Introduction
The north-eastern region of India, comprising seven states, supports the highest diversity (11 species) of primates in the country (Kumar et al., Reference Kumar, Mishra and Sinha2005; Sinha et al., Reference Sinha, Datta, Madhusudan and Mishra2005; Srivastava, Reference Srivastava2006). Amongst these states, Arunachal Pradesh (Fig. 1) is arguably the country's richest region in terms of its terrestrial biodiversity. A wide altitudinal range (1,000 to > 6,000 m), an associated diversity of habitats (tropical rainforests, subtropical and temperate forests, alpine meadows) and a unique location at the junction of the Eastern Himalaya and Indo-Burma biogeographical zones have contributed to the rich diversity of mammalian fauna in this state (Mishra et al., Reference Mishra, Madhusudan and Datta2006). Large forest tracts still remain, partly due to the low human density across the state (13 km-2; Government of India, 2003).

Fig. 1 Sighting locations of the Arunachal macaque Macaca munzala in western Arunachal Pradesh, north-east India. The cluster of sightings in north-west Tawang district represents those from the Zemithang area where hunting is prohibited by the village councils. Solid circle on inset indicates location of the main figure in India.
Recent surveys in the mid to high elevations of western Arunachal Pradesh led to the discovery of the Arunachal macaque Macaca munzala (Plate 1; Sinha et al., Reference Sinha, Datta, Madhusudan and Mishra2005). The primate, belonging to the sinica species-group of the genus Macaca, was described as a new species based on its morphology. Subsequent analyses of mitochondrial DNA reconfirmed its distinct identity (Chakraborty et al., Reference Chakraborty, Ramakrishnan, Panor, Mishra and Sinha2007). The species occurs largely at altitudes of 2,000–3,000 m in Arunachal Pradesh's westernmost districts of Tawang (2,172 km2) and West Kameng (7,422 km2; Fig. 1). Given the contiguity of habitat, it should also occur in the bordering areas of central Arunachal Pradesh, as well as in Bhutan and Tibet, although these areas have not yet been surveyed for the species.

Plate 1 The Arunachal macaque Macaca munzala.
Within Arunachal Pradesh we believe that the Tawang district, given its particular ethnic composition and practices, should support the highest density of the macaque and provide the best conservation prospects for the species. In most other areas of the state, hunting, an important part of the traditions of most of Arunachal Pradesh's 26 tribes, seriously threatens wildlife populations (Datta, Reference Datta, Shahabuddin and Rangarajan2007). Primates are commonly hunted for meat and medicine (Borang & Thapliyal, Reference Borang and Thapliyal1993; Singh, Reference Singh2001). However, people of the Buddhist Monpa agro-pastoral tribe inhabiting the Tawang district, and constituting c. 5% of the population of the state, generally do not hunt primates (Mishra et al., Reference Mishra, Madhusudan and Datta2006). Some of the villages in Tawang have voluntarily prohibited the hunting of wildlife in their village forests (Mishra et al., Reference Mishra, Madhusudan and Datta2006). Many of our sightings of the Arunachal macaque have been in the proximity of villages and, although wary of people, they were relatively tolerant of human presence (Sinha et al., Reference Sinha, Datta, Madhusudan and Mishra2005). This is in contrast to most other areas of Arunachal Pradesh, including adjoining West Kameng, where primates are less frequently sighted and are extremely shy of human presence.
We have, nevertheless, recorded a certain level of persecution of the Arunachal macaque even within Tawang district, largely in retaliation for crop damage (Mishra et al., Reference Mishra, Madhusudan and Datta2006). The flesh of the macaque is considered to be of medicinal value locally, particularly for livestock (C. Mishra & A. Sinha, pers. obs.). We have also observed the practice of keeping juvenile macaques as pets amongst the Monpa (Sinha et al., Reference Sinha, Datta, Madhusudan and Mishra2005).
Here we report the first ever assessment of the conservation status of the Arunachal macaque in western Arunachal Pradesh. Our first objective was to map its current distribution and estimate its abundance in the Tawang and West Kameng districts. Our second objective, following on from earlier observations (Mishra et al., Reference Mishra, Madhusudan and Datta2006), was to develop an understanding of the nature and extent of conflict between the Arunachal macaque and people.
Study area
Tawang and West Kameng districts together encompass a wide altitudinal gradient (150–7,000 m), south of the Great Himalayan Range. The region is drained by the Tawang Chu, Nyamjang Chu and their tributaries in Tawang, and the Bhareli and its tributaries in West Kameng. The main vegetation types, where primates occur, include broad-leaved forests (up to 3,000 m, with Rhododendron, Acer, Alnus and Quercus as the dominant species), mixed conifer broad-leaved forests (3,000–4,200 m, with Abies densa, Juniperus, Larix, Picea, Rhododendron and Quercus), and forest clearings (pastureland created by clearing and burning broad-leaved forests and mixed conifer broad-leaved forests, with shrubs such as Rosa and Berberis, and forbs such as Anaphalis, Potentilla, Sambucus, Rumex and Senecio). At low to mid elevations the broad-leaved forests are degraded in the vicinity of human habitations and form secondary scrub, with reduced tree cover, and dominated by species such as Erythrina, Rhus, Elaeagnus and Debregeasia.
The other primates in the region include the capped langur Trachypithecus pileatus, Assamese macaque M. assamensis, rhesus macaque M. mulatta, and the slow loris Nycticebus bengalensis. Amongst macaques, the Arunachal macaque was the predominant species in the altitudinal range that we surveyed. We did not sight any other macaque species in Tawang, although some troops of the Arunachal macaque at lower altitudes appeared to have morphologically variant individuals. The region's wildlife includes mountain ungulates such as the serow Nemorhaedus sumatraensis, three species of goral Nemorhaedus spp., takin Budorcas taxicolor, and carnivores such as the dhole Cuon alpinus, Himalayan black bear Selenarctos thibetanus, and the snow leopard Uncia uncia at higher altitudes.
The people of the region are largely agro-pastoralists, with a few villages at high altitudes being predominantly pastoral. Millet and maize are the major crops, followed by wheat, buckwheat, potatoes and chillies. Vegetable crops and fruits such as apple, peach and plum are also grown in kitchen gardens and near settlements. People in Tawang and West Kameng districts mainly practice settled agriculture, often up to 3,000 m, unlike in most other districts of the state where shifting cultivation is predominant.
Whereas the Monpa tribe is the predominant community inhabiting Tawang district (with a density of 16 people km-2; Government of India, 2003), the adjoining areas of West Kameng (10 people km-2; Government of India, 2003) also has Sherdukpen, Khowa, Aka and Miji communities, in addition to the Monpa.
Methods
Distribution and abundance
Between April 2004 and August 2005 we conducted vehicular surveys, covering a distance of c. 1,000 km thrice, stopping to scan regularly for macaques at selected vantage points. Surveys were also made on foot, covering c. 400 km along existing trails and paths. At every sighting we recorded information on troop size and composition, altitude, location (with a global positioning system, GPS) and habitat features. These data gave an estimate of the minimum population of the Arunachal macaque and its distribution in western Arunachal Pradesh. In cases where macaques were repeatedly sighted in the same area, and where there was a possibility of multiple counts, we used data only from the sighting in which the maximum number of individuals was recorded. In most instances (86%), we could not enumerate all the individuals in a troop because of poor visibility. The data, therefore, represent partial counts and provide a conservative estimate of the population.
The Zemithang region of Tawang district, from where the holotype and paratypes of the Arunachal macaque were first reported (Sinha et al., Reference Sinha, Datta, Madhusudan and Mishra2005), has a relatively high macaque density and the least hunting pressure of the sites surveyed because most of the village councils in this region have prohibited hunting (Mishra et al., Reference Mishra, Madhusudan and Datta2006). We therefore extensively surveyed a 10.6-km2 area in this region to estimate the density of the species.
Human-macaque relationships
To study the conflict between people and the Arunachal macaque we initially conducted rapid assessments in 54 and 10 villages in Tawang and West Kameng districts, respectively. In each village at least 1–6 individuals or groups of individuals were interviewed. Without invoking the macaques, we asked the interviewees to list the factors they believed were responsible for crop losses. The potential factors considered were frost, disease, insect pests, rodents and wildlife. The interviewees’ perceptions of the importance of each factor were graded as very serious, serious, moderate, and of low or no concern. The interviewees were then asked about the wildlife species causing crop losses and their responses graded in a similar fashion. For simplicity, during analysis, the two highest categories of importance (very serious and serious) were combined into a single category (high). The wildlife categories in this survey included wild pig Sus scrofa, barking deer Muntiacus muntjak, birds, Arunachal macaques, porcupines Hystrix sp., and rodents. Altitude, location with a GPS, and crops grown in each of these villages were also recorded.
From the 64 villages originally surveyed we conducted door-to-door interviews in six selected villages (Rho, Shyro, Jangda and Thingbu in Tawang; Morshing and Berichi in West Kameng) to collect further information on human-macaque conflict. The selection of villages accommodated (1) adequate coverage of altitudinal zones, (2) inclusion of high-conflict villages based on the initial rapid surveys, and (3) logistic feasibility. Five of the surveyed villages are large, whereas Berichi is small, with only 13 families. In each village we interviewed at least one member in each family. We asked each interviewee about the extent of crop losses due to macaques, graded their response, and estimated the extent of conflict by examining the proportion of respondents in each category (low, medium, high). The interviewees were also asked about which crops were damaged, how often macaques raided the crop fields, and the extent of crop loss in monetary terms. Each of the interviewees was asked whether and how many macaques they had killed, what techniques they had employed, and what they had done with the carcasses.
Results
Distribution and abundance
Of the total of 35 Arunachal macaque troops sighted, with at least 569 individuals (Table 1), 32 troops (540 individuals) were seen in Tawang, and 3 troops (29 individuals) in West Kameng district (Fig. 1). Information from local people indicated the possible occurrence of at least 25 other troops in the region. Most of the macaques were sighted at altitudes of 2,000–2,250 m, although we recorded them up to 3,000 m in fir Abies densa forests (Fig. 2). Local people reported seasonal occurrence of macaques up to 3,500 m, and we accordingly estimated the total potential macaque habitat (all areas below 3,500 m) within Tawang district to be c. 800 km2 (one third of the district's total area). In the Zemithang area of Tawang district we recorded 10 troops (234 individuals), and estimated a density of 0.94 troops and 22.01 individuals km-2.
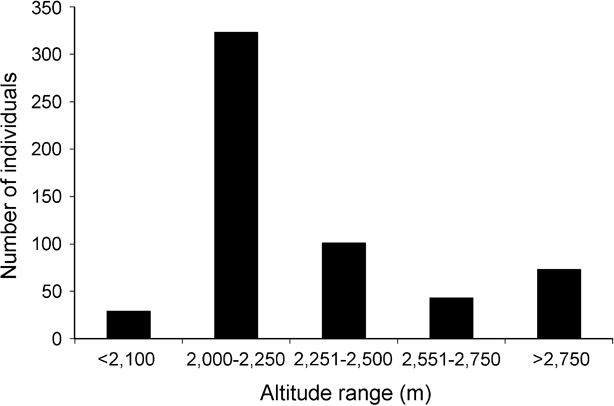
Fig. 2 Altitudinal distribution of the sightings of the Arunachal macaque in Tawang and West Kameng districts of western Arunachal Pradesh (Fig. 1).
Table 1 Distribution of Arunachal macaque troops and individuals by vegetation type.

Over 75% of all individual Arunachal macaques sighted were in human-modified landscapes and forests (Table 1); more than half of these individuals were in degraded broad-leaved forests and open scrub in the vicinity of human habitations. These degraded forests had moderate to high levels of anthropogenic disturbance in the form of felling, livestock grazing, lopping and leaf litter collection.
Troop size ranged from solitary to > 60 individuals. Troops were generally multimale-multifemale, with an average size of 16.3 ± SD 13.4 individuals. In the troops where a majority of individuals could be classified (n = 22), the overall adult sex ratio was 52 males per 100 females, with 17 infants per 100 adult females, and 110 juveniles per 100 females. Our preliminary demographic data on six troops observed in some detail between August 2003 and May 2006 indicate that the adult females in these troops may have an inter-birth interval of 2 years but longer term observations are required to confirm this.
Human-macaque relationships
In 69% of the 64 villages where rapid assessments were conducted people reported wildlife to be the most common cause of high crop losses (Fig. 3a). Crop diseases, insect pests, and rodents were reported to cause high crop losses in only two, one, and two of the surveyed villages, respectively. Amongst wildlife, Arunachal macaques, wild pigs and porcupines caused 55, 50 and 23% of crop losses in the surveyed villages, respectively (Fig. 3b). Other wildlife species reportedly causing crop losses were the Himalayan black bear, goral, and birds such as the kaleej pheasant Lophura leucomelana and blackbirds Turdus spp..

Fig. 3 Main causes of crop losses identified by people during perception surveys of human-primate conflict in 64 villages in western Arunachal Pradesh.
The extent of crop damage by macaques was reportedly highest at altitudes of 2,000–2,500 m, possibly because of the relatively greater abundance of villages and agricultural fields in this zone (Fig. 4). Patterns in the intensity of crop damage, however, was similar across altitudinal zones (Fig. 4), with the exception of the high altitude zone where agriculture is limited.

Fig. 4 Altitudinal distribution of the intensity of crop damage by the Arunachal macaque. The number of villages surveyed in each altitudinal zone is indicated next to the bars.
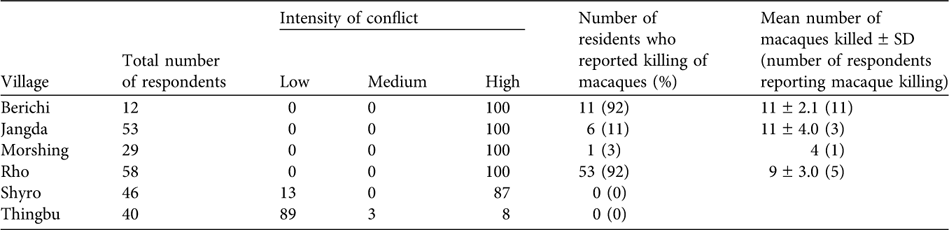
In five of the six surveyed villages, 87–100% of the respondents reported a high intensity of conflict with macaques (Table 2). The only exception was the high altitude village of Thingbu, a largely pastoral settlement that has little cultivation and where, because of religious beliefs, the village council imposes a fine of INR 1,000 on anyone hunting macaques. In the detailed door-to-door surveys in the six villages only 3% of a total of 238 respondents could not offer an opinion.
Table 2 Intensity of human-macaque conflict in six villages of western Arunachal Pradesh (see Fig. 1 for locations). The intensity is depicted as the percentage of the total of 238 respondents reporting each category of conflict (Low, Medium, High, see text for details). The persecution of macaques in retaliation for crop damage is shown as the percentage of respondents from each village that confirmed the killing of macaques and the mean number of instances of macaque killing reported over 2000–2005.
Half of the respondents reported damage to all crops by the Arunachal macaque, while the other half reported damage mainly to maize and millet. Buckwheat, which is limited in cultivation, was reportedly the least affected crop. Crop damage was reported to occur throughout the year, with maximum damage in July–September. We could not reliably estimate the extent of crop losses in terms of yield or money but eight people estimated the average loss to be INR 3,250–4,600 (c. USD 70–100) per family per year.
Amongst the five villages with high levels of conflict, 92% of people acknowledged the occurrence of retaliatory killing of macaques in two villages, people completely denied macaque persecution in one, and 3 and 11% of respondents acknowledged its occurrence in the other two villages, respectively. Only three of the respondents admitted to killing macaques. In the four villages where persecution was confirmed, people reported an average of 35 macaques killed over the last 5 years (Table 2). Snaring was the most commonly reported method employed, although guns and bows and arrows were also used. In most cases, the carcass was thrown away. We twice came across carcasses displayed prominently close to habitation, presumably to deter further crop raiding. We also recorded one instance in which the carcass of an adult male that had been killed after it had entered a house was to be cooked for treating sick livestock.
We recorded eight captive individuals of the Arunachal macaque (a juvenile male and seven females: an adult, five juveniles and an infant) kept as pets.
Discussion
Tawang has the highest human density of all Arunachal Pradesh districts, and has widespread cultivation with considerable forest degradation and loss over its entire area (Mishra et al., Reference Mishra, Madhusudan and Datta2006). Yet, it supports a significant population of the Arunachal macaque. The fact that > 75% of our sightings of the Arunachal macaque were in human-modified landscapes suggests that the species is tolerant of some level of anthropogenic habitat modification. This is consistent with the ecological adaptability and behavioural flexibility that characterize a majority of the 21 species of Macaca. The genus occupies a very wide range of habitats and, amongst primates, is second only to humans in terms of its global geographical range (Fa & Lindburg, Reference Fa and Lindburg1996). Species such as the rhesus and bonnet macaque M. radiata represent extreme examples of macaques that can continue to persist even inside towns and cities (Malik & Johnson, Reference Malik and Johnson1994; Sinha, Reference Sinha2001; Imam et al., Reference Imam, Yahya and Malik2002). The ability of these species to use extra food from crop fields or anthropogenic waste from human settlements can offset the effects of habitat degradation and result in the stability or even growth of such populations (Zhao, Reference Zhao1999). The population density attained even by the Arunachal macaque, as exemplified in the Zemithang area, can be remarkably high, comparable with the highest densities reported for macaques in general (e.g. Macaca mulatta: 9–17 individuals km-2, Southwick & Siddiqi, Reference Southwick and Siddiqi2001; M. fascicularis: 15–39 km-2, M. nemestrina: 5 km-2, Chapman, Reference Chapman1995; M. thibetana: 24–56 km-2, Zhao, Reference Zhao1999; M. sylvanus: 20–29 km-2, Camperio Ciani et al., Reference Camperio Ciani, Mouna and Arhou2000; M. fuscata: 13–27 km-2, Hanya et al., Reference Hanya, Zamma, Hayaishi, Yoshihiro, Tsuriya and Sugaya2005).
Although we confirmed the presence of the Arunachal macaque up to 3,000 m, and its seasonal occurrence was reported up to 3,500 m, it appears to be a mid to high elevation species. We recorded a maximum abundance at 2,000–2,500 m (Fig. 2) but this was largely due to the high density (c. 41% of sighted individuals) in an area of only 10.6 km2 in Zemithang, where it is not hunted. We therefore believe that, if hunting could be controlled, the species would attain much greater abundance at other altitudes.
Crop damage by macaque species is not commonly reported as a problem from other parts of Arunachal Pradesh, possibly because macaques are eaten in most of these areas and the surviving troops are extremely shy and retiring. Traditionally, shifting cultivators have combined cultivation with hunting of crop-raiding animals for food (Naughton-Treves et al., Reference Naughton-Treves, Treves, Chapman and Wrangham1998). As hunting for food and sport is not prevalent in Tawang, conflicts over crop damage by wildlife are common. Our data indicate that the intensity of crop damage by the Arunachal macaque across altitudinal zones does not appear to be correlated with its abundance. Crop-raiding animals generally do not give up feeding on wild food but expand their diet to include crops (Naughton-Treves et al., Reference Naughton-Treves, Treves, Chapman and Wrangham1998). Given that crops generally have greater nutritive and lower toxin levels compared to their wild relatives, agricultural fields presumably represent attractive patches of seasonally abundant, high quality food. Therefore, even under conditions of low density and high per capita availability of natural food, the macaques can be expected to raid crops and come into conflict with people as long as crops are available close to their natural habitats.
Despite low levels of hunting in Tawang, our surveys confirmed the occurrence of retaliatory persecution of macaques. The practice of keeping infants and juveniles as pets also acts as an incentive to hunting (Chapman & Peres, Reference Chapman and Peres2001). Furthermore, the local people reported that members of other tribes, living or posted to Tawang, hunt primates for food and sport. Thus, although seemingly adaptable to habitat change, the Arunachal macaque is threatened by hunting, a situation that needs to be addressed urgently. The low infant to adult female ratio recorded suggests that not all adult females breed at any given time, and that females do not give birth every year, making the species yet more vulnerable to hunting.
Conservation of the Arunachal macaque will need to focus on a landscape that has already undergone considerable anthropogenic impacts. A better understanding of the patterns and intensity of crop-raiding will be required for the design of appropriate conflict mitigation strategies (such as the adoption of alternate buffer crops, use of deterrents, better crop protection measures, habitat management in the vicinity of villages, and the introduction of crop compensation or insurance programmes).
The Arunachal macaque is the first new macaque species to be described in over a century, the last macaque to be described being the Pagai macaque M. pagensis in 1903. Appropriate conservation education efforts are required to communicate the global significance of the Arunachal macaque to the communities in the state. The species has the potential to become a tourism resource as well as a conservation flagship, as has been noted for other primates (Hill, Reference Hill2002). Given the Buddhist beliefs of the Monpa community and that they do not have a strong hunting culture, we believe that a multifaceted conservation programme will be able to garner local support for the conservation of the Arunachal macaque.
Acknowledgements
We are grateful to the Arunachal Pradesh Forest Department for their continued support and encouragement of our research and conservation efforts, K.D. Singh, N.N. Zhasa and the Divisional Forest Officers of Tawang and West Kameng districts, Pekyom Ringu for his excellent support, Dorje Raptan and Nima Shiring for assistance with fieldwork, Rohan Arthur and M.D. Madhusudan for planning help and discussions, and the Rufford Maurice Laing Foundation, Whitley Fund for Nature and the Wildlife Conservation Society-India Program for funding.
Biographical sketches
R. Suresh Kumar is interested in the natural history and conservation of the little-known Himalayan birds and mammals. He has studied pheasants for many years and was responsible for the discovery of a new sub-species of Sclater's monal. Nabam Gama is a veterinary doctor from Arunachal Pradesh, with an interest in studying and protecting the wildlife of this region. He has been involved in assessments of human-wildlife conflicts in western Arunachal Pradesh. R. Raghunath is interested in the application of mapping and GIS to understand and address issues in wildlife conservation. He provides mapping support to a variety of research and conservation projects. Anindya Sinha studies behavioural ecology and cognitive psychology of primates, population and molecular genetics, evolutionary biology, conservation science and the philosophy of biology. He is also interested in biology education and the popularization of science in India. Charudutt Mishra studies grazing systems, pastoralism, large herbivore community ecology, carnivore ecology, and human-wildlife conflicts, focusing in particular on high-altitude ecology and conservation.











