Introduction
Background
Bentonite will be used as an engineered barrier in radioactive waste repositories which are designed such that the maximum temperature does not exceed 100°C (NUMO, 2021). One of the main purposes for using bentonite in radioactive waste repositories is to retard radionuclide migration; the properties of bentonite which allow this are its low permeability and self-sealing ability (Sellin and Leupin, Reference Sellin and Leupin2013; NUMO, 2021). The low permeability and self-sealing ability can be attributed to the amount and properties of montmorillonite, the main constituent of bentonite.
Cementitious materials are often installed around bentonite as engineered barriers or peripheral components (Tyupina et al., Reference Tyupina, Kozlov and Krupskaya2023 and references therein), and they produce alkaline leachate with a pH range of 10–13.5 (Andersson et al., Reference Andersson, Allard, Bengtsson and Magnusson1989; García Calvo et al., Reference García Calvo, Hidalgo, Alonso and Fernández Luco2010). As montmorillonite is known to dissolve under alkaline conditions (Cama et al., Reference Cama, Ganor, Ayora and Lasaga2000; Yokoyama et al., Reference Yokoyama, Kuroda and Sato2005), there is a concern that the alkaline alteration of bentonite induced by leachate from cementitious materials may increase permeability. In reality, however, the alteration of bentonite is a complex reaction that includes not only the dissolution of montmorillonite but also other reactions, including precipitation of secondary phases. Furthermore, the formation of secondary phases has a significant impact on permeability changes of bentonite.
The evaluation of alkaline alteration of bentonite is necessary for the performance assessment of radioactive waste repositories. However, it is impossible to conduct experiments in which bentonite and cementitious materials interact over thousands of years. Therefore, the long-term alteration of bentonite must be estimated using reactive transport (RT) modeling, which considers the dissolution of primary minerals in bentonite and the precipitation of secondary phases. Of these, data have been reported on mineral dissolution, including its kinetics (Bandstra et al., Reference Bandstra, Buss, Campen, Liermann, Moore and Hausrath2008; Cama and Ganor, Reference Cama and Ganor2015), and the kinetics of mineral dissolution appears to be ready to be implemented in RT modeling. Conversely, with regard to the precipitation of secondary phases, the chemical environment assumed in radioactive waste repositories is spatiotemporally complex, and the potential secondary phase species are established in various manners accordingly. Furthermore, knowledge of the subsequent mineralogical transition of the secondary phases formed in the RT modeling is lacking, which leads to uncertainty in the modeling results; therefore, it is important to examine how to select secondary phases and parameters related to these secondary phases in RT modeling.
To understand the alkaline alterations of bentonite, including the formation of secondary phases, several laboratory and in situ experiments have been conducted (Sánchez et al., Reference Sánchez, Cuevas, Ramírez, León, De Fernández and Vigil2006; Fernández et al., Reference Fernández, Ruiz and Cuevas2016; Fernández et al., Reference Fernández, Torres, Ruiz, Cuevas, Alonso and García Calvo2017; Yokoyama et al., Reference Yokoyama, Shimbashi, Minato, Watanabe, Jenni and Mäder2021). However, there is a significant disparity in the timescales of reactions in those experiments and actual radioactive waste repositories. To understand the reaction of secondary phases formed by long-term geochemical reactions and examine how to select secondary phases to be considered in the RT modeling, it is necessary to observe geochemical reactions under alkaline conditions in nature along with laboratory and in situ experiments. In this review, the reactions forming the secondary phases in diverse natural alkaline environments at low temperatures (<100°C) were reviewed to understand species, formation conditions, and mineralogical transition of secondary phases that have the potential to form during cement–bentonite interactions in radioactive waste repositories. In the following sections, the alkaline conditions derived from cementitious materials, the impact of secondary phases on the permeability change of bentonite, and the advantages of investigations on natural geochemical reactions are comprehensively discussed.
Alkaline conditions derived from cementitious materials
Alkaline leachate is generated by the interaction between cementitious materials and groundwater in radioactive waste repositories. The composition of alkaline leachate varies with the stage of cement degradation. For example, the overall evolution of the chemical composition of leachate from ordinary Portland cement (OPC) can be divided into four stages, as follows (Fig. 1): (1) Stage I: the leachate is dominated by highly soluble alkali hydroxides (Berner, Reference Berner1992; Glasser, Reference Glasser and Ojovan2011), which has a pH of ~13 and is rich in K and Na ions (García Calvo et al., Reference García Calvo, Hidalgo, Alonso and Fernández Luco2010); (2) Stage II: the leachate is dominated by Ca(OH)2, and the pH is buffered at ~12.5 (Berner, Reference Berner1992; Glasser, Reference Glasser and Ojovan2011); (3) Stage III: the leachate is determined by the C–S–H gel, depending on the Ca:Si ratio of the gel; the pH decreases continuously to 11 (Berner, Reference Berner1992; Glasser, Reference Glasser and Ojovan2011); and (4) Stage IV: the leachate is controlled by alteration products (Glasser, Reference Glasser and Ojovan2011). Estimates in given conditions predicted that Stage I can last ~10,000 y and Stage II can last from 10,000 to 200,000 y (Sun et al., Reference Sun, Chen and Ye2022 and references therein), although the leachate evolution period can be affected by the chemical composition of the groundwater (Atkinson et al., Reference Atkinson, Goult and Hearne1985; Berner, Reference Berner1992). In Savage et al. (Reference Savage, Noy and Mihara2002), the chemical compositions of the simulated leachates in Stages I–III were set out as illustrated in Table 1.
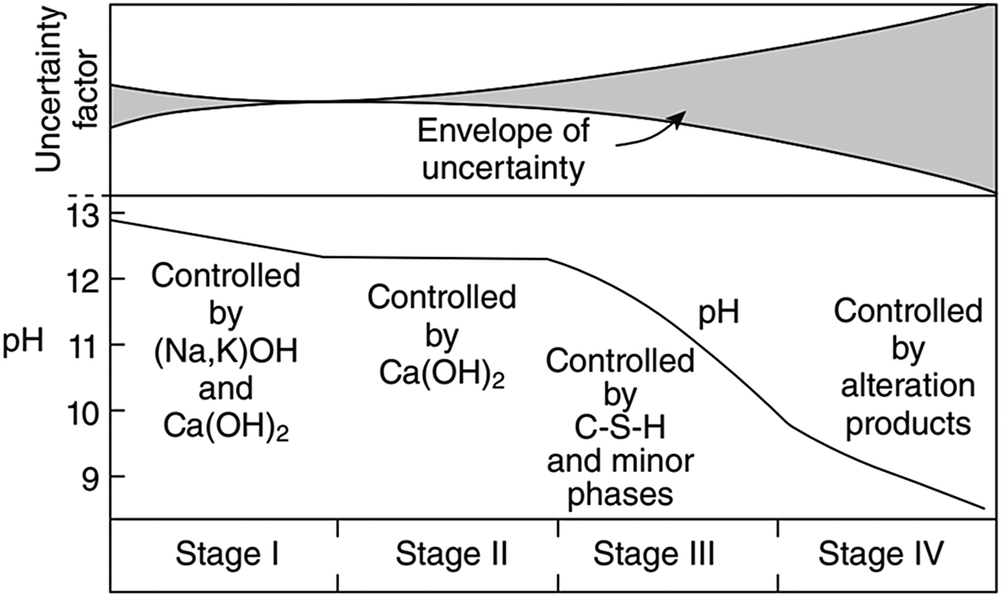
Figure 1. Evolution of ordinary Portland cement leachate and associated uncertainties (Glasser, Reference Glasser and Ojovan2011).
Table 1. Examples of chemical compositions of cement leachates
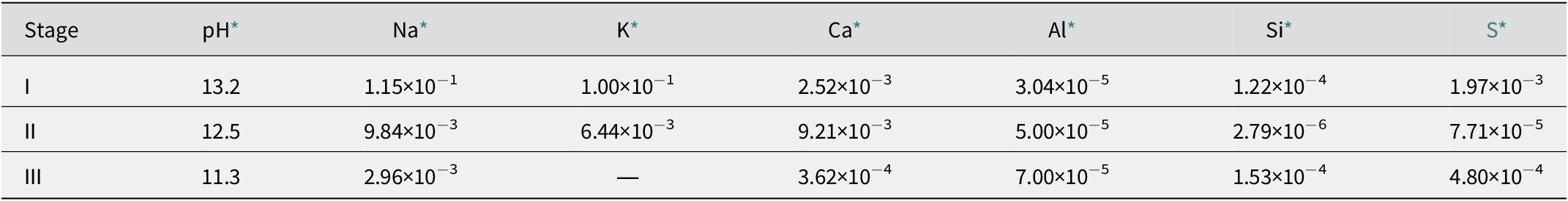
* Data sourced from Savage et al. (Reference Savage, Noy and Mihara2002). The concentrations are expressed in mol dm−3.
Furthermore, the composition of alkaline leachate varies with the type of cementitious material. For example, the chemical composition of the alkaline leachate changes when supplementary cementitious materials, such as silica fume or fly ash, are mixed with OPC (García Calvo et al., Reference García Calvo, Hidalgo, Alonso and Fernández Luco2010; Vollpracht et al., Reference Vollpracht, Lothenbach, Snellings and Haufe2016). García Calvo et al. (Reference García Calvo, Hidalgo, Alonso and Fernández Luco2010) reported that low-alkali cement, in which silica fume and fly ash were added to OPC, produced leachates containing smaller amounts of K and Na ions and larger amounts of SO4 ions. Additionally, an increase in supplementary cementitious materials decreases pH (Vollpracht et al., Reference Vollpracht, Lothenbach, Snellings and Haufe2016), and therefore, there is growing interest in using low-alkali cement with an initial pH of 9–11 as an alternative to OPC due to concerns about bentonite alteration under the highly alkaline conditions generated by OPC degradation (Milodowski et al., Reference Milodowski, Norris and Alexander2016).
Impact of secondary phases on permeability change of bentonite
During cement–bentonite interactions, the formation of secondary phases can promote or inhibit the permeability increase of bentonite. For example, the formation of secondary phases can contribute to a decrease in the porosity of bentonite, thereby decreasing its permeability (Yokoyama et al., Reference Yokoyama, Nakamura, Tanaka and Hironaga2011). Conversely, in some cases, the formation of Si-containing secondary phases decreases the Si concentration in the pore solution. As a decrease in the Si concentration in a pore solution enhances the dissolution of montmorillonite due to the effect of the degree of undersaturation (Cama et al., Reference Cama, Ganor, Ayora and Lasaga2000; Cappelli et al., Reference Cappelli, Yokoyama, Cama and Huertas2018), this may increase the permeability. Furthermore, the precipitation of secondary phases changes the pH of the pore solution, which affects the dissolution kinetics of the montmorillonite (Rozalén et al., Reference Rozalén, Huertas, Brady, Cama, García-Palma and Linares2008). Notably, the effect of secondary-phase precipitation on the pore solution chemistry and porosity depends on the species of the secondary phases; therefore, the species of secondary phases formed during bentonite alteration could impact the permeability change of bentonite.
Additionally, metastable phases, which are not thermodynamically stable, can be formed during the alteration process as the dissolution of the primary minerals and precipitation of secondary phases are governed by kinetics. For example, in the alkaline alteration of tuffaceous sedimentary rocks, the rate of dissolution of the glass is so quick that it is supersaturated for the metastable phase. This results in the precipitation of metastable phases with a fast precipitation rate, followed by a transition to thermodynamically more stable minerals with a slower precipitation rate over time (Dibble and Tiller, Reference Dibble and Tiller1981). A similar mineralogical transition is expected in the alkaline alteration of bentonite (Savage et al., Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007). In other words, during the long-term alteration of bentonite under alkaline conditions, not only is a mineral transition owing to changes in the chemical environment caused by external factors (e.g. changes in the solution composition of pore water from cementitious materials) but also a transition from a metastable phase to stable minerals even in a stable chemical environment is expected. The mineralogical transitions after the precipitation of secondary phases may further alter permeability. This indicates that the mineralogical transitions of the secondary phases within the evaluation period may need to be considered when evaluating the long-term permeability changes in bentonite.
Advantages of investigations on natural geochemical reactions
As mentioned previously, the evaluation period of bentonite alterations in radioactive waste repositories is extremely long, and the chemical conditions to be assumed are diverse. Laboratory experiments, in situ experiments, and natural analog (NA) studies differ in the amount of data produced, diversity of the chemical conditions that can be considered, and observation period of the phenomena (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Yoshikawa, Minato and Miyakawa2022a; Fig. 2). To understand bentonite alteration under diverse chemical conditions over long periods of time, it is important to combine knowledge from these three research methods.
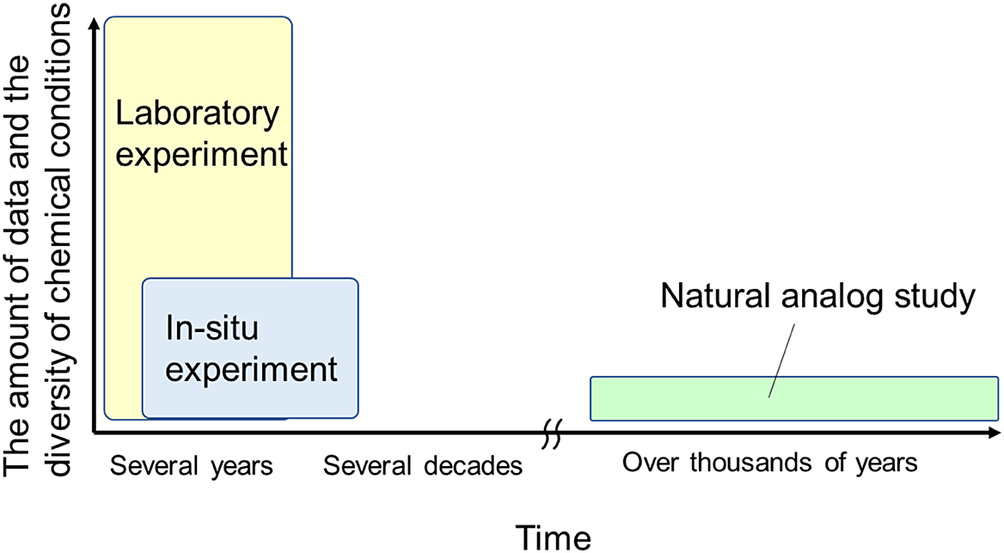
Figure 2. Conceptual diagram of the reaction times and diversity of data and chemical conditions covered by each research method. Adapted from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Yoshikawa, Minato and Miyakawa2022a).
Natural analog studies involve the observation of systems in nature that are analogs of the phenomena concerned (i.e. conditions in radioactive waste repositories). The advantage of this approach is that phenomena can be observed over a long period (Fig. 2); however, relatively few locations exist that can be set up as study sites with significant similarity to the system of radioactive waste repositories. Indeed, previous NA studies at sites where bentonite has interacted directly with alkaline fluids in nature are limited to two case studies, reported by Fujii et al. (Reference Fujii, Yamakawa, Shikazono and Sato2014) and Milodowski et al. (Reference Milodowski, Norris and Alexander2016). Therefore, it is important to proceed with NA studies with the stance that localized analog systems are acceptable as long as the target to be reflected in the radioactive waste repository system is clear (Yoshida et al., Reference Yoshida, Kitayama, Sato and Kobayashi2010). This review compiles geochemical reactions, including the precipitation reactions of secondary phases at several alkaline environments in nature, that are chemically similar to the alkaline leachate of cementitious materials. Although there have been previous reviews compiling natural geochemical reactions to understand cement–bentonite or cement–clay rock interactions (Gaucher and Blanc, Reference Gaucher and Blanc2006; Savage et al., Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007; Savage, Reference Savage2011), the present review is unique in that it organizes geochemical reactions under a broader range of natural sites as described in the next section and includes the latest findings since previous reviews. Furthermore, this review provides implications for the species and mineralogical transitions of secondary phases resulting from long-term bentonite–cement interactions, taking into account the spatial distribution of bentonite in radioactive waste repositories and changes in the chemistry of alkaline leachate from cementitious materials over time. This will play an important role in selecting secondary phases to be considered in the RT modeling of bentonite-cement interactions and parameters related to these secondary phases.
Natural sites covered in this literature review
In this review, three types of natural sites that produce alkaline fluid at low temperatures were compiled, near pyrometamorphic rocks, ophiolites, and alkaline saline lakes. As noted above, this is unique because previous reviews compiled natural geochemical reactions near pyrometamorphic rocks (Gaucher and Blanc, Reference Gaucher and Blanc2006; Savage et al., Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007; Savage, Reference Savage2011) and in alkaline saline lakes (Savage et al., Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007; Savage, Reference Savage2011) but not for ophiolite.
Here, the mechanisms of formation of natural alkaline fluids at the three types of sites and the chemical compositions of the alkaline fluid produced at each site type are described. Furthermore, the analogy of the chemical environments at each site type for cement–bentonite interactions in radioactive waste repositories is discussed.
Formation sites and mechanisms of natural alkaline fluids
Alkaline fluids with a pH of ~12.5 were recognized near pyrometamorphic rocks in the western and eastern springs in the Maqarin area of Jordan (Khoury et al., Reference Khoury, Salameh, Clark, Fritz, Bajjali and Milodowski1992). In Maqarin, natural cements are produced by the pyrometamorphism of marls, and alkaline fluids are produced by interactions between these natural cements and groundwater (Kamei et al., Reference Kamei, Alexander, Clark, Degnan, Elie and Khoury2010; Pitty and Alexander, Reference Pitty and Alexander2011).
The formation of alkaline fluids in ophiolite aquifers has been recognized worldwide (Barnes and O’Neil, Reference Barnes and O’Neil1969; Barnes and O’Neil, Reference Barnes and O’Neil1978). The alkaline fluids found near ophiolites can be divided into two types: Ca-OH and Mg-HCO3 types (Barnes and O’Neil, Reference Barnes and O’Neil1969). Several Ca-OH types have a pH of >11 (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli and Longinelli2002; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020), whereas the Mg-HCO3 type has a pH of ~9 (Okland et al., Reference Okland, Huang, Dahle, Thorseth and Pedersen2012; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020). The production of alkaline fluids of intermediate quality by mixing these two types of alkaline fluids has also been reported (mix type; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020). These types of alkaline fluids are considered to be of meteoric origin, and previous studies suggested water–rock interaction mechanisms that could produce them (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli and Longinelli2002). Specifically, the interaction of serpentinite with immature Mg-rich SO4–Cl water, an early product in the evolution of rainwater, produces Mg-HCO3-type alkaline fluids under open-system conditions for atmospheric CO2. Under closed-system conditions with respect to atmospheric CO2, these Mg-HCO3-type alkaline fluids evolve into Ca-OH-type alkaline fluids via interactions with serpentinite (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli and Longinelli2002). Here, the question arose as to why Ca-OH-type alkaline fluids are rich in Ca and low in Mg despite the fluids interacting with MgO-rich and CaO-poor rocks, such as serpentinites. The former question can be answered by the role of calcite precipitation in depleting the aqueous solution of C species, allowing the increase in Ca supplied by the dissolution of serpentinite despite its relatively low CaO content (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli and Longinelli2002). Regarding the latter question, precipitation of brucite and serpentine depletes Mg in the fluids (Marques et al., Reference Marques, Carreira, Carvalho, Matias, Goff and Basto2008). With these water–rock interactions, the pH of the fluids increased, and fluids evolved into Ca-OH-type.
The chemical composition of alkaline saline lakes is attributed to the evaporation of lake water (Darragi and Tardy, Reference Darragi and Tardy1987; Barbiéro et al., Reference Barbiéro, De Queiroz Neto, Ciornei, Sakamoto, Capellari and Fernandes2002). For example, in the case of an alkaline saline lake in the Pantanal Wetland, Brazil, it was suggested that some ions, such as Na+, and carbonate alkalinity increase linearly during evaporation, whereas Ca2+ and Mg2+ ions are consumed by precipitation. The pH value increased from ~7 to 10 during this process (Barbiéro et al., Reference Barbiéro, De Queiroz Neto, Ciornei, Sakamoto, Capellari and Fernandes2002).
Chemical compositions of natural alkaline fluids
To compare the chemical compositions of the alkaline fluids at each site type, a Piper diagram and scatter plots are shown in Fig. 3 and Fig. 4, respectively. The plots are based on the following reported chemical compositions: near pyrometamorphic rocks (Khoury et al., Reference Khoury, Salameh, Clark, Fritz, Bajjali and Milodowski1992), near ophiolites (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli and Longinelli2002; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020; Okland et al., Reference Okland, Huang, Dahle, Thorseth and Pedersen2012), and near alkaline saline lakes (Taylor and Surdam, Reference Taylor and Surdam1981). Cited values of chemical compositions of the alkaline fluids to create the diagrams are provided in Appendix A of the Supplementary material.
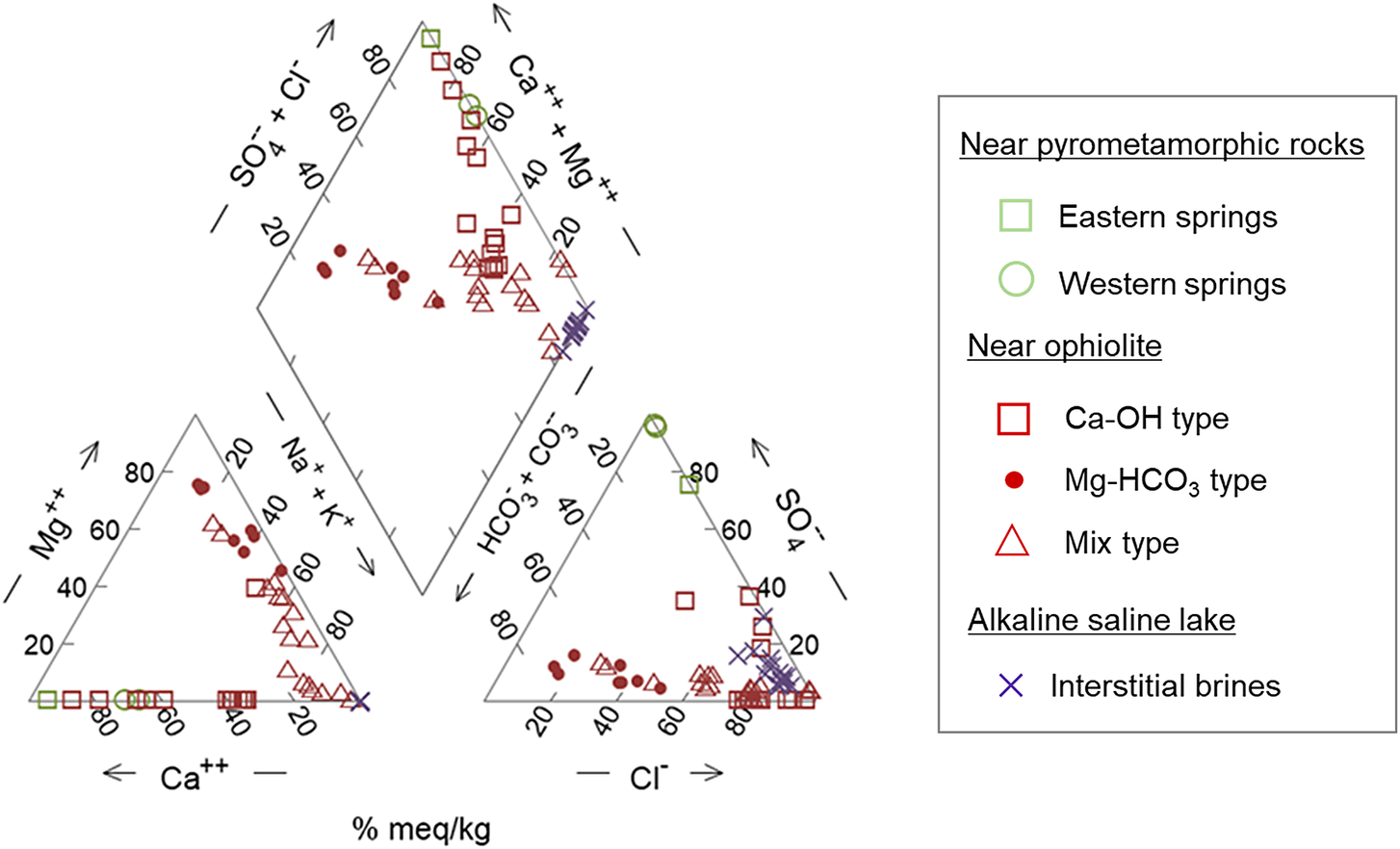
Figure 3. Piper diagram showing the chemical compositions of the alkaline fluids produced at each site. Values of chemical compositions of the alkaline fluids to create the Piper diagram are provided in Appendix A of the Supplementary material.
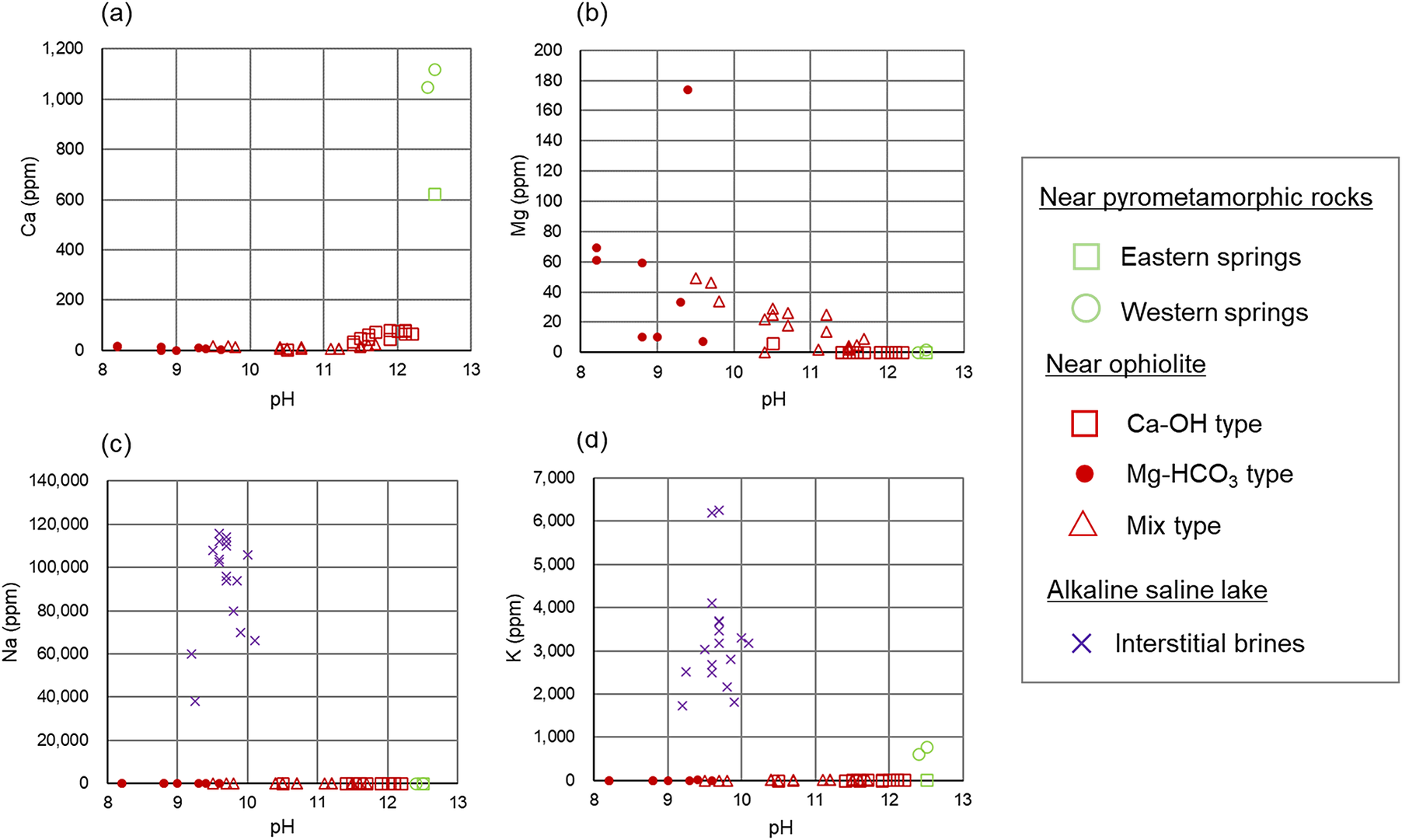
Figure 4. Dissolved ion concentrations at different pH values of the alkaline fluids: (a) Ca ion; (b) Mg ion; (c) Na ion; (d) K ion. Values of chemical compositions of the alkaline fluids to create the scatter plots are provided in Appendix A of the Supplementary material.
The alkaline fluids with a high pH of >11 are produced near pyrometamorphic rocks and near ophiolites (Ca-OH-type). A common feature of these alkaline fluids is that they are rich in Ca2+ ions, with a certain amount of Na+ or K+ ions also observed, but low concentrations of Mg2+ ions (Figs 3 and 4). Moreover, the alkaline fluids near pyrometamorphic rocks are rich in SO42− ions, while the Ca-OH-type alkaline fluids near ophiolites are rich in Cl− ions. Both of these alkaline fluids were found to contain low concentrations of HCO3− and CO32− ions (Fig. 3).
The Mg-HCO3-type alkaline fluids near ophiolite and alkaline fluids in alkaline saline lakes have a lower pH. The Mg-HCO3-type alkaline fluids were rich in Mg2+ ions and HCO3− or CO32− ions, compared with the alkaline fluids described above. The alkaline fluids in alkaline saline lakes are characterized by a lack of Mg2+ and Ca2+ ions and a high concentration of Na+ or K+ ions (Figs 3 and 4).
The chemistry of mix-type alkaline fluids near ophiolite exhibited characteristics intermediate between the Ca-OH-type and Mg-HCO3-type (Fig. 3).
Analogs to radioactive waste repositories
The alkaline environment expected in a radioactive waste repository varies spatiotemporally. In particular, the chemical composition of the alkaline leachate from cementitious materials is expected to change over time (Fig. 1). The spatial distribution of pH at bentonite is expected to differ owing to the decrease in pH of alkaline leachate caused by migrating and potentially chemically reacting with the bentonite (Savage, Reference Savage2011). Furthermore, the chemical composition of the groundwater and the design of radioactive waste repositories also affect bentonite–cement interactions. Different designs for radioactive waste repositories depending on waste type have been proposed in various countries. For example, in one option for a spent fuel repository in Switzerland (Sellin and Leupin, Reference Sellin and Leupin2013) or in an option for TRU waste in Japan (Ichikawa and Hamamoto, Reference Ichikawa and Hamamoto2021), the tunnel would be supported by shotcrete, and bentonite would be placed inside the tunnel. On the other hand, for long-lived low- and intermediate-level waste (LL-LILW) in Sweden, bentonite materials would be placed around concrete (Elfwing et al., Reference Elfwing, Evins, Gontier, Grahm, Mårtensson and Tunbrant2013). For high-level radioactive waste in Japan, bentonite would be placed in a deposition hole below the tunnel supported by shotcrete (Ichikawa and Hamamoto, Reference Ichikawa and Hamamoto2021). Fluids, after interaction between shotcrete and groundwater, may leach to bentonite in the case of spent fuel in Switzerland and TRU waste in Japan, whereas first groundwater and then alkaline leachates from concrete may leach to bentonite in the case of LL-LILW in Sweden and high-level radioactive waste in Japan. In other words, different fluid chemistries leaching to bentonite make different chemical conditions. Figure 5 shows a conceptual diagram of spatiotemporal bentonite–cement interactions depending on different chemical conditions, Mg-rich or not. As noted earlier, there is more than one design of radioactive waste repository. How the chemistry of alkaline fluids near pyrometamorphic rocks, ophiolites, and alkaline saline lakes is analogous with bentonite interacting with cementitious materials is illustrated in Figure 5.
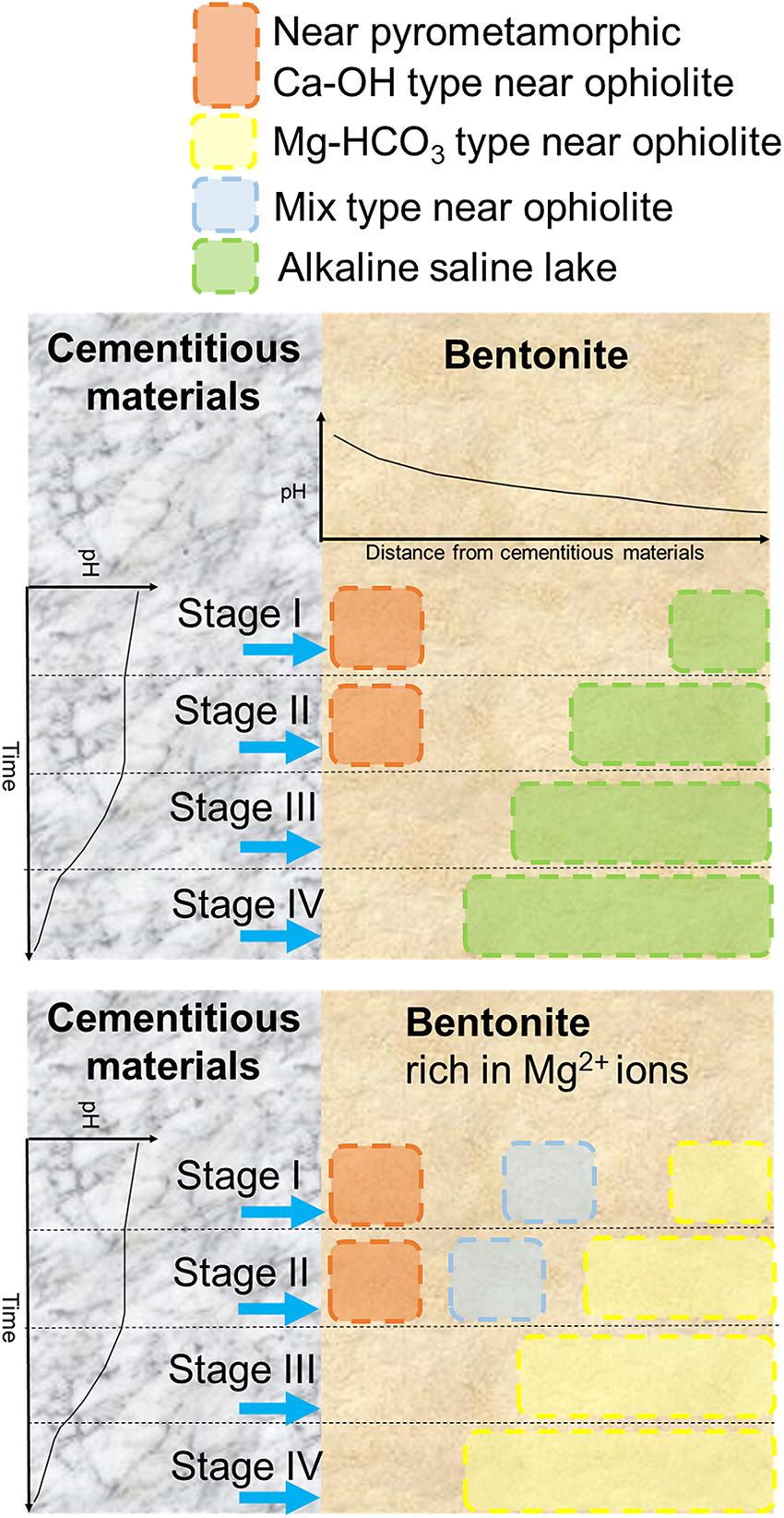
Figure 5. Conceptual diagram showing how the chemistry of alkaline fluids near pyrometamorphic rocks, ophiolites, and alkaline saline lakes are analogous with those of cement–bentonite interactions in radioactive waste repositories at various times and spaces.
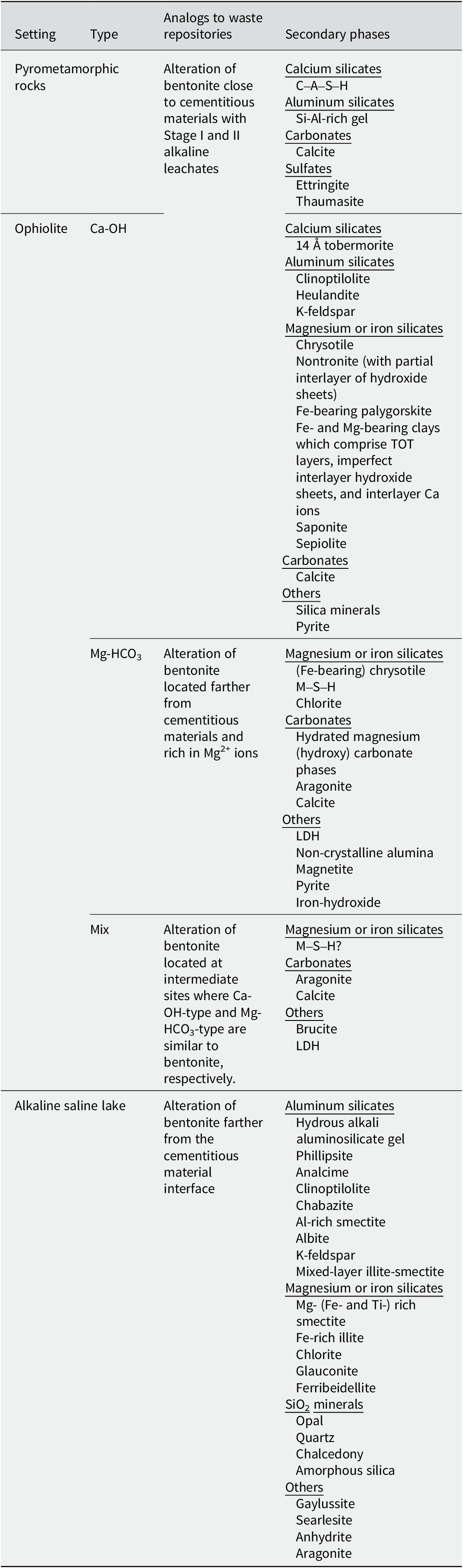
Based on the chemical compositions of alkaline fluids near pyrometamorphic rocks and the Ca-OH-type alkaline fluids near ophiolites (Fig. 3), the chemical environment in which the secondary phases form at these sites would mainly be analogous to the alteration of bentonite close to cementitious materials with Stage II alkaline leachates (Fig. 5). As a certain amount of Na+ or K+ ions are also recognized in these alkaline fluids (Fig. 3), those alkaline fluids may also be analogous to Stage I alkaline leachates, possibly of a relatively late stage within Stage I. Indeed, the alkaline fluids near the pyrometamorphic rocks are considered analogous to the alkaline leachates from cementitious materials (Pitty and Alexander, Reference Pitty and Alexander2011). Moreover, Ca-OH-type alkaline fluids near ophiolites are considered analogous to alkaline leachates from low-pH cement (Fujii et al., Reference Fujii, Yamakawa, Shikazono and Sato2014; Milodowski et al., Reference Milodowski, Norris and Alexander2016), or even from OPC (Fujii et al., Reference Fujii, Yamakawa, Shikazono and Sato2014).
Based on the chemical compositions of alkaline saline lakes (Fig. 3), the chemical environment in which the secondary phases form at these sites may be analogous to the alteration of bentonite that occurs under relatively low pH conditions farther from the interface between cementitious materials and bentonite (Fig. 5). Indeed, geochemical reactions in alkaline saline lakes are believed to be similar to those associated with alkaline leachates that progressively interact with rocks (Savage, Reference Savage2011).
Similarly, the Mg-HCO3-type alkaline fluids near ophiolites may be analogous to the chemical environment in which secondary phases are formed by the alteration of bentonite farther from the cementitious materials and under Mg-rich conditions (Fig. 5), such as when seawater (dissolved at ~1200 mg L–1 of Mg2+ in Kang et al. (Reference Kang, Linga, Park, Choi and Lee2014)) leach to bentonite.
As the chemistry of mix-type alkaline fluids showed characteristics intermediate between the Ca-OH-type and the Mg-HCO3-type (Fig. 3), they may be analogous to the chemical environment at the intermediate part of the bentonite, where Ca-OH-type and Mg-HCO3-type each have similarities (Fig. 5).
In summary, the chemistry of alkaline fluids produced near pyrometamorphic rocks, ophiolites, and alkaline saline lakes is analogous to cement–bentonite interactions in radioactive waste repositories at different times and spaces (Fig. 5); thus, compiling previous literature describing secondary phases at these three types of sites will provide an understanding of potential secondary phases that may be formed during cement–bentonite interactions at various timescales and in various environments in radioactive waste repositories.
Secondary phases under natural alkaline conditions
The secondary phases reported to occur near pyrometamorphic rocks and with Ca-OH-type alkaline fluids in ophiolites (Table 2), with Mg-HCO3-type and mix-type alkaline fluids in ophiolites (Table 3), and in alkaline saline lakes (Table 4) are summarized below.
Table 2. Secondary phases near pyrometamorphic rocks and with Ca-OH-type alkaline fluids in ophiolites
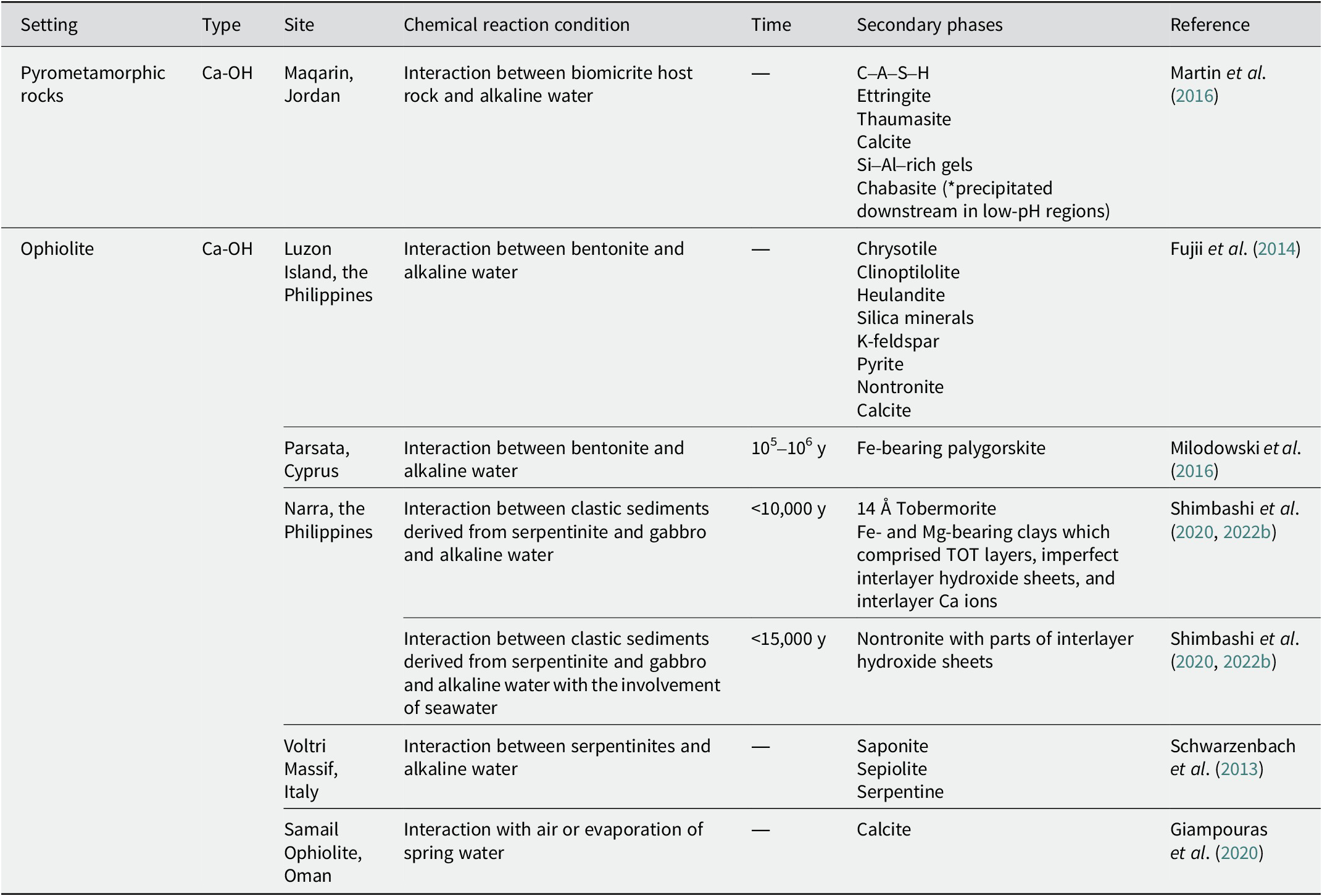
Table 3. Secondary phases with Mg-HCO3-type and mix-type alkaline fluids in ophiolites
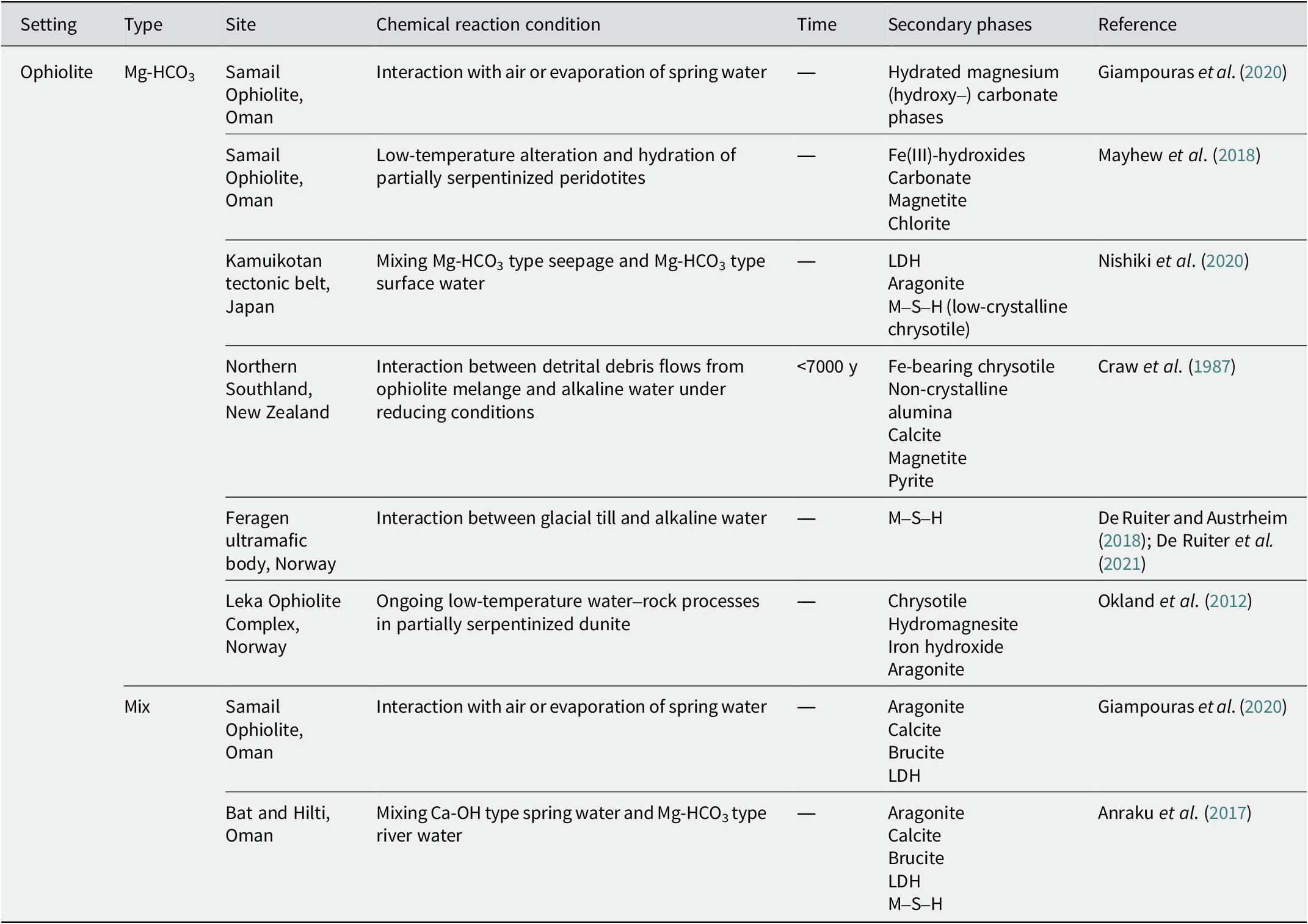
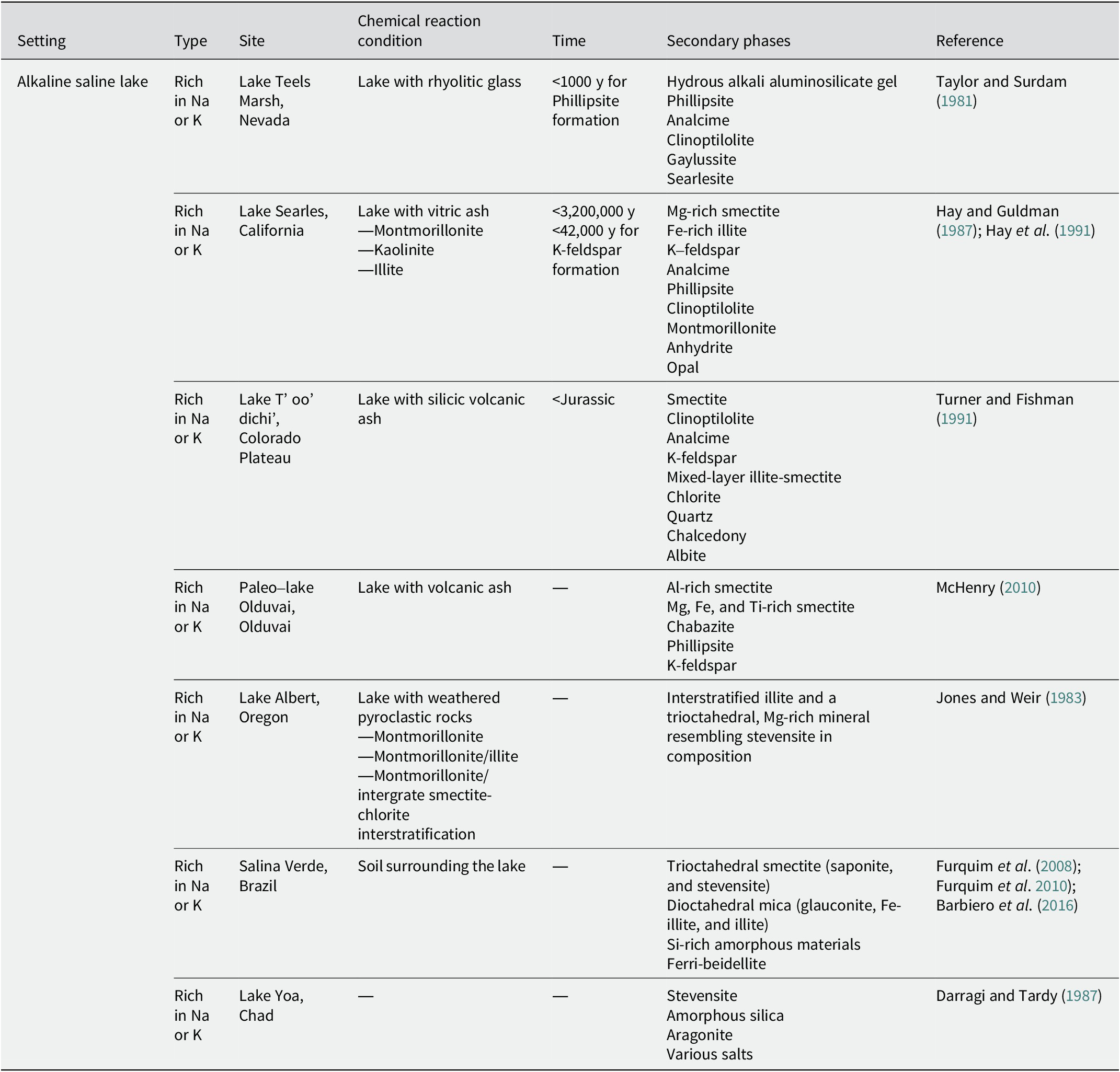
Table 4. Secondary phases in alkaline saline lakes
Near pyrometamorphic rocks
The site in the Maqarin area of Jordan has been studied extensively (Kamei et al., Reference Kamei, Alexander, Clark, Degnan, Elie and Khoury2010; Pitty and Alexander, Reference Pitty and Alexander2011). The alkaline alteration of the host rocks in the western and eastern springs is similar, despite variations in their primary lithologies and mineralogy (Baker et al., Reference Baker, Bateman, Hyslop, Ilett, Linklater and Milodowski2002). For example, Martin et al. (Reference Martin, Leemann, Milodowski, Mäder, Münch and Giroud2016) collected samples from adits through which alkaline fluids flow in the eastern springs of the Maqarin area. Calcium–aluminum–silicate–hydrate (C–A–S–H) precipitated from the alkaline fluids in the veins and the adjacent biomicrite. When the solution evolved to a more S-rich solution, ettringite, and thaumasite precipitated in C–A–S–H veins under Al-rich and Al-poor conditions, respectively (Martin et al., Reference Martin, Leemann, Milodowski, Mäder, Münch and Giroud2016). It is worth noting that thaumasite formation is affected not only by molar SO3/Al2O3 ratio, but also by temperature, with thaumasite formation being favored at temperatures of <8°C (Schmidt et al., Reference Schmidt, Lothenbach, Romer, Scrivener, Rentsch and Figi2008). Zeolite (possibly chabazite) precipitated downstream in the low pH regions of the alkaline fluids. Among the precipitates, C–A–S–H gels are sensitive to carbonation, and the C–A–S–H gels decompose to form a mixture of Si- and Al-rich gels and calcite (Martin et al., Reference Martin, Leemann, Milodowski, Mäder, Münch and Giroud2016).
Near ophiolites
Ca-OH type
Alkaline alteration of bentonite has been reported on Luzon Island in the Philippines (Fujii et al., Reference Fujii, Yamakawa, Shikazono and Sato2014). Fractures of pillow lava connected to bentonite. As chrysotile was recognized in the fractures, it is indicated that alkaline fluids produced in ophiolite may have leached into the bentonite using the fractures as flow paths. At the contact with the fractures filled with chrysotile, bentonite was altered to within 5 mm, and the altered layer was divided into two layers. In the layer on the fracture side, the formation of Ca-zeolite (clinoptilolite and heulandite), silica minerals, and K-feldspar was reported. In the adjacent layer, K-feldspar, pyrite, and nontronite were reported. In other outcrops, chrysotile and calcite were found within the fractures, and calcite was formed at the boundary between the fractures and bentonite.
The alkaline alteration of bentonite has also been reported in the Parsata area of Cyprus (Milodowski et al., Reference Milodowski, Norris and Alexander2016). In the alteration zone, which is believed to have been altered by reactions with alkaline fluids, Fe-bearing palygorskite was formed over 105–106 y.
At Narra in the Philippines, ongoing interactions between clastic sediments and Ca-OH-type alkaline fluids (pH >11) have been observed (Shimbashi et al., Reference Shimbashi, Sato, Yamakawa, Fujii and Otake2018). The sediments originated from a combination of serpentinized rocks and gabbro, and deposition of the sediments began 15,000 y ago (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake and Kikuchi2020). The depositional environment of the sediments probably changed from seawater to freshwater over time, resulting in different species of secondary phases depending on the depositional environment. Specifically, dioctahedral nontronite with part of an interlayer hydroxide sheet was formed, presumably by interactions between the sediments and alkaline fluid interaction with the involvement of seawater (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake and Kikuchi2020; Shimbashi et al., Reference Shimbashi, Yokoyama, Kikuchi, Otake and Sato2022b). When the depositional environment changed to freshwater, 14 Å tobermorite and trioctahedral Fe- and Mg-bearing clays were formed through a similar interaction without seawater infiltration (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake and Kikuchi2020). The trioctahedral Fe- and Mg-bearing clays consisted of tetrahedral–octahedral–tetrahedral layers, imperfect interlayer hydroxide sheets, and interlayer Ca ions. Mineralogical changes, such as the gradual decrease of the interlayer hydroxide sheets, occurred during the interaction. Furthermore, the Fe- and Mg-bearing clays probably oxidized after sample collection (Shimbashi et al., Reference Shimbashi, Yokoyama, Kikuchi, Otake and Sato2022b).
In Voltri Massif, Italy, interactions between serpentinites and alkaline fluids with a pH of 11.8–12.3 (from a spring named BR1) resulted in the formation of saponite and sepiolite, which precipitated together with or replaced serpentine (Schwarzenbach et al., Reference Schwarzenbach, Lang, Früh-Green, Lilley, Bernasconi and Méhay2013).
In the Samail Ophiolite in Oman, calcite was precipitated owing to the uptake of atmospheric CO2 and evaporation in Ca-OH water pools (pH >11.6; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020).
Mg-HCO3 type
In the Samail Ophiolite in Oman, various hydrated magnesium (hydroxy) carbonates have been recognized in Mg-HCO3-type alkaline fluids (7.9 < pH < 9.5; Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020). Nesquehonite was formed via evaporation and was transformed into dypingite and hydromagnesite. In addition, the low-temperature alteration of partially serpentinized, less-altered peridotites was reported by Mayhew et al. (Reference Mayhew, Ellison, Miller, Kelemen and Templeton2018). It is presumed that the alteration occurred via interactions with Mg-HCO3-type alkaline fluids (pH ~8). The alteration included the loss of olivine and Fe(II)-bearing brucite, an increase in the oxidized iron content in the serpentine, and the appearance of Fe(III) hydroxides and carbonate. It was also indicated that chromite was altered to Cr-rich magnetite and subsequently to chlorite at low temperatures.
In the Kamuikotan tectonic belt in Japan, the mixing of Mg-HCO3-type alkaline seepage with a pH of 10.34 and Mg-HCO3-type alkaline surface water with a pH of 9.69 produced layered double hydroxide (LDH) (Nishiki et al., Reference Nishiki, Sato, Katoh, Otake and Kikuchi2020). In contrast, the mixing of Ca-bearing Mg-HCO3-type alkaline seepage with a pH of 10.67 and Mg-HCO3 type-alkaline surface water with a pH of 9.57 produced aragonite. In addition to LDH and aragonite, the formation of magnesium silicate hydrate (M–S–H), considered low-crystalline chrysotile, has also been reported.
In northern Southland, New Zealand, debris flowing from an ophiolite mélange was deposited <7000 y ago (Craw et al., Reference Craw, Landis and Kelsey1987). The interaction between detrital debris flows and alkaline fluids with pH 9 under reducing conditions produced Fe-bearing chrysotile and nanocrystalline alumina (probably pseudoboehmite) in the veins. They often coexisted with calcite as a vein mineral, and magnetite and pyrite were recognized near and adjacent to the veins.
In Norway, the Feragen ultramafic body is covered with felsic glacial sediments mixed with ultramafic rock fragments. It has been reported that the dissolution of quartz in sediments and the evaporation process leads to the precipitation of amorphous silica, which subsequently reacts with Mg-HCO3-type alkaline fluids (pH ~10) to precipitate M–S–H (De Ruiter et al., 2021). The Mg:Si ratio of the M–S–H is between 0.9 and 1.1. M–S–H, which is assumed to be precipitated via association with the dissolution of K–feldspar, contains slightly more Al (De Ruiter and Austrheim, Reference De Ruiter and Austrheim2018).
At the Leka Ophiolite Complex in Norway, Mg-HCO3-type groundwater (8.2 < pH < 9.4) was recognized (Okland et al., Reference Okland, Huang, Dahle, Thorseth and Pedersen2012). It was produced by the dissolution of serpentine, brucite, and calcite and the precipitation of chrysotile and hydromagnesite. The hydromagnesite coexisted with small amounts of aragonite. Moreover, the dissolution of Fe(II)-bearing brucite and the formation of iron hydroxide is presumed.
Mix type
In the Samail Ophiolite in Oman, mix-type water pools (9.6 < pH < 11.5) were recognized, and aragonite was predominantly formed due to the uptake of atmospheric CO2 and evaporation (Giampouras et al., Reference Giampouras, Garrido, Bach, Los, Fussmann and Monien2020). Calcite, brucite, and trace LDH phases were occasionally attached to aragonite.
At Bat and Hilti in Oman, the mixing of Ca-OH-type spring water (pH >11) and Mg-HCO3-type river water (8.46 < pH < 8.59) produced carbonate, LDH, and brucite (Anraku et al., Reference Anraku, Matsubara, Morimoto and Sato2017). The mineral species of carbonate are determined by dissolved Mg ions, which vary with the mixing ratio of river water to spring water. Specifically, calcite is recognized near Ca-OH-type springs and aragonite at locations where Mg-HCO3-type river water is highly mixed. LDH was formed in areas with Ca-OH-type spring water which is relatively rich in Al ions, and brucite was formed in areas with Ca-OH-type spring water which is relatively poor in Al ions. Furthermore, the formation of M–S–H has been speculated about (Anraku et al., Reference Anraku, Matsubara, Morimoto and Sato2017).
Alkaline saline lake
At Teels Marsh, Nevada, an alkaline saline lake with tuffaceous sediments has been reported (Taylor and Surdam, Reference Taylor and Surdam1981). The pH of the brine ranged from 9 to 10. The interaction between rhyolitic glass and alkaline brines produced hydrous alkali aluminosilicate gel and then formed phillipsite within 1000 y. Analcime was also found to be associated with the phillipsite, indicating that it was formed by the reaction of phillipsite with Na+-rich brines. Clinoptilolite was detected only in trace amounts. In addition to phillipsite and analcime, gaylussite and searlesite have been reported as common authigenic phases.
In Searles Lake in California, lacustrine sediments deposited over 3.2 million y have been investigated (Hay and Guldman, Reference Hay and Guldman1987; Hay et al., Reference Hay, Guldman, Matthews, Lander, Duffin and Kyser1991). The drill core, which consisted of 693.4 m of sediments, was divided into three diagenetic zones. In the upper zone (0–291.1 m), detrital montmorillonite and kaolinite reacted with pore water (alkaline brine with a pH of 9–10) to form Mg-smectite, Fe-illite, K-feldspar, and analcime (Hay et al., Reference Hay, Guldman, Matthews, Lander, Duffin and Kyser1991). Phillipsite was recognized at a depth of 19.9 m. In the middle zone (291.1-541.6 m), detrital montmorillonite was probably the major reactant and formed smaller amounts of the same authigenic silicates as those in the upper zone, as the pH of the pore water was lower than that in the upper zone, but still probably exceeded 9. K-feldspar crystallized within ~42,000 y (Hay et al., Reference Hay, Guldman, Matthews, Lander, Duffin and Kyser1991). In the lower zone (541.6–693.4 m), vitric ash reacted with the pore water (pH 7.5–8) at 45–85°C, and the formation of montmorillonite, clinoptilolite, anhydrate, and a trace of opal was identified (Hay and Guldman, Reference Hay and Guldman1987; Hay et al., Reference Hay, Guldman, Matthews, Lander, Duffin and Kyser1991). The lowermost samples also contained analcime, probably indicating that the clinoptilolite was replaced by analcime (Hay and Guldman, Reference Hay and Guldman1987).
In the Colorado Plateau in New Mexico, authigenic minerals were formed in alkaline saline lakes owing to the low-temperature alteration of silicic ash deposited during late-Jurassic volcanism (Turner and Fishman, Reference Turner and Fishman1991). Around the margins of the lakes, where the pore waters are recharged, smectite was recognized as an authigenic mineral. In addition to smectite, clinoptilolite was identified in the central region of the lakes adjacent to these locations. The clinoptilolite is believed to have formed directly from silicic ash. Adjacent to this, but near the center of the lake, analcime, K-feldspar, mixed-layer illite-smectite with variable composition (0–100% expandable layers), chlorite, quartz, and chalcedony were recognized. As the authigenic K-feldspar overgrew on chlorite, it is suggested that the chlorite may have formed earlier than K-feldspar. In the center-most part of the lake, albite, mixed-layer illite-smectite in highly illitic layers (typically 0–30% expandable layers), chlorite, quartz, and chalcedony were recognized. These distributions are attributed to a lateral hydrogeochemical gradient characterized by an increase in pH and salinity owing to the interaction with lacustrine sediments and water.
In the alkaline saline lake of the Olduvai paleolake, a change in the distribution of authigenic minerals toward the center of the lake has also been reported (McHenry, Reference McHenry2010). In the distal lake margin, the smectitic clay minerals were dominated as authigenic minerals with some zeolite. The alteration of the volcanic glass caused an increase in the salinity and alkalinity of the fluids, which led to the initial formation of clay minerals. Chabazite and phillipsite were dominant at the proximal margins. As the salinity and alkalinity fluctuated with the lake water level, chabazite and phillipsite nucleated on the clay surfaces. In the lake center, where the pH of the fluids was high and the K+ activity was strong, phillipsite and K-feldspar were dominant. Although clay minerals were preserved in the proximal margin and lake center, the chemical components of the clay minerals differed from those in the distal lake margin. In the distal lake margin, where the clay minerals were dominant, they were more Al-rich. In contrast, the clay minerals were rich in Mg, Fe, and Ti in the proximal margin and lake center, where zeolite dominated.
Lake Abert in Oregon is an alkaline saline lake with a pH of 9.7 (Phillips and Van Denburgh, Reference Phillips and Van Denburgh1971). Volcanic rocks, ranging from basalt to rhyolite, weathered to give a range of clays, including montmorillonite, montmorillonite/illite, and montmorillonite/intergrade smectite–chlorite interstratified clays, which entered the lake as detritus (Jones and Weir, Reference Jones and Weir1983). These detrital clays were modified by interactions with the alkaline saline water to form authigenic interstratified illite and a trioctahedral, Mg-rich mineral resembling stevensite in composition (Jones and Weir, Reference Jones and Weir1983).
The distribution of clay minerals has been investigated in the soils surrounding an alkaline saline lake in the Pantanal Wetland, Brazil (Furquim et al., Reference Furquim, Graham, Barbiero, de Queiroz Neto and Vallès2008; Furquim et al., Reference Furquim, Barbiéro, Graham, de Queiroz Neto, Ferreira and Furian2010; Barbiero et al., Reference Barbiero, Berger, Rezende Filho, Meunier, Martins-Silva and Furian2016). The soil pH ranged from 9.2 to 10.9. A zone rich in authigenic trioctahedral smectite was found in the topsoil close to the lake, where Fe and Al ions were bonded to organic colloids, preventing these ions from contributing to the formation of mica. In contrast, a zone rich in authigenic dioctahedral mica was found in deeper horizons, a few meters apart, where the mineralization of organic matter and release of available Fe and Al ions allowed for the formation of the mica (Barbiero et al., Reference Barbiero, Berger, Rezende Filho, Meunier, Martins-Silva and Furian2016). The authigenic trioctahedral smectites in the topsoil close to the lake were referred to as saponitic and stevensitic minerals based on differences in their Al content; they were formed by direct precipitation from the water (Furquim et al., Reference Furquim, Graham, Barbiero, de Queiroz Neto and Vallès2008). The authigenic dioctahedral micas in the deeper horizons, a few meters away from the lake, were Fe3+-rich micas (Fe-illite, and glauconite) with small amounts of illite. They coexist with Si-rich amorphous materials and are believed to be neoformed (Furquim et al., Reference Furquim, Barbiéro, Graham, de Queiroz Neto, Ferreira and Furian2010). Moreover, some of them transformed into ferribeidellite owing to the wetting-drying cycles (Furquim et al., Reference Furquim, Graham, Barbiero, de Queiroz Neto and Vallès2008).
Lake Yoa in Chad is an alkaline saline lake with a pH >9.3 (Darragi and Tardy, Reference Darragi and Tardy1987). At this site, the formation of stevensite and aragonite, in addition to the formation of various salts, has been observed. Stevensite is associated with amorphous silica.
Implications for cement–bentonite interactions in radioactive waste repositories
Formation conditions and mineralogical transition of secondary phases
In the previous section, secondary phases reported to occur at natural sites were summarized. In this section, the following secondary phases were selected, and their formation conditions and mineralogical transition in radioactive waste repositories are discussed based on the findings in the previous section, as well as laboratory and in situ experiments.
Calcium silicates
The formation of 14 Å tobermorite was observed near ophiolites where Ca-OH-type alkaline fluids are present (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake and Kikuchi2020). This indicates that 14 Å tobermorite can potentially form during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates (Table 5). Contrary to this, the formation of 14 Å tobermorite has not been reported in previous laboratory and in situ experiments showing alkaline alteration of bentonite or clay, but rather the formation of C–S–H, C–A–S–H, 11 Å tobermorite, or Al–tobermorite (Fernández et al., Reference Fernández, Ruiz and Cuevas2016; Lalan et al., Reference Lalan, Dauzères, De Windt, Bartier, Sammaljärvi and Barnichon2016; Fernández et al., Reference Fernández, Torres, Ruiz, Cuevas, Alonso and García Calvo2017; Lerouge et al., Reference Lerouge, Gaboreau, Grangeon, Claret, Warmont and Jenni2017; Nakarai et al., Reference Nakarai, Shibata, Sakamoto, Owada and Kosakowski2021; Ramírez et al., Reference Ramírez, Vieillard, Bouchet, Cassagnabère, Meunier and Jacquot2005; Sánchez et al., Reference Sánchez, Cuevas, Ramírez, León, De Fernández and Vigil2006; Yokoyama et al., Reference Yokoyama, Nakamura, Tanaka and Hironaga2011; Techer et al., Reference Techer, Bartier, Boulvais, Tinseau, Suchorski and Cabrera2012). The tendency for C–S–H to form at low temperatures was recognized from these experiments, which lasted up to 15 y (Appendix A in the Supplementary material). This may be due to metastable C–S–H formation instead of 14 Å tobermorite in the short term and formation of stable 14 Å tobermorite with a slower precipitation rate in the long term because it is known that C–S–H with a Ca:Si ratio of <1.4 is a 14 Å tobermorite with an incomplete crystal structure (Taylor, Reference Taylor1997). According to natural geochemical reactions, the formation of 14 Å tobermorite has occurred within 10,000 y (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake and Kikuchi2020). Therefore, it is implied that the time required for the formation of 14 Å tobermorite may be more than a few decades but less than a few thousand years at low temperatures. As it is estimated that Stage I can last approximately 10,000 y and Stage II can last from 10,000 to 200,000 y (Sun et al., Reference Sun, Chen and Ye2022 and references therein), the mineralogical transition from C–S–H to 14 Å tobermorite may need to be considered in RT modeling for the performance assessment of bentonite in radioactive waste repositories.
Table 5. List of secondary phases suggested by NA studies at various geological settings
The formation of 11 Å tobermorite was not recognized from natural geochemical reactions at low temperatures (Table 5), while they were reported in previous laboratory and in situ experiments at >70°C (Appendix A in the Supplementary material). It is known that three types of tobermorite exist in different degrees of hydration: 14 Å tobermorite (plombierite), 11 Å tobermorite, and 9 Å tobermorite (riversideite), from most hydrated to least hydrated (McConnell, Reference McConnell1954). Furthermore, 14 Å tobermorite transforms to 11 Å tobermorite by heating at 90°C, and subsequent heating at 300°C causes lattice shrinkage to form 9 Å tobermorite, although some 11 Å tobermorite does not shrink (Mitsuda and Taylor, Reference Mitsuda and Taylor1978). Therefore, 11 Å tobermorite and 9 Å tobermorite may not form at temperatures of <~70°C.
C–A–S–H was recognized at sites near pyrometamorphic rocks, indicating that C–A–S–H can potentially form during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates (Table 5). This is consistent with previous laboratory and in situ experiments demonstrating C–A–S–H formation during the alkaline alteration of bentonite or clay (Yokoyama et al., Reference Yokoyama, Nakamura, Tanaka and Hironaga2011; Fernández et al., Reference Fernández, Ruiz and Cuevas2016; Lalan et al., Reference Lalan, Dauzères, De Windt, Bartier, Sammaljärvi and Barnichon2016; Fernández et al., Reference Fernández, Torres, Ruiz, Cuevas, Alonso and García Calvo2017). In addition, laboratory and in situ experiments exhibited the tendency for the secondary phases to change from C–A–S–H to Al-tobermorite with increasing temperature (Appendix A in the Supplementary material). This tendency may be due to metastable C–A–S–H formation instead of Al‐tobermorite at low temperatures and stable Al-tobermorite formation within a shorter time at higher temperatures. It is worth noting that Al-tobermorite formations have been reported within 16.5 y in cementitious materials at 40–55°C (Maruyama et al., Reference Maruyama, Rymeš, Aili, Sawada, Kontani, Ueda and Shimamoto2021) and within 2000 y in Roman concrete that reacted with seawater at low temperatures (Jackson et al., Reference Jackson, Mulcahy, Chen, Li, Li, Cappelletti and Wenk2017). Therefore, a mineralogical transition from C–A–S–H to Al-tobermorite may also need to be considered in RT modeling for the performance assessment of bentonite, even at low temperatures.
As described above, C–S–H, C–A–S–H, 14 Å tobermorite, and Al-tobermorite have been suggested to occur under alkaline conditions from Stage I and II leachates at low temperatures in radioactive waste repositories. On the other hand, these secondary phases have not been reported to form under lower pH conditions. Therefore, they are likely to become unstable after a decrease in pH over time in radioactive waste repositories.
Zeolite (aluminum silicates)
In alkaline lakes, species of zeolite tend to depend on the pH conditions. Specifically, phillipsite was observed under relatively high pH conditions, while clinoptilolite and chabazite were observed under relatively low pH conditions. Analcime tends to form under relatively high pH conditions though it can form under relatively low pH conditions when following clinoptilolite formation (Hay and Guldman, Reference Hay and Guldman1987). It has been reported that an increase in pH reduces the Si/Al ratio of the solution, resulting in the formation of zeolite with low Si/Al ratios, such as phillipsite and analcime, rather than zeolite with a high Si/Al ratio, such as clinoptilolite (Mariner and Surdam, Reference Mariner and Surdam1970). Therefore, the tendency to form phillipsite and analcime under relatively high pH conditions and clinoptilolite under relatively low pH conditions in alkaline lakes may be influenced by the Si/Al ratios of the fluids. On the contrary, chabazite formed under relatively low pH conditions despite the low Si/Al ratio. Although the conditions that determine the formation of clinoptilolite and chabazite are unknown, the formation of zeolite in alkaline saline lakes suggests that it can potentially form during bentonite alteration farther from the interface between cementitious materials and bentonite, where the chemistry of alkaline saline lake is analogous in radioactive waste repositories (Fig. 5). Furthermore, the distribution of zeolite species even within the locations that are analogous to the chemistry of alkaline saline lake (Fig. 5) is suggested to be dependent on pH conditions.
Several previous laboratory and in situ experiments have reported phillipsite or analcime formation owing to the alkaline alteration of bentonite or clay (Bauer and Velde, Reference Bauer and Velde1999; Vigil de la Villa et al., Reference de la Villa, Cuevas, Ramîrez and Leguey2001; Ramírez et al., Reference Ramírez, Cuevas, Vigil and Leguey2002; Ramírez et al., Reference Ramírez, Vieillard, Bouchet, Cassagnabère, Meunier and Jacquot2005; Sánchez et al., Reference Sánchez, Cuevas, Ramírez, León, De Fernández and Vigil2006; Yokoyama and Nakamura, Reference Yokoyama and Nakamura2010; Lalan et al., Reference Lalan, Dauzères, De Windt, Bartier, Sammaljärvi and Barnichon2016), indicating that they have the potential to form during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates. Compared with the laboratory and in situ experiments, pH conditions under which phillipsite and analcime are formed in alkaline saline lakes are relatively low. These facts indicate that the phillipsite and analcime formed under alkaline conditions from Stages I and II leachates could be stable, even after a decrease in pH over time.
The formation of phillipsite is followed by the formation of analcime in an alkaline saline lake in Teels Marsh (Taylor and Surdam, Reference Taylor and Surdam1981). On the other hand, laboratory experiments showed a tendency for the secondary phases to change from phillipsite to analcime with increasing temperature or interaction time. For example, laboratory experiments under a wide range of temperature conditions show that phillipsite is formed at lower temperatures (75°C) and analcime at higher temperatures (125–200°C) (Sánchez et al., Reference Sánchez, Cuevas, Ramírez, León, De Fernández and Vigil2006). In a laboratory experiment conducted at 90°C, phillipsite was observed after 30 days, and analcime, in addition to phillipsite, was observed after 90 days (Ramírez et al., Reference Ramírez, Cuevas, Vigil and Leguey2002). The tendency indicates the metastable phase of phillipsite and the stable phase of analcime under those conditions. However, the timescales required for these mineral transitions differ depending on the conditions. In particular, while laboratory experiments have reported the formation of analcime within 1 year at low temperatures (23–60°C) (Yokoyama and Nakamura, Reference Yokoyama and Nakamura2010), the formation of phillipsite has been reported to occur within 1000 y in alkaline saline lakes, followed by the formation of analcime (Taylor and Surdam, Reference Taylor and Surdam1981). These different timescales for analcime formation may be caused not only by different temperature conditions but also by different pH and other chemical concentrations; for example, the mineralogical transition in the laboratory experiments at pH 14 was relatively rapid, whereas that in the alkaline saline lakes at pH 9–10 was relatively slow. Because the pH and other chemical concentrations expected in the radioactive waste repositories vary over time and space, it is important to consider temporal and spatial chemical conditions in bentonite when predicting the timescale of the mineralogical transition of zeolite mineral species. Despite the different time scales, considering a mineralogical transition of zeolite mineral species may be important in RT modeling for the performance assessment of bentonite.
Magnesium silicates
In several laboratory experiments wherein bentonite or clay interacted with alkaline fluids, NaOH, Ca(OH)2 and KOH were often used as alkaline fluids based on the simplified composition of pore waters from cementitious materials over time, and calcium silicates and zeolite formation was reported (Ramírez et al., Reference Ramírez, Vieillard, Bouchet, Cassagnabère, Meunier and Jacquot2005; Yokoyama et al., Reference Yokoyama, Nakamura, Tanaka and Hironaga2011). On the other hand, in natural environments with the presence of Mg, Mg-bearing smectite (i.e. smectite enriched in structural Mg such as saponite) was detected at sites with Ca-OH-type alkaline fluids near ophiolites (Table 5). This indicates their potential formation during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates. This was consistent with previous laboratory experiments that considered the Mg effect and in situ experiments showing Mg-bearing smectite formation during the alkaline alteration of bentonite or clay (Fernández et al., Reference Fernández, Mäder, Rodríguez, Vigil de la Villa and Cuevas2009; Lerouge et al., Reference Lerouge, Gaboreau, Grangeon, Claret, Warmont and Jenni2017; Sánchez et al., Reference Sánchez, Cuevas, Ramírez, León, De Fernández and Vigil2006). As Mg-bearing smectite formation was also observed under moderately alkaline conditions in alkaline saline lakes (Table 5), it is suggested that Mg-bearing smectite, which forms under alkaline conditions from Stage I and II leachates in radioactive waste repositories, could be stable even after a decrease in pH over time. Furthermore, this indicates that Mg-bearing smectite can potentially form during bentonite alteration farther from the interface between cementitious materials and bentonite in radioactive waste repositories.
A smectitic phase with imperfect interlayer hydroxide sheets was also found at sites with Ca-OH-type alkaline fluids near ophiolites (Table 5), suggesting that it can potentially form during bentonite alteration close to cementitious materials with Stage I and II alkaline leachates in radioactive waste repositories. This was consistent with previous laboratory and in situ experiments. For example, in in situ experiments on cementitious materials and clay rocks, the formation of Mg-bearing smectite with Mg(OH)2 in the interlayer was discussed (Lerouge et al., Reference Lerouge, Gaboreau, Grangeon, Claret, Warmont and Jenni2017). In addition, intercalation of Mg(OH)2 in the montmorillonite interlayer has been observed in laboratory and in situ experiments (Fernández et al., Reference Fernández, Mäder, Rodríguez, Vigil de la Villa and Cuevas2009; Fernández et al., Reference Fernández, Vigil de la Villa, Ruiz, García and Cuevas2013; Cuevas et al., Reference Cuevas, Ruiz, Fernández, González-Santamaría, Angulo and Ortega2018; Fernández et al., Reference Fernández, González-Santamaría, Angulo, Torres, Ruiz and Turrero2018). As it is known that the intercalation of Mg(OH)2 in the montmorillonite interlayer easily occurs at pH values between 10 and 12 (Xeidakis, Reference Xeidakis1996), the hydroxide sheets in the interlayer could occur under alkaline conditions from Stage I and II leachates and would be unstable after a decrease in pH over time in radioactive waste repositories.
M–S–H was recognized at sites with Mg-HCO3-type alkaline fluids near ophiolites (Table 5), suggesting that it can potentially form during bentonite alteration farther from the interface between cementitious materials and bentonite in radioactive waste repositories. On the other hand, in previous in situ experiments, M–S–H formed during the alkaline alteration of clay rocks (Dauzeres et al., Reference Dauzeres, Achiedo, Nied, Bernard, Alahrache and Lothenbach2016; Bernard et al., Reference Bernard, Jenni, Fisch, Grolimund and Mäder2020). The M–S–H formation was implied to form in clay rocks away from the interface of Portland cement paste (Bernard et al., Reference Bernard, Jenni, Fisch, Grolimund and Mäder2020). This is consistent with the above discussion that M–S–H could be formed by the alteration of bentonite farther from the interface between cementitious materials and bentonite. Notably, the structure of M–S–H is similar to that of Mg-bearing phyllosilicates (Roosz et al., Reference Roosz, Grangeon, Blanc, Montouillout, Lothenbach and Henocq2015; Nied et al., Reference Nied, Enemark-Rasmussen, Hopital, Skibsted and Lothenbach2016; Pedone et al., Reference Pedone, Palazzetti and Barone2017), and M–S–H could be the precursor of the Mg-bearing phyllosilicates, such as smectite, talc, or serpentine (Roosz et al., Reference Roosz, Grangeon, Blanc, Montouillout, Lothenbach and Henocq2015; Nied et al., Reference Nied, Enemark-Rasmussen, Hopital, Skibsted and Lothenbach2016). Their structural similarity makes it difficult to distinguish M–S–H from other Mg-bearing phyllosilicates after several years of interaction. Indeed, for the same samples after a 4.9-year interaction between cementitious materials and clay rocks, M–S–H formation was reported by Dauzeres et al. (Reference Dauzeres, Achiedo, Nied, Bernard, Alahrache and Lothenbach2016), while that of Mg-bearing smectite was reported by Lerouge et al. (Reference Lerouge, Gaboreau, Grangeon, Claret, Warmont and Jenni2017). Therefore, distinguishing between M–S–H and Mg-bearing phyllosilicates and understanding the time scales required for their mineralogical transition is a future challenge. In addition, it is important to clarify which Mg-bearing phyllosilicates will form after the mineralogical transition of M–S–H in a given condition because permeability is expected to change depending on the swelling smectite or non-swelling minerals, such as talc and serpentine.
Summary
Comparison between the findings of secondary phases compiled in this review and findings in previous laboratory and in situ experiments provide an implication for the species and mineralogical transition of secondary phases that have the potential to form during cement–bentonite interactions. Specifically, C–S–H, C–A–S–H, and phillipsite were suggested as potential secondary phases, and the mineralogical transition from C–S–H to 14 Å tobermorite, C–A–S–H to Al–tobermorite, and phillipsite to analcime may need to be considered during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates at low temperatures. After a decrease in pH over time, 14 Å tobermorite and Al-tobermorite would be unstable, while analcime would be stable. Mg-bearing smectite and a smectitic phase with imperfect interlayer hydroxide sheets were also potential secondary phases during the alteration of bentonite close to cementitious materials with Stage I and II alkaline leachates. Note that M–S–H may be the precursor to Mg-bearing phyllosilicates such as Mg-bearing smectite. When the pH decreased over time, Mg-bearing smectite would be stable, while a smectitic phase with imperfect interlayer hydroxide sheets would be unstable.
During bentonite alteration farther from the interface between cementitious materials and bentonite, phillipsite, analcime, clinoptilolite, chabazite, newly formed Mg-bearing smectite, and M–S–H were indicated as potential secondary phases. Among those minerals, phillipsite would form under relatively high pH conditions, which may depend on the distance from the cementitious materials, while clinoptilolite and chabazite would form under relatively low pH conditions. The mineralogical transition from philippisite to analcime would occur, although the time scale required for the transition would be slower than that close to the cementitious materials. Furthermore, the mineralogical transition from clinoptilolite to analcime may also occur.
Conclusion
Understanding the cement–bentonite interactions is important to evaluate bentonite properties in radioactive waste repositories. Investigating natural geochemical reactions has the significant advantage of observing geochemical reactions over a long period of time. In this review, the secondary phases formed at low temperatures (<100°C) and under alkaline conditions were compiled for a broader range of natural sites than in previous reviews. Specifically, studies reporting secondary phases near pyrometamorphic rocks, near ophiolites, and in alkaline saline lakes, were summarized. Furthermore, this review provides implications for the species and mineralogical transition of secondary phases formed by the spatiotemporal alteration of bentonite by adding that information from natural sites to previous laboratory and in situ experiments. This will be useful for selecting secondary phases to be considered in the RT modeling for performance assessment of bentonite by comparing the secondary phases predicted to form by the RT modeling and insights from this review.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cmn.2024.4.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. Code availability: not applicable.
Acknowledgments
The authors thank Professor Tsubasa Otake from Hokkaido University, Assistant Professor Ryosuke Kikuchi from Hokkaido University, and Dr Yuto Nishiki from AIST for their valuable input in performing this research. The authors also thank the two anonymous reviewers for considerably improving the manuscript.
Author contributions
Conceptualization: M.S., S.Y., T.S.; Investigation: M.S.; Writing-original draft: M.S.; Writing-review and editing: S.Y., T.S.; Supervision: T.S.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no competing interests.















