rapidly when they were fed 15% royal jelly supplements compared to the standard diet without supplements (P < 0.05; Table 2; Fig. 2). At day 35, adult females fed royal jelly were 30% heavier on average, had significantly longer abdomens, and had significantly higher fecundity (66.6% more eggs) than did females fed the standard diet (Tables 3 and 4; Fig. 3). Male mass and body size were unaffected by royal jelly both over time and at the end of the experiment (Figs. 2 and 3). Trial was never a significant source of variation (P > 0.05). Summary statistics from the linear model are provided in Supplementary Material, Table S3.
Table 2. Summary statistics from the repeated measures linear mixed model of Gryllodes sigillatus measurements from experiment 2: direct test of 15% royal jelly.

Terms in bold are statistically significant.

Fig. 2. Lifetime development of mass, body size (PC1), and pronotum length over age of Gryllodes sigillatus fed either a standard diet (N = 80) or a diet with 15% royal jelly (N = 84). Smoothed thick lines connect mean values at each time point, and solid circles represent individual crickets and are connected by thin lines over time.
Table 3. Least square means ± standard error of morphology and life history measurements of 35-day-old adult Gryllodes sigillatus from experiment 2: direct test of 15% royal jelly. Values within a column followed by different letters are significantly different (P < 0.05).

Notes: RJ, royal jelly; PC1, first principal component.
Table 4. Summary statistics from the linear model of 35-day-old adult Gryllodes sigillatus measurements from experiment 2: direct test of 15% royal jelly.

PC1, first principal component.
Terms in bold are statistically significant.

Fig. 3. Number of eggs dissected from 35-day-old adult Gryllodes sigillatus females fed standard (N = 17) and 15% (N = 29) royal jelly diets. Auto jitter was applied to the data points in JMP Pro Graph Builder. The middle line of each box represents the median and the outer edges represent the maximum and minimum values.
Discussion
Evidence from several insect orders and nematodes suggests that honey bee royal jelly, when administered orally through diet, can influence life history trait development. Key similarities among insects include increased body size and mass (B. mori – Hayashiya et al. Reference Hayashiya, Kato and Hamamura1965; Nguku et al. Reference Nguku, Muli and Raina2007; D. melanogaster – Kamakura Reference Kamakura2011; Shorter et al. Reference Shorter, Geisz, Özsoy, Magwire, Carbone and Mackay2015; Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016; Arruda et al. Reference Arruda, Gelineau, De Pina, Hatzidis, Nascimento, Hicks and Seggio2017; Gryllus bimaculatus – Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016) and increased fecundity (D. melanogaster – Kamakura Reference Kamakura2011; Kayashima et al. Reference Kayashima, Yamanashi, Sato, Kumazawa and Yamakawa-Kobayashi2012; B. mori – Miyashita et al. Reference Miyashita, Kizaki, Sekimizu and Kaito2016). Despite this body of past work, the present study is the first to our knowledge that tests the effects in an insect species mass reared for food and feed. We found a sex-specific effect of royal jelly in a hemimetabolous insect (G. sigillatus): females grew heavier more quickly and developed more eggs when fed royal jelly, but males were unaffected. These results complicate our understanding of the effects of royal jelly in insects because they both complement and contrast with Miyashita et al.’s (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) finding that both male and female Gryllus bimaculatus – a cricket in the same family as G. sigillatus – grew larger when fed royal jelly. In the same study by Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016), sex-specific differences similar to those we observed in the present study were found in the lepidopteran B. mori; female moth pupae and adults grew larger and developed more eggs when fed royal jelly. Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) suggested that the response differences to royal jelly observed in B. mori and Gryllus bimaculatus were a result of different developmental processes and mechanisms that respond to royal jelly between holometabolous and hemimetabolous insects. Although the theory of insects with different development life histories responding differently to diet is not new (Bernays Reference Bernays1986; Thompson Reference Thompson2019; Neiro Reference Neiro2020; Bertram et al. Reference Bertram, Yaremchuk, Reifer, Villarreal, Muzzatti and Kolluru2021), our results suggest that the effects of royal jelly can be sex specific in Orthoptera. Thus, distantly related holometabolous and hemimetabolous insects can respond similarly to dietary royal jelly, whereas two relatively closely related hemimetabolous orthopterans can respond differently. The signalling mechanisms activated by royal jelly molecules may thus be conserved between holometabolous and hemimetabolous insects, but the ultimate effects of royal jelly may be tied to other life history traits or the dietary or environmental context in which it is experienced by an insect. Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) concluded that royal jelly affected cricket size via upregulation of food consumption; although this would have been an excellent addition to our study, limitations brought on by the COVID-19 pandemic severely restricted access to resources necessary to accurately measure food consumption.
Sex-specific life history differences in response to diet are well documented in insects, especially in crickets. Protein intake is important for optimising fecundity and egg production in females, and carbohydrate intake regulates calling effort in males (Teleogryllus commodus Walker – Maklakov et al. Reference Maklakov, Simpson, Zajitschek, Hall, Dessmann and Clissold2008; Gryllus veletis Alexander and Bigelow – Harrison et al. Reference Harrison, Raubenheimer, Simpson, Godin and Bertram2014; T. oceanicus Le Guillou – Ng et al. Reference Ng, Simpson and Simmons2018; Gryllus assimilis (Fabricius) – Reifer et al. Reference Reifer, Harrison and Bertram2018). Houslay et al. (Reference Houslay, Hunt, Tinsley and Bussière2015) provide a stimulating discussion on the differences in resource acquisition between females and males; females have a straightforward process of gathering resources and converting them into eggs, whereas males exhibit acquisition-related plasticity and must choose how to allocate invested resources (calling effort, competition, etc.). The investment strategy of male G. sigillatus may explain the differences between sexes in the present study; males exhibit acquisition-related plasticity in calling behaviour (Bertram et al. Reference Bertram, Thomson, Auguste, Dawson and Darveau2011; Houslay et al. Reference Houslay, Hunt, Tinsley and Bussière2015), and therefore, compared to females, which become fecund by gathering nutrients and converting those nutrients into eggs, males experience the resource-intensive additional burden of producing a spermatophylax and conserve resources and energy until they perceive a competitor (Mallard and Barnard Reference Mallard and Barnard2003, Reference Mallard and Barnard2004). Investment strategy and resource acquisition may also explain the response differences to royal jelly by G. sigillatus and Gryllus bimaculatus. Both species increase the number of sperm transferred in the presence of apparent competitors; male G. sigillatus significantly increases sperm with a conspecific competitor, whereas Gryllus bimaculatus increases sperm regardless of the competitor species (Mallard and Barnard Reference Mallard and Barnard2003). In a group environment rearing protocol as established by Miyashita et al. (Reference Miyashita, Kizaki, Sekimizu and Kaito2016), it is no surprise that both male and female Gryllus bimaculatus increased body size on a royal jelly (i.e., higher-quality) diet; males were susceptible to competition and likely increased their investment by consuming more food, and females grow heavier under higher population density (El-Damanhouri Reference El-Damanhouri2011). Under the isolated and individual-rearing conditions used in the present study, we suspect that G. sigillatus were less able to detect competition, and therefore no competitor cue increased the crickets’ investment in body size. This hypothesis could be tested by repeating our experiment in a controlled group-rearing and active farm environment to determine whether royal jelly also influences body size of male G. sigillatus in a group environment. If G. sigillatus were fed royal jelly in a mass-rearing environment with a constant presence of conspecific competition, males may demonstrate a response of increased body size regulated by increased investment.
In addition to farm-scale trials, future research should also explore how royal jelly – either fresh or manufactured – impacts male and female growth rates and body size over generations. Warbrick-Smith et al. (Reference Warbrick-Smith, Raubenheimer, Simpson and Behmer2009) showed that lab populations of Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) reared on high-carbohydrate diets initially responded with reduced fitness; however, over 350 generations, they evolved the ability to reduce fat storage. Hall et al. (Reference Hall, Bussière and Brooks2008) reared Australian ground crickets, Pteronemobius sp. Jacobson (Orthoptera: Trigonidiidae) for seven generations on low- and high-quality diets, and although individuals at the beginning of the experiment grew heavier on the high-quality diet, after seven generations, crickets grew significantly heavier on the low-quality diet treatment. Based on these examples, single-generation diet changes may be misleading in their long-term effects but are still valuable for demonstrating the potential power that dietary supplements may have. Further research should test royal jelly across multiple generations of G. sigillatus to determine how it affects overall farm yield and whether such supplements would be financially viable for use in insect farming.
Our study is useful in its contrast to Miyashita et al.’s (Reference Miyashita, Kizaki, Sekimizu and Kaito2016) work regarding species-specific nutritional requirements to optimise fitness. Our study also represents an important step towards application of nutritional supplements in cricket farming. Gryllodes sigillatus is an important economical cricket species currently being farmed for food and feed in North America. Small- and medium-scale cricket farms often use whatever feed is safe, available, and consumed by the insects: if the crickets eat, grow, and reproduce, the feed is deemed acceptable (Lee Bess, Entomo Farms, personal communication). This keeps production costs low but can lead to lower harvest yields and variable nutrient quality of the food products (Weru et al. Reference Weru, Chege and Kinyuru2021). Our results contribute to a growing body of literature that demonstrates the strong potential of using royal jelly as a dietary supplement to optimise the mass rearing of insect species. Although royal jelly is expensive, difficult to produce in large quantities (Ramanathan et al. Reference Ramanathan, Nair and Sugunan2018), and quick to spoil in storage (Tarantilis et al. Reference Tarantilis, Pappas, Alissandrakis, Harizanis and Polissiou2012), research into improving the mass production of natural and artificial royal jelly proteins responsible for enhancing body size and growth is underway (Cao et al. Reference Cao, Zheng, Pirk, Hu and Xu2016; Wang et al. Reference Wang, Dong, Qiao, Zhang and Zhang2020; Ma et al. Reference Ma, Zhang, Feng, Fang, Hu and Han2021). Synthesising royal jelly into mass-reared insect diets must be cost effective but also maintain or increase the gustatory appeal of the diet. Royal jelly is rich in free amino acids (Guo et al. Reference Guo, Wang, Chen, Cao, Tian, Mao and Dong2021), and female G. sigillatus feeding time increases with spermatophylax amino acid concentration (Warwick et al. Reference Warwick, Vahed, Raubenheimer and Simpson2009), thereby increasing the total amount of ejaculate transferred to females. Diet can have a strong influence on male G. sigillatus fitness (Duffield et al. Reference Duffield, Hampton, Houslay, Rapkin, Hunt, Sadd and Sakaluk2020), and male mating success is maximised on diets that are slightly higher in protein than carbohydrates (Rapkin et al. Reference Rapkin, Jensen, House, Sakaluk, Sakaluk and Hunt2017). Future studies testing how dietary royal jelly influences spermatophylax composition, feeding time, and overall mating success in G. sigillatus would therefore be valuable.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2022.39.
Acknowledgements
The authors thank Olivia Gagnon and Kylie Barwise for laboratory assistance and Entomo Farms for providing specimens and diet. This work was supported by funding from the Province of Ontario, NSERC Discovery Grants awarded to both H.A.M. and S.M.B., and the Canadian Foundation for Innovation. M.J.M., H.A.M., and S.M.B. conceived the experiment and contributed to writing the manuscript. M.J.M., E.M., and S.N. conducted the research. M.J.M. and S.M.B. analysed the data.
Declaration of competing interest
M.J.M, H.A.M., and S.M.B. have a research partnership with Entomo Farms.