Introduction
Harvesting of common reed Phragmites australis, a large perennial grass with great economic value, can help to maintain reedbed habitat because it prevents accumulation of organic material and thus colonisation by other plant species (Valkama et al. Reference Valkama, Lyytinen and Koricheva2008). However, extensive stands of common reed are an important habitat for birds, including many rare and vulnerable species, which may be either positively or negatively affected by reed cutting (Bibby and Lunn Reference Bibby and Lunn1982, Kristiansen Reference Kristiansen1998, Barbraud and Mathevet Reference Barbraud and Mathevet2000, Boulord et al. Reference Boulord, Wang, Wang and Song2010). Understanding the potential effects of harvesting on bird communities is thus important in enhancing reedbed management and balancing economic benefits and biodiversity conservation.
A recent review focusing on reedbed management in Europe concluded that bird numbers are 60% lower in harvested stands (Valkama et al. Reference Valkama, Lyytinen and Koricheva2008). For species nesting early in the season, winter harvesting has been shown to reduce vegetation cover and prevent nesting (Baldi and Moskat Reference Baldi, Moskat, Bissonette and Krausman1995, Poulin and Lefebvre Reference Poulin and Lefebvre2002), increase the risk of nest predation and delay clutch initiation (Graveland Reference Graveland1999), and decrease arthropod abundance which may affect food availability for birds (Graveland Reference Graveland1999, Poulin and Lefebvre Reference Poulin and Lefebvre2002).
Most published studies investigating the potential impact of reed harvesting on bird species have focused on breeding density (Kristiansen Reference Kristiansen1998, Barbraud and Mathevet Reference Barbraud and Mathevet2000, Barbraud et al. Reference Barbraud, Lepley, Mathevet and Mauchamp2002, Poulin and Lefebvre Reference Poulin and Lefebvre2002, Trnka and Prokop Reference Trnka and Prokop2006, Boulord et al. Reference Boulord, Wang, Wang and Song2010) but very few have investigated demographic parameters. Of these parameters, reproductive success is of key importance to population viability (Haig et al. Reference Haig, Belthoff and Allen1993), especially in species with short generation times. Understanding harvesting effects on reproductive success is therefore essential in designing reedbed management strategies that are compatible with the conservation of threatened species. Graveland (Reference Graveland1999) showed that nesting success is higher in non-harvested than harvested reedbeds for European populations of Eurasian Reed Warbler Acrocephalus scirpaceus and Sedge Warbler A. schoenobaenus. However, a review by Valkama et al. (Reference Valkama, Lyytinen and Koricheva2008) did not support a generalisation of this pattern.
In this study, we quantify the effects of reed harvesting on the reproductive success of Reed Parrotbill Paradoxornis heudei, a passerine found in reedbeds of the lower Yangtze River, the Yellow Sea coast of north-east China, extreme eastern Mongolia and extreme south-east Russia (Zheng Reference Zheng2002). Its populations are declining, presumably because of habitat loss and degradation, and it is considered ‘Near Threatened’ (BirdLife International 2010). Individuals live in flocks during the winter, form pairs in April, and nest from early May to late September (Ma Reference Ma1988). Population density is significantly higher in non-harvested than in harvested reedbeds during the breeding period (Boulord et al. Reference Boulord, Wang, Wang and Song2010). Furthermore, Boulord et al. (Reference Boulord, Wang, Wang and Song2010) showed that individuals nest in tall reed stands, which are not always available in harvested areas. Our objectives were therefore to evaluate the potential impacts of reed harvesting on the nesting success of the Reed Parrotbill and to determine which environmental factors could mediate these effects.
Materials and methods
Field work was carried out during the 2009 and 2010 breeding seasons in the reedbeds of the Chongming Dongtan National Nature Reserve (31°25’–31°38’N, 121°50’–122°05’E), a complex of coastal wetland ecosystems listed as a Wetland of International Importance by the Ramsar Convention. It is located on Chongming Island, Shanghai Municipality, China. The island has a subtropical monsoon climate with an average annual temperature of 15.3°C (26°C in summer and 3°C in winter). Average humidity is 82% and average annual precipitation is approximately 1,022 mm, with 60% of rainfall occurring between May and September (Tian et al. Reference Tian, Zhang, Wang, Zhou and Zhang2010). In July and August thunderstorms are frequent and wind speed often reaches level 4–5 on the Beaufort scale.
We chose two study areas 1.5 km apart, mainly composed of monospecific stands of common reed (Figure 1). The first study area (NHA) consisted of a reedbed that remained non-harvested for at least two years. The total area is covered by monospecific stands of common reed with patches of smooth cordgrass Spartina alterniflora, an introduced species, that are not occupied by Reed Parrotbill (Boulord et al. Reference Boulord, Wang, Wang and Song2010). The total area of common reed was 3.9 ha in 2009 and 4.2 ha in 2010. The second study area (HA) is a 5 ha monospecific stand of common reed which was completely harvested manually in February-March in each of the two consecutive study years. In both areas, water level was usually under 10 cm, except during several days after heavy rain when water level reached 10–20 cm.

Figure 1. Locations of the harvested (a) and non-harvested (b) study areas in the Chongming Dongtan National Nature Reserve located in the Yangtze River (c)
Nest monitoring
In each area we searched for nests once a month, as each breeding attempt lasts at least 29 days (see below), from mid-May to late-August, by walking 3-m wide transects through reedbeds. Nest position was recorded with a GPS (Garmin Etrex®) and contents were monitored every 3-4 days until fledging or nest failure. In cases of nest failure, adverse weather was considered to be the cause when eggs were found on the ground and/or the nest was found destroyed after strong winds. When eggshells were found in the nest, the nest was found destroyed, or young nestlings disappeared during a period with no strong winds, we considered the nest to have been predated. If cold eggs remained in the nest for several days, the nest was considered abandoned. We used chi-square tests to check for differences in causes of nest failure between years for the NHA, and between NHA and HA in 2010.
For each nest, the first laying day was determined by direct observation or back-dating on nests for which at least the beginning of one nesting stage was witnessed. Our previous research on the species allowed the duration of each nesting phase to be determined, so we used these phases (one day per egg for laying, 13 days for incubation and 12 for the nestling phase) to calibrate the back-dating.
Reed Parrotbill can raise three broods during the nesting season (Boulord pers. obs.). The first laying period occurs from mid-May to mid-July, the second from mid-July to mid-August and the third from mid-August to mid-September. We assigned each nest to a laying period. We also assigned nests that were found empty to one of these three laying periods, assuming that they were built between the dates of the previous and current nest surveys. Using this method, we assumed that there was no substitution attempt due to nest failure, so that the different nests of a laying period belonged to different pairs.
We divided the study areas into 50-m wide quadrats (four quadrats in 2009, five in 2010) and defined nest density in each quadrat by dividing the total number of nests per quadrat by the area of pure common reed. We tested for differences in nest densities between years for NHA, and between NHA and HA in 2010, with Student’s t-tests. In order to examine whether harvesting was correlated with delays in nest initiation, we then used a chi-square test to test for an equal nest distribution among the three laying periods between years in the NHA, and between NHA and HA in 2010.
Vegetation measurements
Each study area was divided into three parallel transects (100 m long in 2009 and 200 m in 2010) separated by a width of 50 m. In each transect, 50 cm x 50 cm quadrats were placed at each 50 m interval (nine quadrats in 2009 in NHA and 15 quadrats in 2010 in NHA and HA). In each quadrat, densities of green stems (shoots of the year) and dry stems (shoots of previous years) were measured at the beginning of September. Each month, from June to August in 2009 and from May to August in 2010, heights of five randomly chosen green stems were measured in each quadrat. We measured the height at the last node of the stem and did not consider terminal leaves. Height of dry stems was measured in June 2009 and May 2010. We examined monthly differences in green stem heights between NHA and HA in 2010 with Bonferroni’s correction. We chose green stems as they were taller than dry ones and presumably contribute more to vegetation cover. In the NHA in May, however, dry reed stems were taller than green stems; we thus included them in the dataset for that month.
Nest survival and nesting success
We estimated the Daily Survival Rate (DSR) of nests, i.e. the probability that a nest survives for one day, using the nest survival model in the program MARK (White and Burnham Reference White and Burnham1999, Dinsmore et al. Reference Dinsmore, White and Knopf2002) following recommendations by Rotella (Reference Rotella, Cooch and White2009). We established 14 May as the first nest day. We defined two groups according to the year of study (2009 and 2010) and used the nest survival model to test for year, time (linear and quadratic) and nest-age (linear and quadratic) effects on DSR. We also tested for effects of the following covariates: habitat (NHA or HA), vegetation height, vegetation density (green stems in HA and sum of green and dry stems in NHA), distance from the nearest nest, and laying period. Vegetation height for each nest was defined as the mean green stem height measured in the nearest quadrat during the month in which laying was initiated. Vegetation density for each nest was defined as the density of reeds measured in the nearest quadrat. Distance from the nearest nest was measured between nests in the same laying period.
We first ran the model for each variable individually. The quality of each model was evaluated using Akaike’s Information Criterion (AIC; Akaike Reference Akaike, Petran and Csáki1973) in order to choose the best-fit model for hypothesis testing (Burnham and Anderson Reference Burnham and Anderson1998, Reference Burnham and Anderson2002). We used the AICc, a correction of AIC for small sample size (Dinsmore et al. Reference Dinsmore, White and Knopf2002). Normalised Akaike weights (wi) were calculated to define the strength of evidence for each model (Burnham and Anderson Reference Burnham and Anderson1998, Reference Burnham and Anderson2002, Dinsmore et al. Reference Dinsmore, White and Knopf2002). The model with the lowest AICc is considered to best fit the data; but, as suggested by Burnham and Anderson (Reference Burnham and Anderson2002), we retained all models with ∆AICc < 2 to test models involving two covariates. Since time and laying period were correlated, we did not run a model including both covariates. As no model involving two covariates provided a better fit than those involving one covariate, we did not perform three-way interaction models.
Nest success, which is the true probability of a nest to survive from laying to fledging (Rotella Reference Rotella, Cooch and White2009), was then estimated as following: nest success = DSRd× 100, where d is the number of days from first laying date to fledging date (29 days on average) and DSR is the estimate obtained with the best fit model.
Nesting success not only depends on the nest fate but also on the number of chicks per nest that survived until fledging. We thus tested for inter-annual and inter-habitat differences in the numbers of surviving chicks produced per nest using Student’s t-tests.
Results
Nest density and nesting period
We found a total of 46 and 70 nests in NHA in 2009 and 2010 respectively, and 46 nests in HA in 2010. In 2010, nest density was significantly higher in NHA (16.4 ± 5.6 nests ha-1) than in HA (9.2 ± 2.9 nests ha-1; Student’s t-test, t 8 = -2.54, P = 0.03). In NHA, nest density was not significantly higher in 2010 (16.4 ± 5.6 nests ha-1) than in 2009 (11.8 ± 4.7 nests ha-1; t 7 = -1.32, P = 0.23). In 2009, the nesting period started in the first week of June in NHA. In 2010, the nesting period started in the third week of May in both HA and NHA. We found no significant difference in the proportion of nests in each laying period between year (χ22 = 0.73, P = 0.69) or between habitat types (χ22 = 1.09, P = 0.58), suggesting a relatively homogeneous chronology of successive broods among years and no laying delay due to harvesting in 2010.
Vegetation characteristics
We found no significant difference in reed height between the harvested and non- harvested reedbeds for each month (Bonferroni’s correction, P = 1 for all tests, Figure 2).

Figure 2. Mean vegetation height in harvested and non-harvested reedbeds from May to August 2010 based on green stems measured (except in May on non-harvested reedbeds when dry stems were higher than green ones and thus included in the data). Error bars show standard deviation from the mean. NS: non-significant difference.
Causes of nest failure
We determined the fate of 98 nests (Table 1). Causes of nest failure did not vary significantly between years for NHA (χ22 = 2.27, P = 0.32) nor between NHA and HA in 2010 (χ22 = 5.27, P = 0.07). In NHA, predation was the most frequent cause of failures in 2009 and 2010 (29% and 18% respectively), followed by adverse weather (14% and 13% respectively) and abandonment (7% and 10% respectively). In HA, the main cause of failure was adverse weather (20%) followed by predation (17%) and abandonment (3%).
Table 1. Causes of nest failures for Reed Parrotbill during the year 2009–2010 in non- harvested (NHA) and harvested (HA) reedbeds. The unknown category includes nests found after their active period.

Nest survival and nesting success
Among all models tested, those with only one variable provided a better fit than those with multiple covariates, and the constant-survival model had the best support (Table 2). Parameter estimates for all co-variables included zero, which indicates that none significantly influenced DSR (Table 3). The DSR of the constant-survival model was 0.961 and the average nest success was 32% in NHA and HA during the two years.
Table 2. Best fit models (∆AICc < 2) of Reed Parrotbill nest survival rate (DSR). The model S(.) represents the constant DSR model which only includes the intercept as parameter. Habitat = non-harvested or harvested reedbeds, AICc = Akaike’s Information Criterion with correction for small sample size, ∆AICc = difference between the given model and the best fit one, K = the number of parameters, wi= estimate of the likelihood of the model given the observed data; all models sum to 1.00.
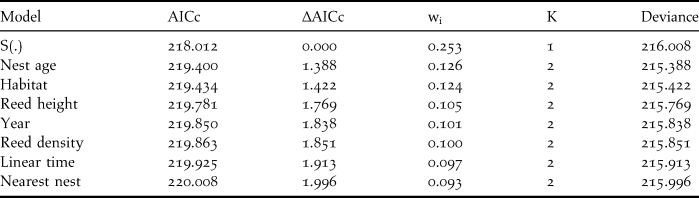
Table 3. Beta estimates, standard error (SE) and 95% confidence intervals (CI) for the best-fit models (∆AICc < 2, one parameter models) of Reed Parrotbill daily nest survival rate. AICc = Akaike’s Information Criterion with correction for small sample size.

In NHA, the mean number of nestlings produced per successful nest was 2.5 ± 1.0 (n = 11) in 2009 and 3.2 ± 1.3 (n = 19) in 2010. In HA it was 3.5 ± 0.9 (n = 13) in 2010. There was no significant difference between years (Student’s t-test, t 28 = -1.47, P = 0.15) or habitats (t 30 = 0.81, P = 0.43)
Discussion
Reed Parrotbill nesting density was significantly lower in the harvested reedbed than in the non-harvested reedbed, as previously reported (Boulord et al. Reference Boulord, Wang, Wang and Song2010). However, our results showed that the nest success rate (32%) was not influenced by harvesting, as all variables tested did not significantly affect daily nest survival in both harvested and non-harvested reedbeds and mean numbers of fledglings per nest were the same in the two types of reedbed. This can be explained by the fact that vegetation cover was not significantly different between the two areas during the whole breeding period and showed few variations in height within each site. As vegetation cover was high enough in the harvested reedbed, no breeding delay was observed. This result is slightly different from a previous study conducted in a different location in the Chongming Dongtan Nature Reserve, where a delay was observed (Boulord et al. Reference Boulord, Wang, Wang and Song2010). At the latter site, the mean height of green stems was 72 ± 20 cm in HA in mid-June, whereas it was 137 ± 20 cm on 3 June in the present study and 111 ± 36 cm in NHA at the end of May in the previous study, against 136 ± 21 cm on 3 June in the present study. These results suggest that impact of harvesting on breeding initiation in harvested reedbeds depends on the growth rate of reeds and thus responses to environmental parameters such as nutrient supply, water level, and climate (Buttery and Lambert Reference Buttery and Lambert1965, Haslam Reference Haslam1971, Reference Haslam1972, Cízková-Koncalová et al. Reference Cízková-Koncalová, Kvet and Lukavská1996, Clevering Reference Clevering1998, Clevering et al. Reference Clevering, Brix and Lukavská2001). In reedbeds where common reed growth is lower, harvesting leads to a delay in egg-laying because the vegetation cover is too low in the early breeding season, as the average height of Reed Parrotbill nests is 79 ± 23 cm (n = 28; unpubl. data).
Potential predators identified in both harvested and non-harvested stands included Long-tailed Shrike Lanius schach, Siberian weasel Mustela sibirica, and an unidentified snake (unpubl. data). Whereas reed harvesting has been shown to affect predation rates in Eurasian Reed Warbler and Sedge Warbler (Graveland Reference Graveland1999), we found no such effect on Reed Parrotbills, possibly because the overall vegetation cover was quite similar in harvested and non-harvested areas. The percentage of nests that failed due to weather did not differ between the harvested and non-harvested areas, but adverse weather seems to have an unusually high impact on Reed Parrotbill breeding success, as it represents 24–35% of total nest failures. For comparison, weather accounts for only 1.2% of nest failures in the Eurasian Reed Warbler, a species which also uses reed stems as nest support (Honza et al. Reference Honza, Øien, Moksnes and Røskaft1998). This large disparity between the two species may be due to climatic differences between East European reedbeds and Chongming coastal reedbeds, where strong winds and rainstorms frequently occur in summer, making adverse weather a major cause of nest failure. Adverse weather thus contributes highly to nest success rate. It suggest that reedbeds that are too high are probably also unfavourable for the Reed Parrotbill and should explain why Ma (Reference Ma1988) observed lower individual densities in reedbeds with deep water such as irrigated reedbeds where reed growth is higher than in reedbeds with shallow water (Cowie et al. Reference Cowie, Sutherland, Ditlhogo and James1992, Ostendorp Reference Ostendorp1999, Vretare et al. Reference Vretare, Weisner, Strand and Granéli2001).
Acknowledgements
This study was supported by the Sciences and Technology Commission of Shanghai Municipality, the Science and Technology Ministry of China (no.10dz1200703; 10dz1211000; 2012BAK69B14), the Shanghai Landscaping and City Appearance Administrative Bureau (no. JB101503) and the French Centre National de la Recherche Scientifique (CNRS-INEE). Many thanks to Dr. Wang Zheng-huan and students Dong Bin and Wu Di for providing useful assistance in field investigation planning, and Pierre Fiquet, Laurent Brucy and Pascal Provost for their invaluable help and advice on bird capture and banding. We appreciate the improvements to the English made by Christina Riehl through the Association of Field Ornithologists’ programme of editorial assistance. Captures, banding and blood sampling were carried out with the authorisation of the Shanghai Forestry Bureau and with the help of the staff of the Chongming Dongtan Nature Reserve, who are authorised by the National Bird Banding Center of China to carry out these activities.







