The National Healthcare Safety Network (NHSN), managed by the Centers for Disease Control and Prevention (CDC), is the nation’s largest and most widely used electronic surveillance system for tracking healthcare-associated infections (HAIs). More than 25,000 healthcare facilities use the NHSN to enter and analyze data on HAIs, adverse events, and healthcare personnel safety. As the NHSN continues to expand its capacity with additional reporting modules, facility types, and location types eligible for participation, national surveillance reports and targeted benchmarks become increasingly vital for healthcare facilities and public health agencies to monitor progress in infection prevention.
The pathogens implicated in HAIs, and their antimicrobial susceptibilities, provide important information about the spread and extent of antimicrobial-resistant infections in the United States. These data can be used to identify emerging resistant pathogens, to provide direction for new drug development, to encourage review and comparison of local pathogen and susceptibility data, and to inform policies such as those designed to interrupt the transmission of antimicrobial-resistant pathogens. This is the fourth summary of the NHSN pathogen and antimicrobial susceptibility data, and it builds upon the methodology from earlier reports.Reference Hidron, Edwards and Patel 1 – Reference Weiner, Webb and Limbago 3 Most of the central line-associated bloodstream infections (CLABSIs) and catheter-associated urinary tract infections (CAUTIs) analyzed in this report were reported to the NHSN under federal requirements for participation in the Centers for Medicare and Medicaid Services (CMS) Quality Reporting Programs (QRPs), which apply to acute-care hospitals, long-term acute-care hospitals (LTACHs), and inpatient rehabilitation facilities (IRFs). 4 – 6 Surgical site infections (SSIs) following colon surgeries and abdominal hysterectomies are also required to be reported under CMS QRPs, and ventilator-associated events (VAEs) became a required LTACH reporting element in January 2016. This report provides expanded pathogen distribution and susceptibility data, stratified by infection type, facility type, location within a facility, and/or surgery type. Publishing data at this level of granularity were made possible by the expansion of CMS and state HAI reporting requirements, as well as the commitment of healthcare facility staff to HAI surveillance.
Methods
The CLABSIs, 7 CAUTIs, 8 select VAEs, 9 and SSIs 10 that occurred between 2015–2017 and had been reported to the NHSN’s Patient Safety Component as of July 1, 2018, were included in this report. These HAIs were reported by acute-care hospitals, critical access hospitals, LTACHs, and IRFs from all US states and territories. Unless otherwise noted, CLABSI data included events classified as mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI). VAE data were limited to events classified as possible ventilator-associated pneumonia (PVAP) because this is the only subtype of VAE for which a pathogen can be reported. Asymptomatic bacteremic urinary tract infections, CLABSIs reported from IRFs, and outpatient SSIs were excluded.
The NHSN protocols provide guidance for attributing device-associated (DA) HAIs (ie, CLABSIs, CAUTIs, and PVAPs) to a CDC-defined location type, and SSIs to a CDC operative procedure code. Due to known differences in pathogens and resistance patterns between adult and pediatric populations,Reference Hocevar, Weiner, Edwards and Magill 11 , Reference Lake, Weiner, Milstone, Saiman, Magill and See 12 this report was limited to DA HAIs attributed to adult location types, and to SSIs that occurred in patients ≥18 years old at the time of surgery. Comparable data from pediatric locations and patients are described in a companion report.Reference Weiner-Lastinger, Abner and Edwards 13
Unless otherwise noted, DA HAIs were stratified into 5 mutually exclusive location categories: hospital wards (inclusive of step-down, mixed acuity, and specialty care areas), hospital intensive care units (ICUs), hospital oncology units (ie, oncology ICUs and wards), LTACHs (ie, LTACH ICUs and wards), and IRFs (ie, freestanding IRFs and CMS-certified IRF units located within a hospital). SSI data were stratified into mutually exclusive surgical categories based on the operative procedure code. Pathogen distributions were also analyzed separately for each operative procedure code and are available in the online supplement. 14
Up to 3 pathogens and their antimicrobial susceptibility testing (AST) results can be reported to the NHSN for each HAI. The AST results for the drugs included in this analysis were reported using the interpretive categories of “susceptible” (S), “intermediate” (I), “resistant” (R), or “not tested.” Instead of “intermediate,” cefepime had the category interpretation of “intermediate/susceptible-dose dependent” (I/S-DD), which was treated as I for this analysis. Laboratories are expected to follow current guidelines from the Clinical and Laboratory Standards Institute (CLSI) for AST. 15 Naming conventions for pathogens generally adhered to the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) Preferred Term. 16 In some cases, pathogens were grouped by genus or clinically recognized group (eg, viridans group streptococci) (Appendices A2–A4 online). Results for Klebsiella spp were limited to K. pneumoniae and K. oxytoca; K. aerogenes was considered part of Enterobacter spp due to the timing of the NHSN’s adoption of its name change.Reference Tindall, Sutton and Garrity 17
Staphylococcus aureus was defined as methicillin-resistant (MRSA) if the isolate was reported as R to oxacillin, cefoxitin, or methicillin. Enterococcus spp isolates were defined as vancomycin-resistant (VRE) if they tested R to vancomycin. VRE data were analyzed for all HAIs except PVAP because Enterococcus spp are excluded from the NHSN’s PVAP surveillance definition under most scenarios. Carbapenem-resistant Enterobacteriaceae (CRE) were defined as Klebsiella spp, Escherichia coli, or Enterobacter spp that tested R to imipenem, meropenem, doripenem, or ertapenem. All other pathogen-antimicrobial combinations (phenotypes) were described using a metric for nonsusceptibility, which included pathogens that tested I or R to the applicable drugs. To be defined as nonsusceptible to extended-spectrum cephalosporins (ESCs), pathogens must have tested I or R to either ceftazidime or cefepime (Pseudomonas aeruginosa) or to ceftazidime, cefepime, ceftriaxone, or cefotaxime (Klebsiella spp and E. coli). For Enterobacter spp, evaluation of nonsusceptibility to ESCs was limited to cefepime due to Enterobacter’s inducible resistance to other ESCs. Fluoroquinolone nonsusceptibility was defined as a result of I or R to either ciprofloxacin or levofloxacin (P. aeruginosa) or to ciprofloxacin, levofloxacin, or moxifloxacin (E. coli). Carbapenem nonsusceptibility in P. aeruginosa and Acinetobacter spp was defined as a result of I or R to imipenem, meropenem, or doripenem. Nonsusceptibility to aminoglycosides was defined as a result of I or R to gentamicin, amikacin, or tobramycin. Finally, multidrug-resistance (MDR) was approximated by adapting previously established definitionsReference Magiorakos, Srinivasan and Carey 18 that require nonsusceptibility to at least 1 agent within 3 different drug classes. For Enterobacteriaceae and P. aeruginosa, 5 classes were considered in the criteria: ESCs (or cefepime for Enterobacter spp), fluoroquinolones, aminoglycosides, carbapenems, and piperacillin (PIP) or piperacillin/tazobactam (PIPTAZ). A sixth class, ampicillin/sulbactam, was included in the criteria for Acinetobacter spp.
Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC). For all HAIs and pathogens, absolute frequencies and distributions were calculated by HAI, location, and surgical category. The 15 most commonly reported pathogens were identified, and their frequencies and ranks within each stratum were calculated. A pooled mean percentage nonsusceptible (%NS) was calculated for each phenotype as the sum of nonsusceptible (or resistant) pathogens, divided by the sum of pathogens tested for susceptibility, and multiplied by 100. Percentage NS was not calculated for any phenotype for which <20 pathogens were tested. Differences in the %NS across location types or surgical categories were assessed for statistical significance using a mid-P exact test, and P < .05 was considered statistically significant. The percentage of pathogens with reported susceptibility results (referred to as “percentage tested”) is defined elsewhereReference Weiner, Webb and Limbago 3 and was calculated for each bacterial phenotype, as well as for select Candida spp. Pathogens and susceptibility data for CLABSIs categorized as MBI-LCBI were analyzed separately and are presented in the online supplement. 14
“Selective reporting” occurs when laboratories suppress AST results as part of antimicrobial stewardship efforts. This practice could contribute to a higher number of pathogens reported to the NHSN as “not tested” to certain drugs. To assess the impact of selective reporting on the national %NS, we applied an alternate calculation for CRE and ESC nonsusceptibility. If a pathogen was reported as “not tested” to carbapenems, susceptibility was inferred as S if the pathogen tested susceptible to at least 2 of the following: ampicillin, ampicillin/sulbactam, amoxicillin/clavulanic acid, PIPTAZ, cefazolin, cefoxitin, or cefotetan. If a pathogen was reported as “not tested” to ESCs, susceptibility was inferred as S if the pathogen tested susceptible to at least 2 of the following: ampicillin, aztreonam, or cefazolin. Therefore, the number of tested isolates increases under the alternate calculation. Percentage NS was calculated using both the traditional (ie, strictly as reported) and alternate approaches.
Statistical analyses were not performed to test for temporal changes in the %NS; thus, this report does not convey any conclusions regarding changes in resistance over time. Due to differences in the stratification levels, inclusion criteria, and patient populations, the %NS values in this report should not be compared to those published in previous iterations of this report.
Results
During 2015–2017, 5,626 healthcare facilities performed surveillance of adult HAIs, and a total of 311,897 HAIs and 356,633 pathogens were reported (Tables 1 and 2). Facilities varied by type and size; 41% had ≤50 beds. SSIs contributed the highest proportion of pathogens (43%) followed by CAUTIs (29%), CLABSIs (25%) and PVAPs (3%). Escherichia coli was the most common pathogen across all HAIs, constituting almost 18% of reported pathogens (Table 3). Staphylococcus aureus (12%) and Klebsiella spp (9%) were the second and third most commonly reported pathogens, respectively.
Table 1. Characteristics of Facilities Performing Adult Healthcare-Associated Infection (HAI) Surveillance in the National Healthcare Safety Network, 2015–2017
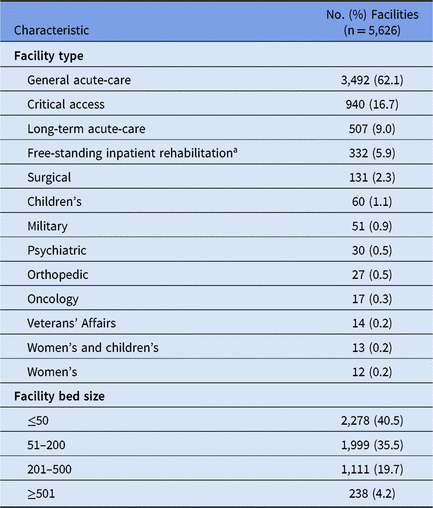
a Does not include inpatient rehabilitation facilities reporting to the NHSN as locations within acute-care hospitals.
Table 2. Frequency of Adult Healthcare-Associated Infection (HAI) Events and Pathogens, by HAI Type, 2015–2017
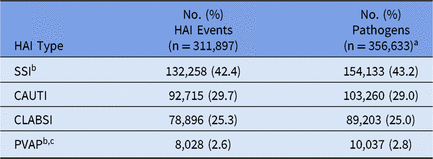
Note. SSI, surgical site infection; CAUTI, catheter-associated urinary tract infection; CLABSI, central line-associated bloodstream infection; PVAP, possible ventilator-associated pneumonia.
a Up to 3 pathogens can be reported for each HAI.
b SSIs and PVAPs can be reported to the NHSN without an associated pathogen. In total, 27,237 (20.6%) SSIs and 43 (0.5%) PVAPs were reported to the NHSN in 2015–2017 without a pathogen.
c PVAP is the only type of ventilator-associated event (VAE) for which a pathogen can be reported.
Table 3. Distribution and Rank Order of the 15 Most Frequently Reported Pathogens Across All Types of Adult Healthcare-Associated Infections (HAIs), 2015–2017
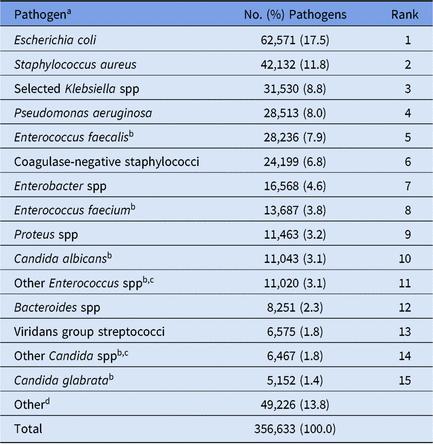
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The following species were frequently reported to the NHSN but are considered part of a larger pathogen group for this table: Staphylococcus epidermidis (11,482; 47.4% of coagulase-negative staphylococci); Enterobacter cloacae complex group (11,886; 71.7% of Enterobacter spp); Proteus mirabilis (10,662; 93.0% of Proteus spp).
b When analyzed on the genus level, Enterococcus ranks #2, and Candida ranks #7.
c The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported. The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species were not reported.
d A complete distribution of all pathogens reported from adult HAIs can be found in the 2015–2017 Adult Antimicrobial Resistance Report Online Supplement (https://www.cdc.gov/nhsn/datastat/index.html).
The highest proportion of CLABSI and CAUTI pathogens were reported from hospital wards, with 9,648 individual wards reporting at least 1 CLABSI pathogen and 11,850 wards reporting at least 1 CAUTI pathogen (Table 4). Most PVAP pathogens (92%) were reported from hospital ICUs.
Table 4. Frequency of Adult Device-Associated Healthcare-Associated Infection (HAI) Pathogens, by HAI and Location Type,a 2015–2017
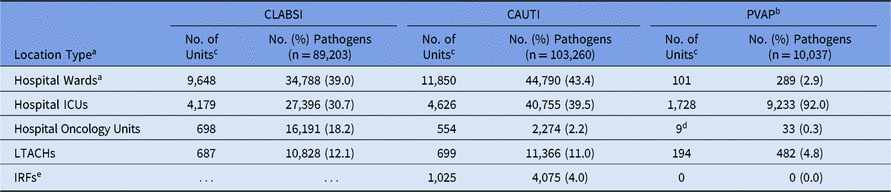
Note. CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; PVAP, possible ventilator-associated pneumonia; ICUs, intensive care units; LTACHs, long-term acute-care hospitals; IRFs, inpatient rehabilitation facilities.
a Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
b PVAP is the only type of ventilator-associated event (VAE) for which a pathogen can be reported.
c Number of units that reported at least 1 pathogen.
d Reported from oncology ICUs only.
e Consists of free-standing IRFs and rehabilitation wards located within hospitals and defined as IRFs per the Centers for Medicare and Medicaid Services (CMS). CLABSI data reported from IRFs were excluded from this report.
Pathogen distributions
Staphylococcus aureus and coagulase-negative staphylococci were among the most frequently reported CLABSI pathogens from wards, ICUs, and LTACHs (Table 5). However, when grouped at the genus level, Candida spp became the most common CLABSI pathogen in hospital wards and ICUs, making up 25% of ICU pathogens. Enterococcus faecalis made up a higher proportion of CLABSI pathogens in LTACHs (12%) compared to all other location types. In contrast, E. coli was the most common CLABSI pathogen in oncology units (17%) and was more likely to be reported from those units than non-oncology units (eg, 4% from ICUs and LTACHs). Viridans group streptococci were also reported more frequently in oncology-unit CLABSIs (9%) than non–oncology-unit CLABSIs (≤1%). Notably, half of all CLABSIs in oncology units met the definition of an MBI-LCBI (Supplementary Table S1).14
Table 5. Distribution and Rank Ordera of the 15 Most Frequently Reported Adult Central Line-Associated Bloodstream Infectionb (CLABSI) Pathogens, by Location Typec, 2015–2017
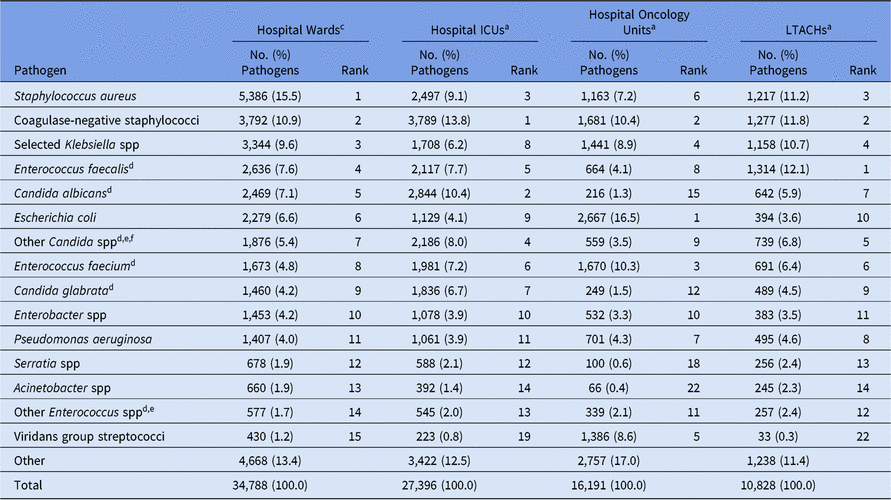
Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The 15 most frequently reported pathogens from hospital wards are shown, along with their distribution and rank within the other location types. Some rankings within the other location types are not shown: hospital ICUs #15 (Yeast, not otherwise specified); hospital oncology units #13 (Rothia mucilaginosa), #14 (Bacteroides spp); LTACHs #15 (Proteus spp).
b Supplemental tables are available in the 2015–2017 Adult Antimicrobial Resistance Report Online Supplement that show pathogen rankings separately for mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) and non–MBI-LCBIs (https://www.cdc.gov/nhsn/datastat/index.html).
c Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
d When analyzed on the genus level, Candida and Enterococcus resulted in the following rankings: Candida: hospital wards (#1), hospital ICUs (#1), hospital oncology units (#7), LTACHs (#2); Enterococcus: hospital wards (#3), hospital ICUs (#2), hospital oncology units (#1), LTACHs (#1).
e The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported. The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species was not reported.
f Candida parapsilosis was reported with the following frequencies: hospital wards (846); hospital ICUs (810); hospital oncology units (122); LTACHs (446).
Escherichia coli, Klebsiella spp, and P. aeruginosa were the 3 most frequently reported CAUTI pathogens for all location types analyzed (Table 6). Frequencies of E. faecium and E. faecalis as CAUTI pathogens differed by location type, with E. faecium rarely identified in IRFs (1%) and E. faecalis commonly reported by oncology units (12%). Several pathogens appeared among the most frequently reported PVAP pathogens that were not frequently reported from other DA infections, such as Haemophilus influenzae and Stenotrophomonas maltophilia. Staphylococcus aureus, P. aeruginosa, and Klebsiella spp were the 3 most frequently reported PVAP pathogens across all location types (Table 7).
Table 6. Distribution and Rank Ordera of the 15 Most Frequently Reported Adult Catheter-Associated Urinary Tract Infection (CAUTI) Pathogens, by Location Type,b 2015–2017

Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; IRFs, inpatient rehabilitation facilities; Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The 15 most frequently reported pathogens from hospital wards and ICUs are shown, along with their distribution and rank within the other location types. Some rankings within the other location types are not shown: hospital oncology units #14 (Stenotrophomonas maltophilia); IRFs #15 (Pseudomonas, not otherwise specified).
b Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
c When analyzed on the genus level, Enterococcus resulted in the following rankings: hospital wards and ICUs (#2), hospital oncology units (#2), LTACHs (#4), IRFs (#4).
d The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported.
Table 7. Distribution and Rank Ordera of the 15 Most Frequently Reported Adult Possible Ventilator-Associated Pneumoniab (PVAP) Pathogens, by Location Type,c 2015–2017
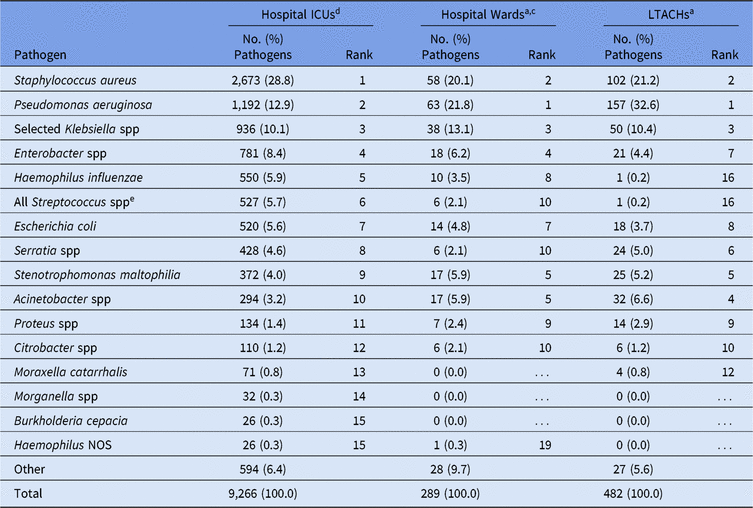
Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; Selected Klebsiella spp, K. oxytoca and K. pneumoniae; NOS, not otherwise specified.
a The 15 most frequently reported pathogens from hospital ICUs are shown, along with their distribution and rank within the other location types. Some rankings within the other location types are not shown: hospital wards #13 (Providencia stuartii), #14 (Achromobacter NOS and Corynebacterium NOS); LTACHs 3 species tied for rank #13, each was reported twice.
b PVAP is the only type of ventilator-associated event (VAE) for which a pathogen can be reported.
c Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
d Includes oncology ICUs.
e The group ‘All Streptococcus spp’ includes all members of genus Streptococcus, including those not identified to the species level. Streptococcus pneumoniae was frequently reported in hospital ICUs (277, 52.6% of the Streptococcus group).
More than half of SSI pathogens (54%) were reported following abdominal surgeries, and 24% were reported following orthopedic surgeries (Table 8). The type of SSI varied by surgical category; for example, 58% of abdominal SSI pathogens were identified as organ/space infections, 43% of orthopedic SSI pathogens were deep incisional, and 53% of obstetrical/gynecological (ob/gyn) SSI pathogens were superficial incisional. Staphylococcus aureus was the most commonly reported pathogen for all SSIs overall (18%), and was the most common pathogen for orthopedic, ob/gyn, and cardiac SSIs (Table 9). As expected, E. coli was the most commonly identified pathogen in abdominal SSIs, accounting for almost 20% of reported pathogens. Notably, coagulase-negative staphylococci were frequently identified as cardiac and orthopedic SSI pathogens (15% and 13%, respectively).
Table 8. Frequency and Types of Adult Surgical Site Infection (SSI) Pathogens, by Surgical Category, 2015–2017

Note. Ob/Gyn, obstetrical and gynecological.
a Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
b Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
c Cesarean section, abdominal hysterectomy, ovarian surgery, and vaginal hysterectomy.
d Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
e Abdominal aortic aneurysm repair, shunt for dialysis, carotid endarterectomy, and peripheral vascular bypass surgery.
f Craniotomy and ventricular shunt.
g Kidney surgery and kidney transplant.
h Neck surgery, and thyroid and/or parathyroid surgery.
Table 9. Distribution and Rank Order of the 15 Most Frequently Reported Adult Surgical Site Infection (SSI) Pathogens, by Surgical Category,a 2015–2017

Note. Ob/Gyn, obstetrical and gynecological; Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The 4 most frequently reported surgical categories by pathogen volume are shown. Supplemental tables are available in the 2015–2017 Adult Antimicrobial Resistance Report Online Supplement that show pathogen rankings separately for each NHSN operative procedure code (https://www.cdc.gov/nhsn/datastat/index.html).
b Consists of all NHSN operative procedure codes and is not limited to the 4 surgical categories shown.
c Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
d Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
e Cesarean section, abdominal hysterectomy, ovarian surgery, and vaginal hysterectomy.
f Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
g When analyzed on the genus level for all surgery types, Enterococcus ranks #2 and Candida ranks #9.
h The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported.
Most SSIs reported to the NHSN occurred after a procedure with a primarily closed incision; the surgical categories with the highest proportion of non-primarily closed incisions were amputation (12% non-primary closure), colon surgeries (8% non-primary closure), and exploratory laparotomy (8% non-primary closure). SSI pathogens varied by closure technique, with the non-primarily closed colon SSIs having a higher proportion of Candida spp (7%) compared to SSIs identified from primarily closed procedures (5%) (data not shown).
Percentage tested and percentage nonsusceptibility
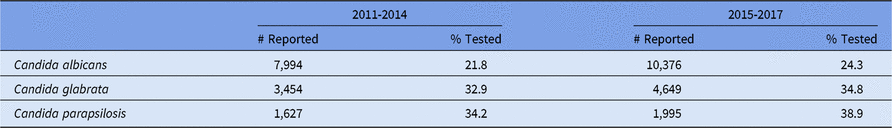
Most facilities reported AST results across all strata for the following pathogen/drug combinations: S. aureus with oxacillin/cefoxitin/methicillin, Enterococcus spp with vancomycin, E. coli with fluoroquinolones, and P. aeruginosa with aminoglycosides, fluoroquinolones, and ESCs (Tables 10–15). Overall, facilities reported low testing frequencies (<85%) for Klebsiella spp, E. coli, and Enterobacter spp with carbapenems, ESCs, and/or cefepime. Pseudomonas aeruginosa and Acinetobacter spp also had low testing frequencies to carbapenems. Overall, fluconazole susceptibility testing for Candida spp was relatively rare, with Candida parapsilosis having the highest percentage tested at 39% in 2015–2017 (Appendix A1 online). Given this low testing frequency, a national %NS was not calculated for any Candida spp.
Table 10. Percentage of Pathogens Reported from Adult Healthcare-Associated Infections (HAIs) in Acute-Care Hospitalsa that Tested Nonsusceptibleb (NS) to Selected Antimicrobial Agents, by Infection Category, 2015–2017
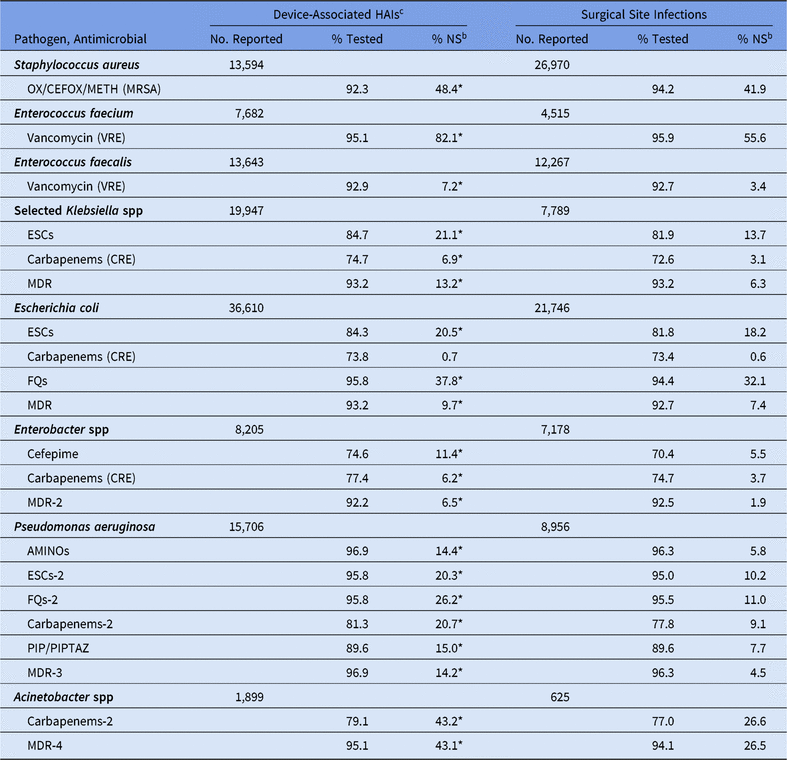
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS in surgical site infections; P < .05.
a Long-term acute-care hospitals (LTACHs) and inpatient rehabilitation facilities (IRFs) are excluded from this table.
b MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant).
c Consists of central line-associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), and possible ventilator-associated pneumonias (PVAPs).
For all phenotypes analyzed except CRE E. coli, the national %NS was statistically significantly higher in DA HAIs compared to SSIs, with the greatest difference appearing among E. faecium resistant to vancomycin (82% resistant in DA HAIs vs 56% resistant in SSIs) (Table 10). Among CLABSIs, the %NS for most phenotypes was significantly higher in LTACHs and ICUs compared to wards (Table 11). Several phenotypes had a substantially higher prevalence of nonsusceptibility in LTACHs than hospital wards: MRSA, Klebsiella spp nonsusceptibility to ESCs, CRE Klebsiella spp and Enterobacter spp, all MDR phenotypes, P. aeruginosa nonsusceptibility to aminoglycosides and PIP/PIPTAZ, and Acinetobacter spp nonsusceptibility to carbapenems. Significant differences in the CLABSI %NS were also observed between oncology units and hospital wards. Klebsiella spp, Acinetobacter spp, and some P. aeruginosa phenotypes identified in oncology units had a lower %NS than those in hospital wards, whereas E. coli nonsusceptibility to fluoroquinolones was significantly higher in oncology units (65% vs 47%). Within oncology units, MBI-LCBIs had a higher %NS for all pathogens analyzed compared to non–MBI-LCBIs, several of which were statistically significantly different. For example, the percentage of E. coli displaying nonsusceptibility to fluoroquinolones was 71% among MBI-LCBIs, compared with 44% in non–MBI-LCBIs (Supplementary Table S5).14
Table 11. Percentage of Pathogens Reported from Adult Central Line-Associated Bloodstream Infectionsa (CLABSIs) that Tested Nonsusceptibleb (NS) to Selected Antimicrobial Agents, by Location Type,c 2015–2017
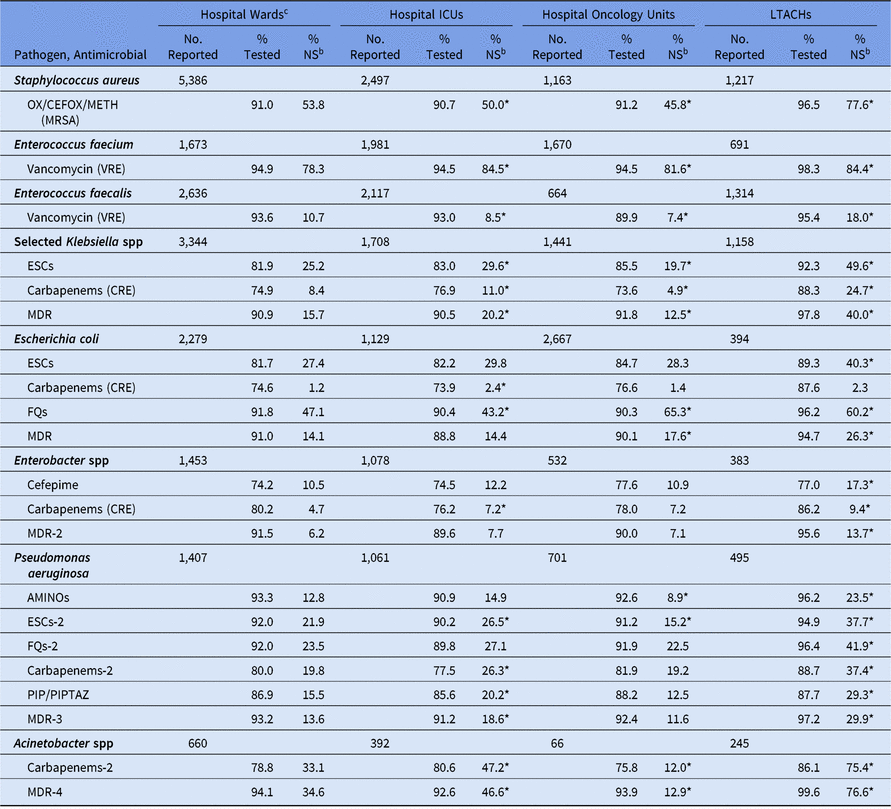
Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs: fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS in hospital wards; P < .05.
a Supplemental tables are available in the 2015–2017 Adult Antimicrobial Resistance Report Online Supplement that show the %NS separately for mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) and non–MBI-LCBIs within hospital oncology locations (https://www.cdc.gov/nhsn/datastat/index.html).
b MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant).
c Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
Among CAUTIs, LTACHs had a significantly higher %NS than hospital wards for all pathogens (Table 12). For example, 23% of Klebsiella spp were CRE in LTACHs, compared with 7% in wards. Hospital ICUs had a lower %NS than hospital wards for all Klebsiella spp and E. coli phenotypes among CAUTIs. There was almost no difference in the %NS between CAUTIs in oncology units and those in hospital wards, but E. coli nonsusceptibility to fluoroquinolones was an exception. Among PVAPs, Klebsiella spp and P. aeruginosa pathogens had a lower %NS in hospital ICUs compared with hospital wards, most of which were statistically significantly different (Table 13). In addition, a lower proportion of S. aureus was identified as MRSA among PVAPs from ICUs (37%), compared with CLABSIs (50%) and CAUTIs (41%) from the same setting.
Table 12. Percentage of Pathogens Reported from Adult Catheter-Associated Urinary Tract Infections (CAUTIs) that Tested Nonsusceptiblea (NS) to Selected Antimicrobial Agents, by Location Type,b 2015–2017
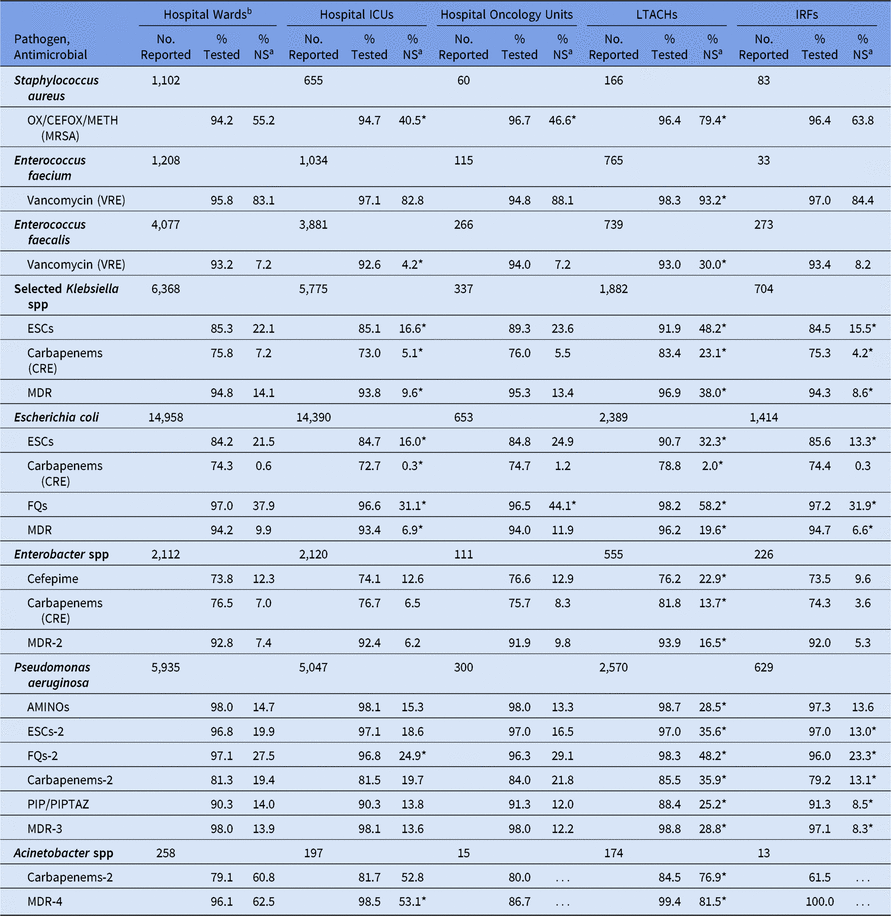
Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; IRFs, inpatient rehabilitation facilities; Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS in hospital wards; P < .05.
a MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested.
b Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas.
Table 13. Percentage of Pathogens Reported from Adult Possible Ventilator-Associated Pneumoniasa (PVAPs) that Tested Nonsusceptibleb (NS) to Selected Antimicrobial Agents, by Location Type,c 2015–2017

Note. ICUs, intensive care units; LTACHs, long-term acute-care hospitals; Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, Carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS in hospital wards; P < .05.
a PVAP is the only type of ventilator-associated event (VAE) for which a pathogen can be reported.
b MRSA and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested. In most cases, Enterococcus species are excluded organisms from VAE surveillance, and therefore VRE data are not displayed.
c Location types are mutually exclusive. “Hospital wards” includes step-down units, mixed acuity units, and specialty care areas. For this table, “hospital ICUs” includes oncology ICUs.
For SSIs following abdominal surgeries, the %NS varied by wound closure technique, with all pathogens showing a higher %NS among non-primarily closed procedures compared to primarily closed procedures (Table 14). The percentage of P. aeruginosa with nonsusceptibility to ESCs was 22% among non-primarily closed abdominal procedures, compared to 12% among those primarily closed. In general, when compared to the abdominal surgical category, SSIs following ob/gyn surgeries had a significantly lower %NS for all phenotypes, particularly E. coli nonsusceptibility to fluoroquinolones (19% vs 35%), MRSA (39% vs 56%), and VRE E. faecium (36% vs 54%) (Table 15). SSIs following orthopedic surgeries also had a lower %NS than SSIs from abdominal surgeries for most phenotypes. However, the percentage of E. faecium resistant to vancomycin was significantly higher in SSIs following orthopedic surgeries (78%) and cardiac surgeries (79%) compared to abdominal surgeries (54%).
Table 14. Percentage of Pathogens Reported from Adult Abdominala Surgical Site Infections (SSIs) that Tested Nonsusceptibleb (NS) to Selected Antimicrobial Agents, by Procedure Closure Technique, 2015–2017
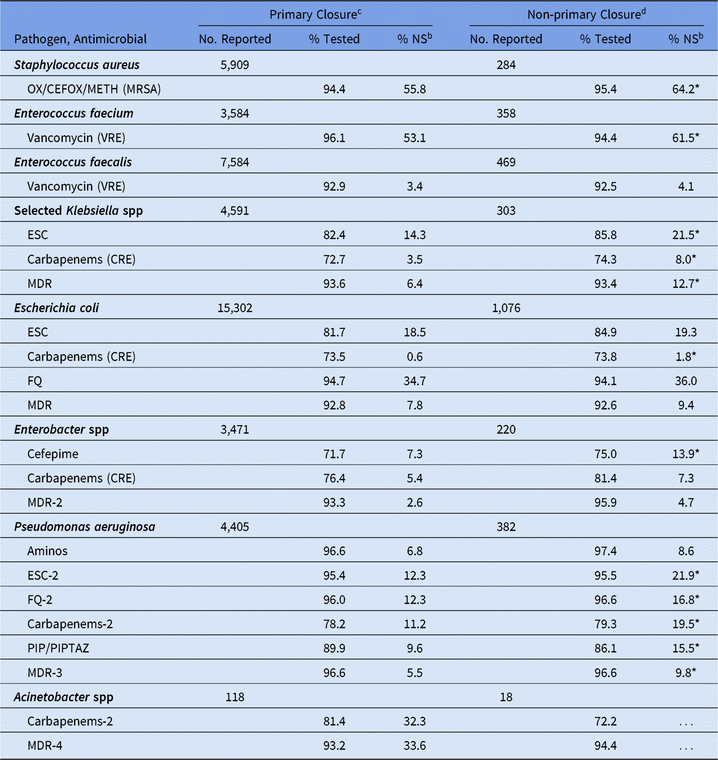
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS among SSIs following primarily closed procedures; P < .05.
a Consists of appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
b MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested.
c Primary closure technique is defined as the closure of a surgical wound in which the skin level has been closed, by any means, during the original surgery. In this table, 79% of primary closure abdominal SSI pathogens are from colon procedures.
d Non-primary closure technique is defined as the closure of a surgical wound in which the skin level has been left completely open. In this table, 87% of non-primary closure abdominal SSI pathogens are from colon procedures.
Table 15. Percentage of Pathogens Reported from Adult Surgical Site Infections (SSIs) that Tested NonSusceptiblea (NS) to Selected Antimicrobial Agents, by Surgical Category,b 2015–2017
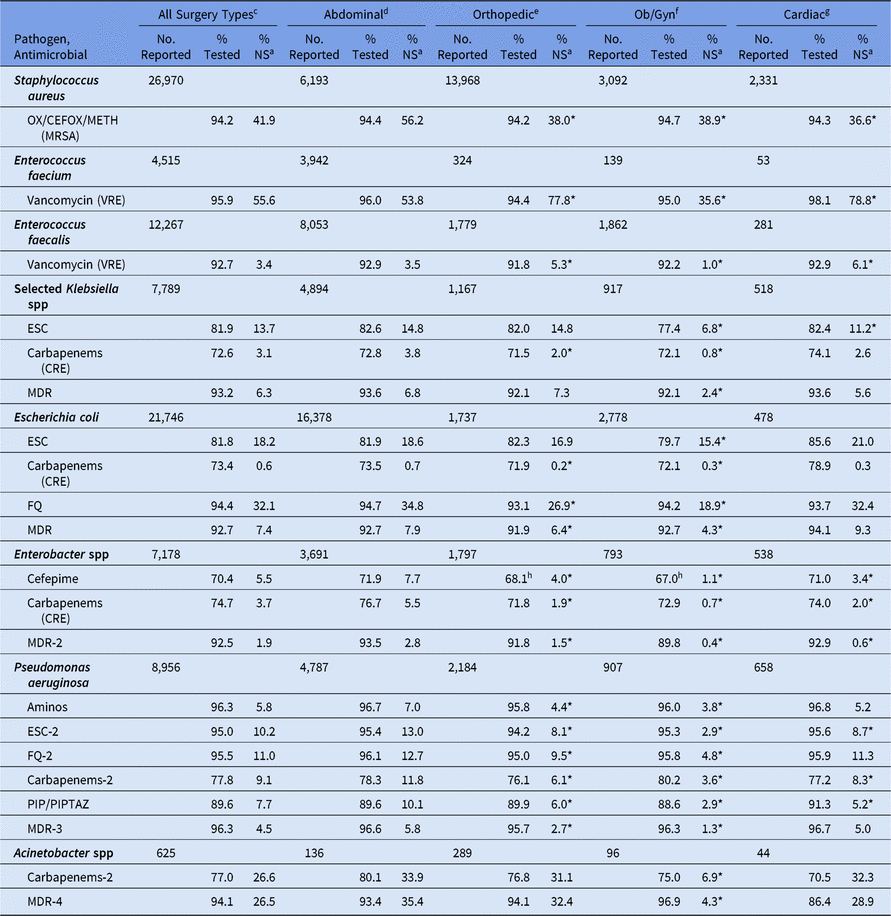
Note. Ob/Gyn, obstetrical and gynecological; Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
h If the percentage tested is <70%, caution should be used when interpreting the %NS.
* Statistically significantly different than %NS among abdominal SSIs; P < .05.
a MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant).
b The 4 most frequently reported surgical categories by pathogen volume are shown.
c Consists of all NHSN operative procedure codes, and is not limited to the 4 surgical categories shown in this table.
d Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
e Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
f Cesarean section, abdominal hysterectomy, ovarian surgery, and vaginal hysterectomy.
g Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
Less than 75% of Klebsiella spp, E. coli, and Enterobacter spp combined from DA HAIs were reported with AST results for carbapenems, and <85% were reported with results for ESCs (Table 16). Inferring susceptibilities for those pathogens reported as “not tested” increased the overall percentage tested to 90% for carbapenems and ESCs, which resulted in minimal decreases in the corresponding %NS.
Table 16. Estimated Effect of Incomplete Reporting of Carbapenem and Extended-Spectrum Cephalosporin Susceptibility Results on the Nationala Percentage Nonsusceptibleb (%NS), by Pathogen and Phenotype, 2015–2017

Note. CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); ESC-NS, extended-spectrum cephalosporin nonsusceptible (cefepime, cefotaxime, ceftazidime, or ceftriaxone); Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a This table consists of adult device-associated infections from acute-care hospitals: central line-associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), and possible ventilator-associated pneumonias (PVAPs). Data from inpatient rehabilitation facilities (IRFs) and long-term acute-care hospitals (LTACHs) were excluded.
b CRE data are presented as %R (ie, includes only those pathogens that tested resistant). ESC-NS data are presented as %NS (ie, includes pathogens that tested intermediate or resistant).
c If a pathogen was reported as “not tested” to carbapenems, susceptibility was inferred as susceptible (S) if the pathogen was susceptible to at least 2 of the following antimicrobials: ampicillin, ampicillin/sulbactam, amoxicillin/clavulanic acid, piperacillin/tazobactam, cefazolin, cefoxitin, or cefotetan. Inferring susceptibility effectively raises the number of tested isolates (ie, the denominator of %NS).
d If a pathogen was reported as “not tested” to ESCs, susceptibility was inferred as susceptible (S) if the pathogen tested susceptible to at least 2 of the following antimicrobials: ampicillin, aztreonam, or cefazolin. Inferring susceptibility effectively raises the number of tested isolates (ie, the denominator of %NS). Due to Enterobacter spp’s inducible resistance to some ESCs, the inferred susceptibility method for ESC-NS was not applied to Enterobacter spp.
Discussion
Due to HAI reporting requirements driven by state mandates and the CMS QRPs, the HAI data reported to the NHSN and included in this report provide a comprehensive national picture of the most common pathogens implicated in HAIs, as well as the percentage of pathogens displaying nonsusceptibility to selected antimicrobials. This report expands upon the NHSN’s prior reports by providing national %NS values stratified by facility type, location type, and surgical category, and is the first report to publish frequent pathogens and/or resistance profiles in MBI-LCBIs, PVAPs, and SSIs following procedures with a non-primary closure technique. This report provides an unprecedented level of detail about the national epidemiology of pathogens and important resistance phenotypes across the healthcare spectrum.
More than 5,000 healthcare facilities conducted surveillance of adult HAIs between 2015 and 2017; almost 80% had <200 beds. In 2015, the CMS QRP requirements for CLABSI and CAUTI surveillance in acute-care hospitals expanded to include infections from medical, surgical, and medical/surgical wards, in addition to the previously required ICU locations. This resulted in a substantial increase in the proportion of CLABSI and CAUTI pathogens reported from ward locations compared to the NHSN’s previous report.Reference Weiner, Webb and Limbago3 In addition, the 2016 expansion of CMS QRP requirements to include LTACH reporting of VAEs allowed us to assess differences in PVAP pathogens and resistance profiles across facility types.
In general, the pathogens associated with DA HAIs varied by location. Similar to previous studies, oncology units in particular had a different pathogen distribution than hospital ICUs and wards.Reference See, Freifeld and Magill19 Escherichia coli and viridans group streptococci were common CLABSI pathogens in oncology units. These pathogens are included in the MBI-LCBI definition, which is used to identify a subset of BSIs that are often the result of mucosal barrier injury from cytotoxic chemotherapy, rather than the result of a contaminated central line.
A lower %NS was seen for several CLABSI pathogens in oncology units compared to hospital ICUs and wards. Although the reason for this difference is not known, it may be related to differences in underlying illnesses and antimicrobial exposures between patients in oncology and non-oncology units. The high prevalence of E. coli nonsusceptibility to fluoroquinolones in oncology units, and particularly in MBI-LCBIs within oncology units, is likely the result of widespread use of fluoroquinolone prophylaxis in this population.
Enterococcus spp have historically been reported as the most common resistant organism isolated from LTACH patients.Reference McKinnell, Singh and Miller20 , Reference Munoz-Price and Stemer21 The results of our analysis were consistent with those of earlier publications: Enterococcus spp represented the most common LTACH CLABSI pathogen. LTACHs consistently had a higher %NS for most phenotypes compared with other settings. This heightened antimicrobial resistance in LTACHs is well established and has been attributed to the longer patient length of stay, the acuity of patients, patient exposure to hospital ICUs and antimicrobials prior to their LTACH admission, and challenges in the implementation of infection control practices in LTACHs.Reference Woodworth, Walters and Weiner22 , Reference Weiner, Fridkin and Aponte-Torres23 Transmission of resistant organisms in these settings can contribute to an increase in the overall regional prevalence of these organisms due to frequent patient transfers. A coordinated approach across healthcare facilities connected by patient sharing has the greatest potential to reduce the spread of resistant infections in the region.Reference Slayton, Toth and Lee24
SSI pathogens varied by surgical category and procedure closure technique. The high frequency of S. aureus and coagulase-negative staphylococci reported from orthopedic and cardiac SSIs could be partially related to the use of implants, such as orthopedic implants, during these procedures.Reference Campoccia, Montanaro and Arciola25 The differences between primarily closed and non-primarily closed abdominal procedures may reflect differences in the underlying pathophysiology that might lead a surgeon to choose one closure technique over another. In addition, patients with a non-primarily closed incision might be more likely to receive antimicrobial treatment for existing infections and/or prolonged prophylaxis prior to the procedure, both of which could contribute to an increase in yeast growth and a higher likelihood for the development of antimicrobial resistance. Pathogen susceptibility varied by surgical category, with ob/gyn procedures having a lower %NS for all phenotypes compared to general abdominal procedures. It is possible that more ob/gyn procedures are elective compared to other abdominal procedures, and patients undergoing ob/gyn procedures may be generally younger and healthier than those receiving nonelective abdominal surgeries.
Consistent with earlier reports, this analysis found a higher %NS in DA HAIs compared to SSIs. Patients with a CLABSI, CAUTI, or PVAP have often been admitted to an inpatient unit for several days prior to the infection, and therefore pathogens contributing to these infections are more likely to have been acquired during a patient’s hospitalization, when patients are exposed to antimicrobials and where there is significant transmission pressure for resistant organisms. Alternatively, SSIs are often caused by bacterial contamination from the patient’s endogenous skin flora,Reference Owens and Stoessel26 and thus may be less likely to show nonsusceptibility to antimicrobials.
Statistically significant differences existed in the %NS between hospital wards and ICUs, with ICUs tending to have a higher %NS for CLABSI pathogens and a lower %NS for CAUTI pathogens. Previous studies reported higher resistance percentages in ICUs, noting several contributing factors such as the severity of patient illness and the common use of prophylactic antimicrobials in ICUs.Reference Archibald, Phillips, Monnet, McGowan, Tenover and Gaynes27 – Reference Streit, Jones, Sader and Fritsche29 However, among CAUTIs, the %NS values were higher in wards than ICUs. This could reflect differences in the frequency and duration of urinary catheter use in these settings. In the past few years, reductions in device utilization have been greater on wards than ICUs30; the remaining ward patients with catheters might represent patients who are more likely to have long-term catheters that are challenging to discontinue, and thus could be more likely to have infections with resistant organisms.
We continue to notice low reporting frequencies of AST results for Klebsiella spp, E. coli, and Enterobacter spp with carbapenems and/or ESCs. To account for this, we compared the %NS for these phenotypes to an alternate %NS in which susceptibility results were imputed for those pathogens without AST results. The slight decrease in %NS that resulted from this method is evidence that the NHSN’s typical %NS calculations may be a small overestimation of the true nonsusceptibility in the population, which has been suggested in prior NHSN reports. Given that any overestimation appears to be minimal (0.5% decrease for CRE and 1.2% decrease for ESC nonsusceptibility), the NHSN will continue to publish %NS under the traditional definitions that are based strictly on the data reported. Comprehensive reporting of AST results is important to public health surveillance efforts and can allow for the consistent identification and tracking of emerging phenotypes.
Increases over time in the proportion of Candida spp tested for fluconazole susceptibility are encouraging, particularly for C. parapsilosis. The 2016 national guidelines for management of Candida infections recommend that all bloodstream and clinically relevant Candida isolates are tested for azole susceptibility.Reference Pappas, Kauffman and Andes31 As more hospitals and laboratories adopt these recommendations, and data entry for antifungal susceptibility increases in the NHSN, we will be able to track a national resistance metric for Candida spp.
Our results have some limitations. The HAI, pathogen, and AST results are self-reported to the NHSN and are not validated by the CDC. Only the final AST interpretations are reported to the NHSN; therefore, differences may have existed among laboratory testing practices, reporting methods, and breakpoint interpretations that could not be accounted for in this analysis. This report does not account for any community-acquired infections or antimicrobial resistance that occurs outside of an inpatient healthcare facility. In addition, DA HAIs were classified as “adult” based on the location in which the patient resided; data from pediatric patients housed in adult location types at the time of their infection are thus included in this analysis.
This report provides updated information on pathogens and resistance phenotypes most commonly reported from HAIs in US healthcare facilities, and it facilitates a greater understanding of the national burden of HAIs and antimicrobial-resistant pathogens. These data have the potential to inform infection prevention strategies and, when tracked over time, to provide feedback on the impact of these strategies. Healthcare facilities are encouraged to regularly review their facility-specific pathogen and antimicrobial resistance data, particularly for high-risk units, to implement targeted prevention efforts. Aggressive support from public health is necessary to reduce the spread of resistant organisms in healthcare settings, particularly those with high acuity patients and longer lengths of stay, such as hospital ICUs and LTACHs.
Acknowledgments
We thank NHSN-participating healthcare facilities and the infection control community for their diligent efforts to monitor infections, reduce antimicrobial resistance, and improve patient safety. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Diseases Registry.
Financial support
The NHSN surveillance system is supported by the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
APPENDICES
A1. Candida Species Reported From Selected Adult Healthcare-Associated Infections1 (HAIs) in Acute Care Hospitals and Tested for Susceptibility to Fluconazole, 2011-2017
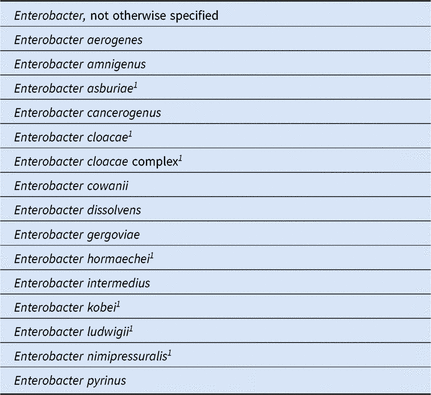
A2. Organisms Included in NHSN’s ‘Enterobacter spp’ Group for this Report
A3. Organisms Included in NHSN’s ‘Coagulase-Negative Staphylococci’ Group for this Report

A4. Organisms Included in NHSN’s ‘Viridans Group Streptococci’ Group for this Report

























