Introduction
Foxtail millet (Setaria italica (L.) Beauv.) (2n = 18) is an important minor millet in the world, grown under different environmental conditions as a drought-tolerant crop (Reddy et al., Reference Reddy, Srinivasulu, Reddy, Sekhar and Shanthi2020). It is an annual and self-pollinating crop of the Setaria genus (Rodiansah and Puspita, Reference Rodiansah and Puspita2020), and suggested to have been domesticated nearly 8700 years ago (Zohary et al., Reference Zohary, Hopf and Weiss2012) from wild green foxtail (Setaria viridis), that is native to temperate areas of Asia and Europe (Hu et al., Reference Hu, Mauro-Herrera and Doust2018). The crop has a short-life cultivation of around 3 months (Martin et al., Reference Martin, Messager, Bedianashvili, Rusishvili, Lebedeva, Longford, Hovsepyan, Bitadze, Chkadua and Vanishvili2021), which provides an opportunity for growing this crop in drought-prone and heat-stressed areas. Foxtail millet grain contains high protein of 12.3–12.5% (Ji et al., Reference Ji, Liu, Ge, Zhang and Wang2019), 5.5% oil, 60.9% carbohydrates (Dhumketi et al., Reference Dhumketi, Singh and Rajput2017) and an ash content of 3.0% (Ch et al., Reference Ch, Patro, Anuradha, Triveni and Jogarao2020), and has many health and medicinal benefits (Ghimire et al., Reference Ghimire, Joshi, Gurung and Sthapit2018).
Currently, different parts of the world, including Iraq, are facing water scarcity. Up to 40% of the world's population will live under severely water-stressed condition by 2035 (Guppy et al., Reference Guppy, Anderson, Mehta and Nagabhatla2017). One of the solutions for food and nutritional security in such regions is the introduction of new staple and minor crops into agricultural production. Millet is a strong candidate crop in the context of climate-resilient agriculture (Muthamilarasan et al., Reference Muthamilarasan, Singh and Prasad2019). It offers better adaptation and reasonable yield under uncertain conditions compared to major crops, having well-developed adventitious root systems (Ceasar et al., Reference Ceasar, Ramakrishnan, Vinod, Roch, Upadhyaya, Baker and Ignacimuthu2020). Development of crop varieties with lower water requirement and higher drought tolerance is an important component of water-use efficiency, to satisfy food demand of the steadily increasing world population (Fahad et al., Reference Fahad, Bajwa, Nazir, Anjum, Farooq, Zohaib, Sadia, Nasim, Adkins and Saud2017; Ierna, Reference Ierna2021). Drought stress limits the availability of water that is needed for germination and subsequently compromises the establishment of seedlings (Pavli et al., Reference Pavli, Foti, Skoufogianni, Karastergiou, Panagou and Khah2020; Kintl et al., Reference Kintl, Huňady, Vymyslický, Ondrisková, Hammerschmiedt, Brtnický and Elbl2021). The ability of plants to persist under drought conditions depends on their capacity to initiate internal signal transduction at different stress levels (Gull et al., Reference Gull, Lone and Wani2019; Yang et al., Reference Yang, Lu, Wang, Wang, Liu and Chen2021). Short-term drought stress has been reported to affect plant growth, leading to declining growth and crop yield (Niu et al., Reference Niu, Song, Xiao and Ge2018; Kapoor et al., Reference Kapoor, Bhardwaj, Landi, Sharma, Ramakrishnan and Sharma2020). Selection of drought-tolerant cultivars through in vitro screening is an important approach to improving foxtail millet. PEG-6000 as a high molecular weight that has been reported to induce uniform water stress for drought stress screening, with no physiological damage to the plant (Shivakrishna et al., Reference Shivakrishna, Reddy and Rao2018).
Studying the genetic relationships of the available genotypes is highly relevant to preserving the germplasm of foxtail millet and ensuring sustainable production (Trivedi et al., Reference Trivedi, Arya, Verma, Hemantaranjan, Verma, Sharma and Saha2018). Rapid identification of heterozygosity in segregated populations (Ahmad et al., Reference Ahmad, Redjeki, Ho, Aliyu, Mayes, Massawe, Kilian and Mayes2016) and true hybrids at an early growth stage (Ahmad, Reference Ahmad2014) can be achieved simply with simple-sequence repeats (SSRs). Determining the level of polymorphism and genetic diversity among foxtail millet genotypes is important to preserve the genetic base and conserve the species (Kim et al., Reference Kim, Sa, Park and Lee2012). Although the origin and domestication process of foxtail millet (S. italica subsp. italica (L.) P. Beauv.) have been studied by some groups, characterization is still ambiguous and detection of a broader genetic base of the crop is essential. The objectives of this study were to evaluate drought stress tolerance in foxtail millet at an early growth stage and to detect the extent of genetic diversity between the genotypes using SSR marker systems.
Materials and methods
The study was conducted in the central laboratory of the College of Agricultural Engineering Sciences, University of Sulaimani. The genetic materials comprised of 18 millet genotypes (16 foxtail millet with two green foxtail accessions) were selected based on their origin, response to a wide range of drought stress (according to previous studies) and their cultivation in different arid areas of Iraq. They were obtained from different national and international research centres, as described in online Supplementary Table S1.
In vitro experiment (osmotic stress)
Foxtail millet genotypes were analysed for drought stress tolerance at germination and early growth stage. Seeds from the 18 genotypes were surface sterilized with 70% ethanol for 5 min, and then rinsed with distilled water. They were allowed to germinate in 9 cm diameter Petri dishes, using two layers of Whatman no. 1 filter paper. A factorial experiment was conducted in a completely randomized design, for genotypes and osmotic solutions. Different osmotic solutions of 0, 10, 20 and 30% PEG-6000 (Bheemesh et al., Reference Bheemesh, Rao, Sekhar and Latha2018; Shivhare and Lata, Reference Shivhare and Lata2019) were prepared and run for all the genotypes with three replicates. Twenty uniform seeds were allowed to germinate in each Petri dish. An imbibition period of 24 h with distilled water was allowed by placing the Petri dishes in an incubator at 25°C before treating with different solutions. Every 2 d, 2.5 ml of distilled water was supplemented to prevent drying out of the Petri dishes. The Petri dishes were placed in an incubator (M 7040 R Elektro-mag) at 25 ± 2°C for 12 d. Germination per cent, shoot length (cm), root length (cm), root/shoot ratio and seed vigour index (SVI) were taken after 12 d of incubation according to the International Seed Testing Association (ISTA, 1993). Seeds with a 0.2 cm length of radicle were considered as germinated. SVI was estimated according to the formula: (root length + shoot length) × germination percentage (Uddin et al., Reference Uddin, Ullah and Nafees2021).
Data obtained from the stress experiment were subjected to an analysis of variance (ANOVA), using XLSTAT 2016 software and the comparisons of trait's means for both factors and their interactions were made using Least significant Difference test (LSD) at the 5% level of probability. Principal component analysis (PCA) and principal coordinate analysis (PCoA) were conducted for the genotypes using rank correlation matrix of two-way data from the studied criteria. Cluster analysis was also performed based on squared Euclidean distance to classify the genotypes relatedness.
DNA extraction
Fresh leaf samples of 14 d-old seedlings were used for DNA extraction. Total Genomic DNA was isolated from 3–4 g of bulked leaves for each genotype using the protocol of Dellaporta et al. (Reference Dellaporta, Wood and Hicks1983). The quality and concentration of the DNA were measured on 1% (w/v) agarose gel in 1× TBE buffer, using known concentrations of DNA lambda, and the equivalence reaction concentration of 20 ng/μl were produced for the genotypes' DNA by adding sterile distilled water.
Polymerase chain reaction (PCR) amplification
Thirty-seven SSR primer sets, previously described (Lin et al., Reference Lin, Chiang, Chang and Kuoh2011; Venkata Suresh et al., Reference Venkata Suresh, Muthamilarasan, Misra and Prasad2013; Zhang et al., Reference Zhang, Tang, Zhao, Li, Yang, Qie, Fan, Li, Zhang and Zhao2014), were chosen from all the nine chromosomes of the millet genome (online Supplementary Table S2). Annealing temperature was tested for each primer at a range of temperatures (from 50 to 65°C), using a pooled DNA template from the genotypes used in the study. Primer amplifications were performed by using a PCR machine (MultiGene OptiMax Thermal Cycler, Labnet Company) in a total reaction volume of 20 μl. PCR mix was provided from CinnaGen Company (Iran) which contained 10× assay buffer, 2.5 mM MgCl2, 400 μM dNTP (Fermantas) and 1 U of Taq DNA polymerase. Each amplification contained 10 μl of the reaction mix, 2–4 μl of genomic DNA (20–50 ng in total), 2 μl (20 ng/μl) of each forward and reverse primers and the final volume was made up to 20 μl with distilled water (ddH2O). PCR programme was set up for one initial denaturation cycle at 94°C for 4 min, followed by 40 cycles of 60 s at 94°C and then 60 s at the appropriate annealing temperature (between 50 and 61°C), followed by 2 min of extension at 72°C. The reaction was terminated with a final extension step of 72°C for 10 min. A 10 μl aliquot of the PCR product was mixed with 5 μl of electrophoresis 6× loading buffer. The samples were loaded onto the agarose gel (2% concentration in 1× TBE buffer). The agarose gel used here was with a low electroendoosmotic, for satisfactory scoring resolve. An amount of 3–5 μl of ethidium bromide (0.5 μg/ml) was added to the gel before pouring into the tray. The gels of PCR products were run on 80 V for 120 min. The fragments were separated and visualized by a UV-light using electrophoresis (ENDURO™ GDS Touch, Labnet, model no. GDST1302). PCRs were repeated for the primers with smeary or unclear bands on the agarose gel.
SSR data analysis
Fragments amplified with the SSR primers were scored manually; alleles were scored as present (1) or absent (0). Fragment bands of less than 100 bp length on the gel, out of expected size (which could be primer dimer) were excluded from the scoring. The polymorphism percentage was calculated for all polymorphic SSR markers according to the method of Blair et al. (Reference Blair, Panaud and McCouch1999):

The informativeness of the markers was quantified through the estimation of polymorphic information content (PIC) (Botstein et al., Reference Botstein, White, Skolnick and Davis1980) which also depends on the scored binary data as follows:

where pi and pj = frequency of the marker allele i and j, respectively, in the genotypes tested, and n = number of different alleles at a certain locus.
Marker index (MI) was estimated to indicate the overall utility of the maker system (Chesnokov and Artemyeva, Reference Chesnokov and Artemyeva2015), as follows:
where n p is the number of polymorphic loci, and n is the total loci number.
The amplification products at each SSR locus were converted to binary matrix data and they were utilized to estimate the genetic distance between the genotypes in the study based on Jaccard's coefficient, followed by the unweighted pair group method with the arithmetic mean algorithm (UPGMA). PCA and PCoA were analysed and clusters algorithm on the genetic distances were expressed as a dendrogram with the help of XLSTAT 2016 software.
Results
In vitro drought experiment
Different concentrations of PEG-6000 were used to evaluate the stress-tolerance response of foxtail millet genotypes. Genotype differences under water-stress conditions were determined based on seed germination and early growth parameters. The ANOVA shows highly significant differences (P ⩽ 0.01) for the traits studied in the foxtail genotypes at different osmotic potentials induced by different concentrations of PEG-6000 (online Supplementary Table S3). ISe 869 and ISe 1851 had the most germination rate compared to other genotypes for the different osmotic stress levels (Table 1). ISe 869 also surpassed the others in root length, shoot length and SIV. Both green foxtail genotypes (PI223677 and PI230134) had a reasonable performance for the traits studied. The lowest ratios were recorded by SiA 2644 for all the traits studied except R/S ratio. Maximum reduction was recorded at the highest level of water stress for all the traits.
Table 1. Effect of the genotypes and different drought stress conditions on germination and seedling characters for 18 foxtail millet genotypes
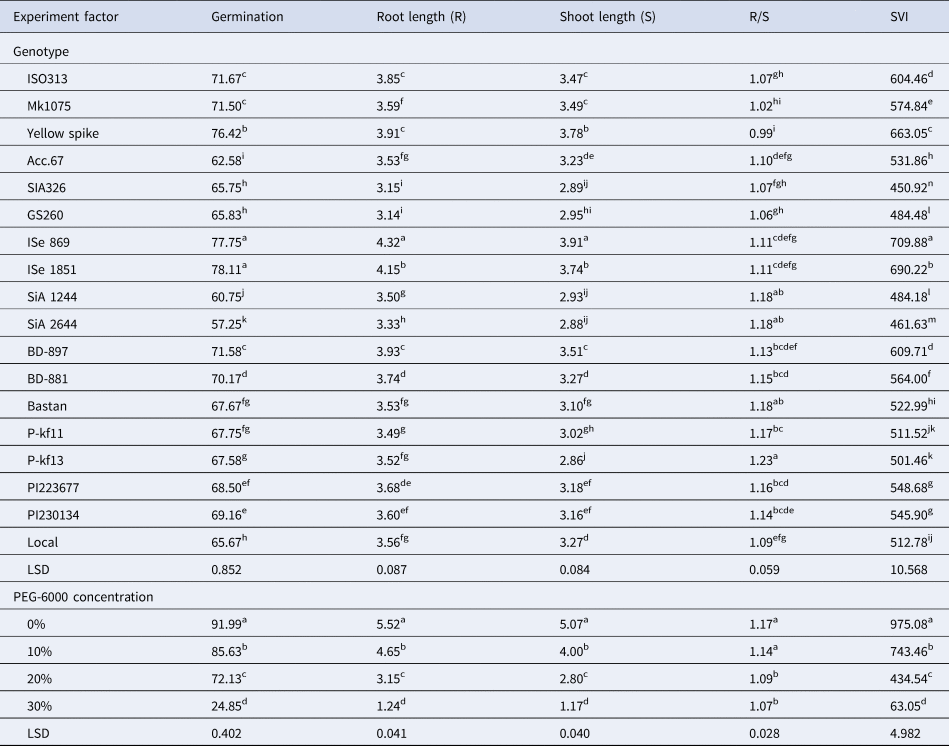
Note: Mean values in columns with different letters are significantly different according to LSD test at the 5% level of probability.
Percentage of germination
The ability to tolerate the chemical dehydration of PEG was investigated for 18 foxtail millet genotypes during germination and early growth stages. The genotypes had varied responses to germination at different osmotic potentials as induced by PEG-6000 (online Supplementary Fig. S1). Reduction in germination per cent was recorded for all the genotypes at different negative osmotic solutions compared to the control. The solution of 10% PEG had less effect on germination reduction. The higher concentration of PEG (30%) caused a maximum and significant reduction in germination up to 90.5% in SiA 2644 (standard deviation of 0.68) compared to the control.
Root length (cm)
Drought stress imposed a significant reduction on root length in all the genotypes at early growth stages. Root length of the genotype SiA 2644 was reduced to the minimum level by the increased PEG concentration in the irrigation solutions (online Supplementary Fig. S2), while both ISe 869 and ISe 1851 were found to be the most resistant genotypes for the PEG concentrations given the least reduction in root length at the highest concentration of PEG (30%), followed by local genotype.
Shoot length (cm)
A similar pattern for shoot length was realized for the genotypes under different PEG concentrations. An osmotic solution of 30% PEG-6000 had the highest effect on the reduction in shoot length for the genotypes. SiA 2644 had the highest reduction in shoot length at 30% PEG-6000, while ISe 869 and ISe 1851 were highly resistant to negative water potentials. The last two genotypes recorded the highest shoot length along the concentrations of 10, 20 and 30% PEG-6000 compared to other genotypes, followed by yellow spike (online Supplementary Fig. S3).
Root/shoot ratio (R/S)
Variable effects of R/S ratio were recorded for the genotypes at different PEG concentrations. Most of the genotypes had an increased ratio at the most negative osmotic solutions (online Supplementary Fig. S4). Less fluctuation in this ratio for different PEG concentration was observed for some genotypes such as ISe 869 and local, showing non-significant differences in R/S ratios at different negative solutions compared to the control.
Seed vigour index
It was observed that SVI was reduced by increasing the PEG concentration. The genotypes SiA 2644 and SiA 1244 had lower values for 30% PEG, while ISe 869 and ISe 1851 showed the minimum decline in SVI value for the same solution (30% PEG-6000), see online Supplementary Fig. S5. Yellow spike was similar in ISe 1851 in terms of its resistance to the reduction in SVI value with the increased concentration of PEG-6000.
PCA for morphological data
The relationships among different parameters were displayed in a graphical biplot of PCA. The first and second components accounted for 95.91% of the total variation (Fig. 1). The genotypes ISe 869 and ISe 1851 were shown to be highly associated, having high values for germination, root and shoot length and SVI, while they both recording lower R/S ratio based on the biplot diagram.
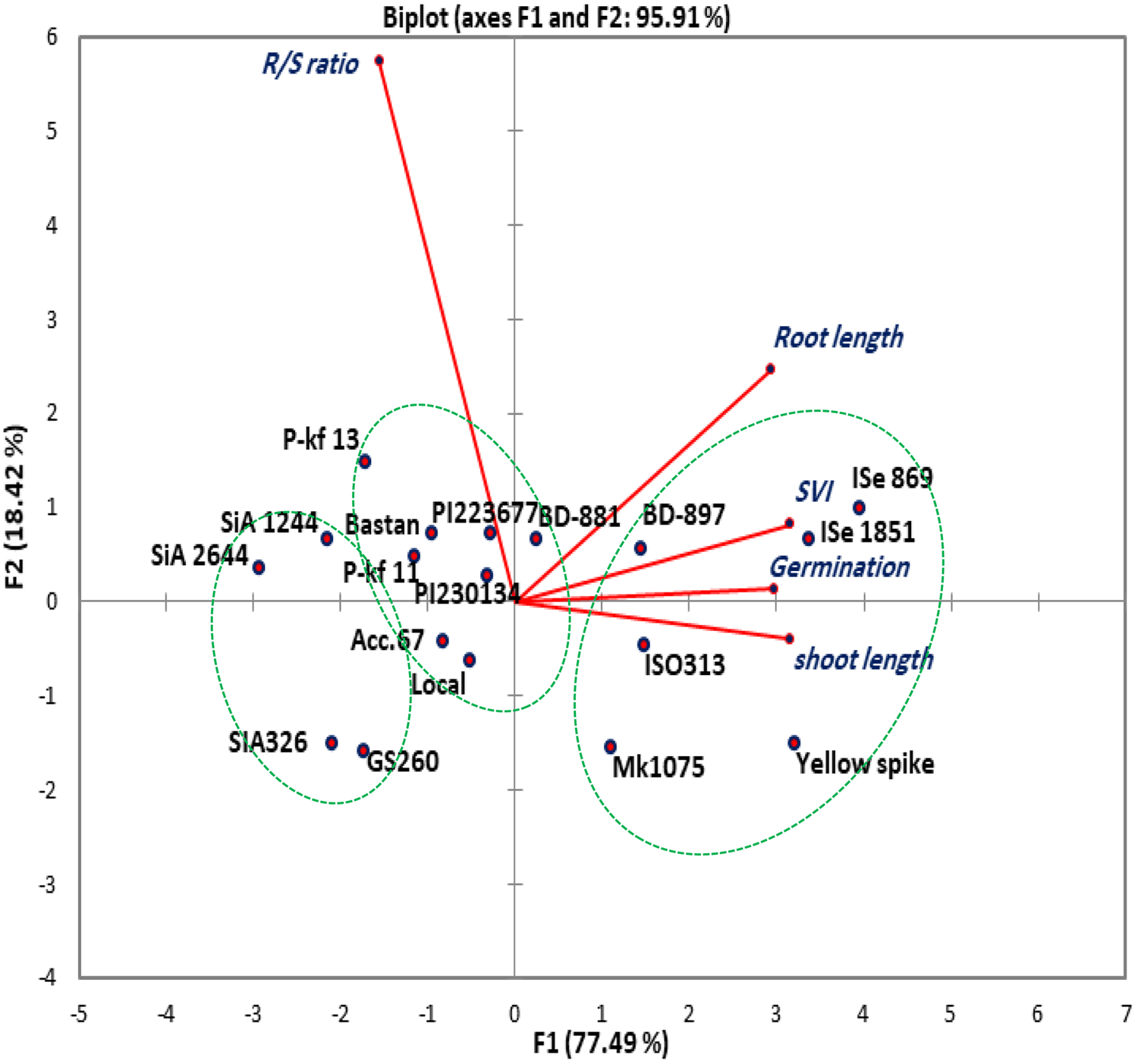
Fig. 1. Scatter plot of PCA for the 18 foxtail millet genotypes. The biplot demonstrates the relationship between the genotypes based on the morphological traits.
Agglomerative hierarchical clustering using morphological data
All the genotypes were included in pairwise comparisons, the average dissimilarity values were calculated based on data of germination and seedling growth stage. Dissimilarity values for the proximity matrix ranged from 0.36 between Bastan and P-kf11, to 6.75 between ISe 869 and SiA 2644 (data not shown).
Cluster analysis based on the morphological data
A dendrogram was created for the foxtail millet genotypes based on the data of germination and early growth characteristics. Clustering was verified using Euclidean distance of Ward's method for dissimilarity matrix. The genotypes were divided into three main groups (Fig. 2). The first group contains the genotypes SiA 2644, SiA 1244, GS 260 and SiA 326. The second group consists of eight genotypes, both green foxtail millets with the other six foxtail millet genotypes. While the third group includes the genotypes yellow spike, Mk1075, ISO313 from Australia that clustered in a subgroup with the genotype BD-897 from Bangladesh, ISe 869 and ISe 1851 from India were in the second subgroup of the third clade.
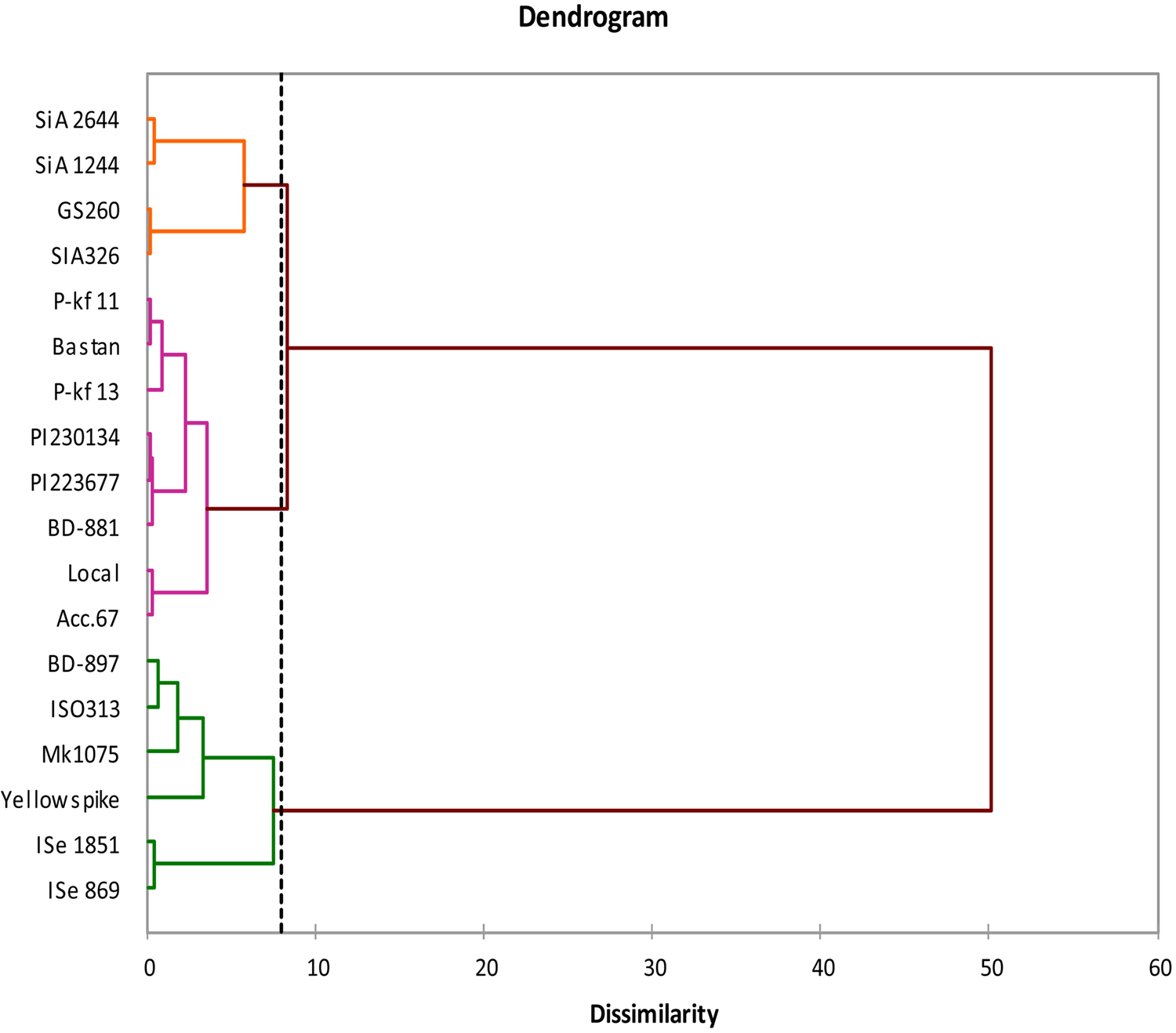
Fig. 2. Phylogenetic relationship between the 18 foxtail millet genotypes based on morphological data at germination and early growth stage. The genotypes were divided into three main groups. Clustering could be demonstrated by Euclidean distance of Ward's method for dissimilarity matrix at 8 (vertical line).
SSR analysis
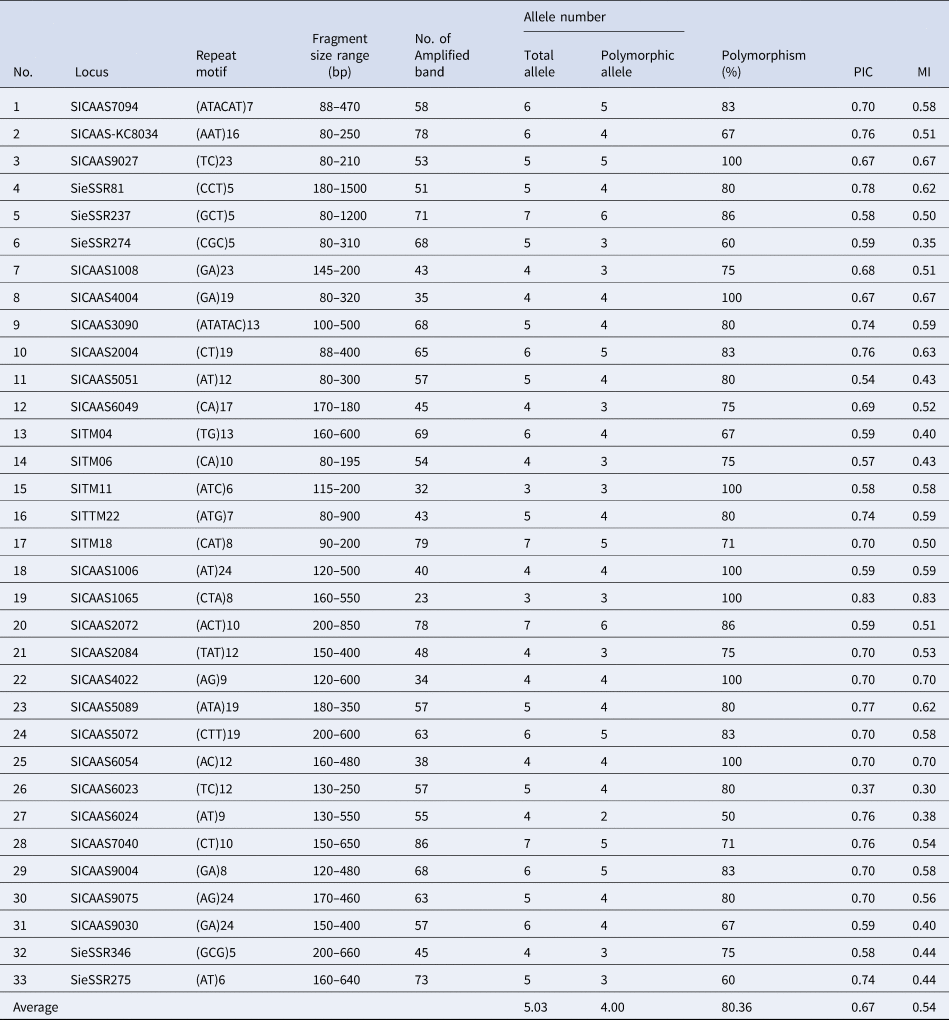
Of the 37 primers used in this study, 33 amplified genomic DNA from the genotypes and revealed the existence of genetic variation between them. A total of 33 polymorphic primers showed the presence of 132 polymorphic alleles (out of 166 loci) at the rate of 4.00 alleles per primer. PIC had the average value of 0.67 for the primers used. The highest value was recorded for primer SICAAS1065 with value 0.83, while the lowest PIC is referred to primer SICAAS6023 with the value of 0.37 (Table 2). The same patterns of the highest MI value and the least value were recorded for primers SICAAS1065 and SICAAS6023, respectively.
Table 2. Motif repeat, number of alleles, PICs and MI of the SSR primers used in the genetic divergence analysis of the 18 foxtail millet genotypes
PCoA for SSR data
The relation between the foxtail genotypes was determined using PCoA based on the SSR genotypic data (Fig. 3). The biplot clearly shows the distribution of the genotypes based on SSR data. ISe 869 and ISe 1851 were found very close to each other with yellow spike. Both green foxtail millet genotypes (PI230134 and PI223677) were positioned close to each other in a group. The genotypes SiA 2644 and SiA 1244 were placed close to the local genotype based on the SSR data.
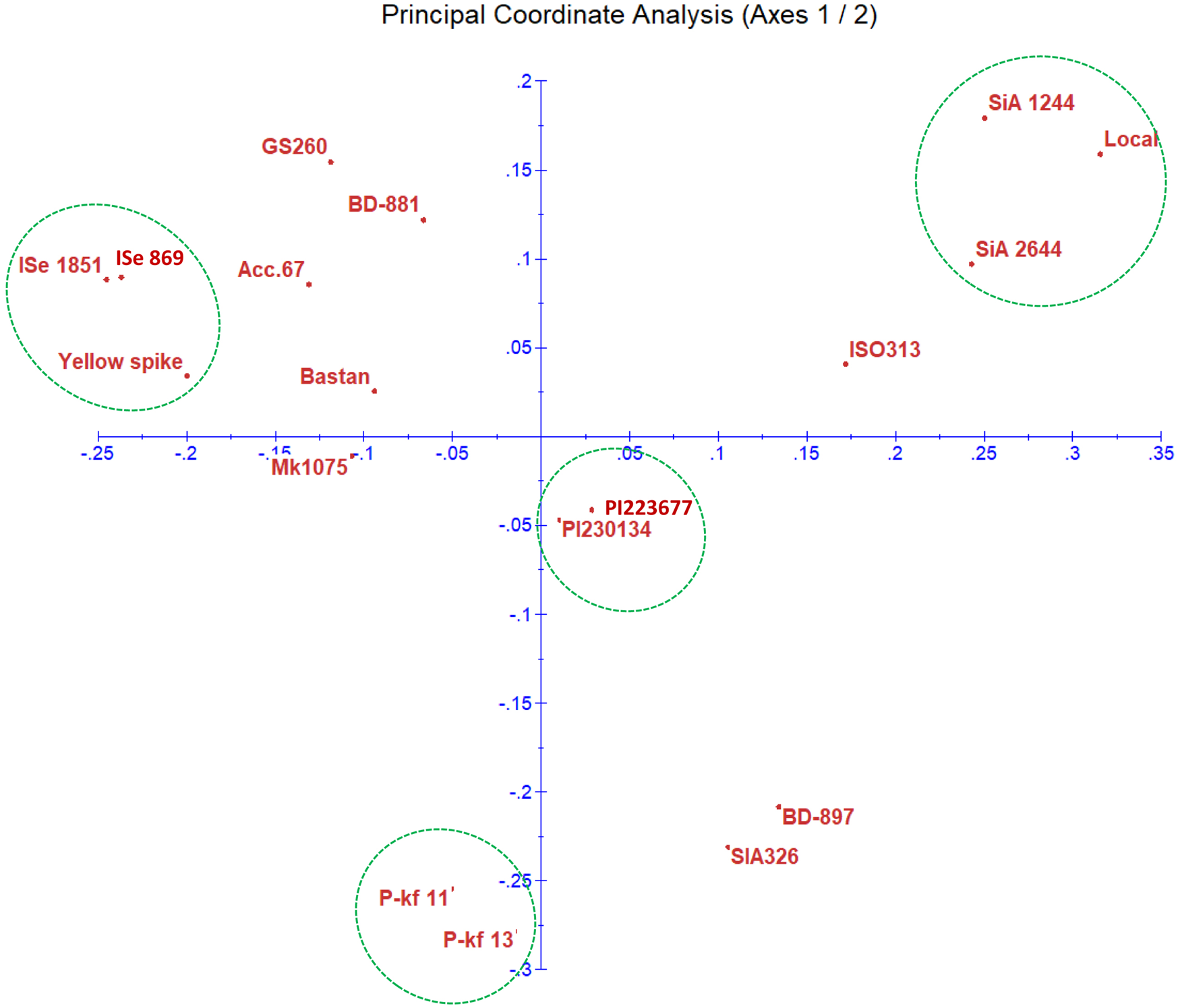
Fig. 3. Scatter plot of PCoA for the 18 millet genotypes. The biplot demonstrates the relationship between the genotypes based on the SSR data.
Agglomerative hierarchical clustering using SSR markers
Genetic distance was estimated for the 18 foxtail millet genotypes using the amplification products of SSR markers to produce a dissimilarity matrix (data not shown). The results showed the highest similarity was between the two green foxtail millet, PI223677 and PI230134 genotypes, those sourced from Iran, giving the lowest value of genetic distance (5.32). While the lowest percentage of genetic similarity (corresponds to the largest genetic distances 19.17) was obtained between ISe 1851 (an Indian origin) and local genotypes.
Cluster analysis based on SSR marker data
Genetic relationships between the 18 genotypes of foxtail millet from different geographical regions identified five main clusters (each with two to five genotypes) in the UPGMA scheme (Fig. 4). The first main cluster includes the genotypes Bastan, MK1075, Acc.67, BD-881 and GS 260. Two green foxtail genotypes (PI223677 and PI230134) were clustered in the second group. P-KF11 and P-KF13 were clustered in the subgroup of the third cluster, while the other subgroup represents the genotypes BD-897, SiA326 and ISO313. The fourth cluster includes the local foxtail genotypes with the other two Indian genotypes (SiA 2644, SiA 1244). Other two Indian genotypes (ISe 1851, ISe 869) were grouped with the Australian foxtail millet yellow spike in the fifth cluster.

Fig. 4. Phylogenetic relationship between the 18 foxtail millet genotypes based on 132 allele data. The genotypes were divided into five main groups. Clustering could be demonstrated at a Jaccard's coefficient, following UPGMA for dissimilarity matrix at 215 (vertical line).
Discussion
In vitro experimental analysis
PEG technique was used successfully for current foxtail millet genotypes to identify potentially more stress-tolerant genotypes. Effects of genotypes, different water-stress conditions and their interaction, induced by PEG-6000 indicated highly significant differences in the response of these genotypes in vitro. This outcome might be a good indication for rapid identification of tolerant foxtail millet genotypes to drought stress. The measurement of seed germination and early seedling growth characteristics have been shown to be useful evaluation for selecting stress tolerance in plants (Islam et al., Reference Islam, Kayesh, Zaman, Urmi and Haque2018; Cai et al., Reference Cai, Chen, Han, Wu, Zhang, Li, Nazir, Zhang and Zeng2020). Different foxtail millet genotypes and simulated water-deficit conditions had distinct effects on the decrease in both germination per cent and seedling growth (Yu et al., Reference Yu, Zhao, Wang, Cheng, Zhang, Tian, Liu, Guo, Du and Wang2020). The current genotypes are found to have a wide response to the characteristics measured at early growth stages. Both ISe 869 and ISe 1815 genotypes showed better performance than all the others for germination and early growth traits at the different stress levels of 10, 20 and 30% PEG, while SiA 2644 had poorer response. High PEG concentrations affected the traits with a highly significant reduction and enabled the discrimination putative of tolerant genotypes among the foxtail millet genotypes. The increased negative water potential at 30% PEG-6000 caused the greatest reduction in germination; however, it had not reached the upper limit of the physiological activity to stop germination totally. A higher dosage of 40% of PEG-6000 caused germination of pearl millet reported to be stopped (Sani and Boureima, Reference Sani and Boureima2014). However, the reduction in germination is due to the development of osmotically enforced dormancy and enzymatic activity reduction under water-deficit conditions (Muscolo et al., Reference Muscolo, Sidari, Anastasi, Santonoceto and Maggio2014; Pérez-Hernández et al., Reference Pérez-Hernández, Navarro-Boulandier, Rojas-Sánchez, Fuentes-Alfonso and Sosa-del Castillo2018), the genotypes ISe 869, ISe 1851 and yellow spike were indicated as resistant to the osmotic stresses, while the most sensitive genotype was SiA 2644 at the treatment levels of 10, 20 and 30% PEG.
Root length is among the major determinant indicators in screening genotypes for dryland conditions. Root length of the current genotypes was also affected by the increased PEG concentration in the irrigation solutions. Like germination, root length was reduced to a minimum value for SiA 2644 at 30% PEG, while the least reduction in root length at the high PEG concentration (30%) was ISe 869 and ISe 1851. Local genotype was also identified as a good resistant type, producing a reasonable root length at a maximum concentration of 30% PEG. An association between root length and drought tolerance at early growth stress in millet was reported previously (Lata, Reference Lata2015; Passot, Reference Passot2016). Seedlings with longer roots are more adapted to drought would be more able to cope with water-deficit conditions better than those with shorter roots (Ghatak et al., Reference Ghatak, Chaturvedi, Bachmann, Valledor, Ramšak, Bazargani, Bajaj, Jegadeesan, Li and Sun2021).
ISe 869 and ISe 1851 genotypes were realized to be highly persistent for shoot length in the negative water potential induced with PEG-6000. They consistently gave the highest length for control and different PEG concentrations compared to the others. The greatest reduction in root length was within the susceptible genotypes such as SiA 2644 and SiA 1244 at 30% PEG. Influence of high PEG concentrations on the reduction of root and shoot lengths in foxtail millet genotypes was also reported by Bheemesh et al. (Reference Bheemesh, Rao, Sekhar and Latha2018); however, shoot length was more inhibited than root length by increasing the concentration of PEG, because root length has the strongest dehydration tolerance (Yu et al., Reference Yu, Zhao, Wang, Cheng, Zhang, Tian, Liu, Guo, Du and Wang2020). Drought stress also led to a reduction in the length of both root and shoot for all the genotypes, which is consistent with the observations made by Shivhare and Lata (Reference Shivhare and Lata2019) and Baloch et al. (Reference Baloch, Dunwell, Khakwani, Dennett, Jatoi and Channa2012). Decrease in seedling growth under drought stress is reported to be the result of the reduced cell division and cell elongation (Hasanuzzaman et al., Reference Hasanuzzaman, Shabala, Brodribb, Zhou and Shabala2019; Abro et al., Reference Abro, Memon, Abro, Sam, Ry, Rind, Solangi, Muhammad, Ali and Ahmed2020).
Non-significant differences in the R/S ratio were observed for both genotypes ISe 869 and local across the different negative solution strengths. The least fluctuation in R/S ratio indicates that physiological activities of the root system in such genotypes are less sensitive to low relative water content of the plant (Sani and Boureima, Reference Sani and Boureima2014). This case was not indicated for other genotypes, as their R/S ratios were decreased by increasing the PEG contents. The most effected genotype for the R/S ratio is identified for SiA 1244 which is also the most sensitive to the other studied traits.
SVI was reduced for all the genotypes with different rates for the different PEG solutions. ISe 869 and ISe 1851 were shown to have a lower reduction in SVI than others with the increased PEG concentration of 30% PEG, and they could be regarded as more drought tolerance genotypes (Baloch et al., Reference Baloch, Dunwell, Khakwani, Dennett, Jatoi and Channa2012). The maximum decline in SVI values for the genotypes SiA 2644 and SiA 1244 indicating their sensitivity to drought stress conditions. High seedling vigour at early seedling growth is important and it is associated with rapid and high emergence rate, and quick establishment of crop stand (Abati et al., Reference Abati, Brzezinski, Zucareli, Foloni and Henning2018).
Selection of genotypes based on the seedling characteristics could be marked as a selection criterion for identification of the right genotypes under water-stress conditions. Characterization of genotypes based on different seedling traits under the induced drought stress caused by PEG-6000 was also reported previously in foxtail millet (Niu et al., Reference Niu, Song, Xiao and Ge2018), finger millet (Mundada et al., Reference Mundada, Nikam, Kumar, Umdale and Ahire2020) and other cereals such as wheat (Ahmad et al., Reference Ahmad, Kareem, Mustafa and Ahmad2017; Abro et al., Reference Abro, Memon, Abro, Sam, Ry, Rind, Solangi, Muhammad, Ali and Ahmed2020), rice (Purbajanti et al., Reference Purbajanti, Kusmiyati, Fuskhah, Rosyida, Adinurani and Vincēviča-Gaile2019), maize (Magar et al., Reference Magar, Parajuli, Sah, Shrestha, Sakh, Koirala and Dhital2019) and tomato (Ali et al., Reference Ali, Schwarzenberg, Yvin and Hosseini2018).
The first two principal components (PC), PC1 and PC2 covered nearly all phenotypic variations (95.91%), across the eighteen genotypes, for germination and early growth characteristics. This result might indicate a high contribution of these two components in determining the relationship between the foxtail genotypes (Fig. 1). The cosine of the angle between the studied traits except R/S ratio indicating their positive association, also indicate their negative relation to the R/S ratio. Such correlations will also be helpful in predicting the traits through others associated traits that are more easily measured (Harrison and LaForgia, Reference Harrison and LaForgia2019). The genotypes have a different degree of relationship based on the morphological traits. ISe 869 and ISe 1851 were realized to be highly associated, having high values for germination, root and shoot lengths and SVI, while they both recorded lowered R/S ratio based on the biplot diagram. These two genotypes also performed very well and highly associated under field and greenhouse conditions for an investigation of phenotypic responses and phosphate content in India (Ceasar et al., Reference Ceasar, Ramakrishnan, Vinod, Roch, Upadhyaya, Baker and Ignacimuthu2020). The designated angles here reflect the importance of the largest contributor to the total variation and association between the genotypes at each axis of differentiation (Abdi and Williams, Reference Abdi and Williams2010).
Reasonable variability was indicated based on the wide range of dissimilarity values (0.36–6.75) between the genotypes using the morphological matrix data, applying Euclidean distance dissimilarity of Ward's method of agglomerative hierarchical clustering analysis. Determination of the genetic distance will be highly helpful in conducting hybridization programme to involve highly distinct genotypes, while for the genotypes with low genetic distance selection programmes could be followed to emphasize additive alleles for desirable traits (Govindaraj et al., Reference Govindaraj, Vetriventhan and Srinivasan2015). To further understanding the relationship between the studied genotypes, UPGMA clustering analysis was able to classify the foxtail millet genotypes into three groups based on the morphological data. According to the studied characteristics most of the genotypes located in the first group are identified to be the most sensitive to the induced drought stress especially SiA 2644 and SiA 1244. Grouping the green foxtail millet genotypes with other foxtail millets in the second group indicates their origin similarity or ancestral relationship, and both could be regarded as the same species (Diao and Jia, Reference Diao and Jia2017). While the third cluster includes the genotypes with the most drought-resistant based on the traits studied. ISe 869 and ISe 1851 were superior for germination and other seedling traits under induced drought stress. Yellow spike, Mk1075, ISO313 and BD-897 are also clustered together within a subgroup from the third cluster, showing moderate tolerance to stress conditions. It has been identified that based on screening the genotypes under drought-induced stress that foxtail millets were separated into these groups independent of their origins. Biplot results of the current study are in agreement with cluster analysis of the morphological data in identifying the same tolerant genotypes for the induced water stress. Cluster analysis has been utilized previously to describe the variation and grouping foxtail millet genotypes based on agronomic traits (Brunda et al., Reference Brunda, Kamatar, Naveenkumar and Hundekar2015; Yadav et al., Reference Yadav, Adhikari, Gautam, Ghimire and Dhakal2018; Kumar et al., Reference Kumar, Prasad and Reddy2019).
Molecular data analysis
SSRs are useful markers for studying genetic diversity, the hypervariable nature of SSRs results in high levels of allelic variation, even among very closely related varieties (Vieira et al., Reference Vieira, Santini, Diniz and Munhoz2016; Ma et al., Reference Ma, Sa, Hong and Lee2019). In this study, we have demonstrated a successful application of SSR analysis to study genetic distance among the genotypes of foxtail millet from different geographic backgrounds and to be in association with drought stress tolerance. Obtaining relatively high polymorphism of SSR primers average (four alleles/locus, Table 2) indicates the utility of these markers in determining unique genetic profiles of the individual foxtail millet genotypes. The majority of primers used in this study were previously applied on foxtail millet genotypes and tested for polymorphism by various researchers (Lin et al., Reference Lin, Chiang, Chang and Kuoh2011; Zhang et al., Reference Zhang, Tang, Zhao, Li, Yang, Qie, Fan, Li, Zhang and Zhao2014); however, seven primers were taken from genic SSR of expressed sequence tags (NCBIdbEST) (Venkata Suresh et al., Reference Venkata Suresh, Muthamilarasan, Misra and Prasad2013) of the foxtail millet marker database (FmMDb; http://www.nipgr.res.in/foxtail.html), and used successfully in this study for the first time. Five out of the seven genic SSR primers were polymorphic giving three to six alleles per locus. Fewer alleles per locus (2.52) were observed in the foxtail millet crop in a previous study (Trivedi et al., Reference Trivedi, Arya, Verma, Hemantaranjan, Verma, Sharma and Saha2018), and a higher mean number of alleles per locus (4.62 alleles) were produced in pearl millet from a total of 74 SSR markers (Kumar et al., Reference Kumar, Hash, Singh, Basava and Srivastava2020). In green foxtail millet 6.1 alleles per locus were detected (Hsieh et al., Reference Hsieh, Chen, Liao, Lin and Chen2021), while, in foxtail millet a higher number of alleles per primer (10.6 and 17.87 alleles/primer) were obtained by Kim et al. (Reference Kim, Sa, Park and Lee2012) and Jia et al. (Reference Jia, Liu, Schnable, Niu, Wang, Li, Wang, Wang, Liu and Guo2015), respectively. Similar ranges of alleles per primer for SSR markers were obtained in other diverse studies of foxtail millet (Chander et al., Reference Chander, Bhat, Kumari, Sen, Gaikwad, Gowda and Dikshit2017), pearl millet (Adeoti et al., Reference Adeoti, Djedatin, Ewedje, Beulé, Santoni, Rival and Jaligot2017), finger millet (Lee et al., Reference Lee, Yoon, Shin, Lee, Cho, Lee, Ma and Lee2017) and barley (Tahir et al., Reference Tahir, Ahmad, Mustafa and Kareem2021). Polymorphism of the current primers used here was reasonably high (80.36%), demonstrating the effectiveness of such SSR markers in the assessment of genetic diversity in foxtail millet (Chander et al., Reference Chander, Bhat, Kumari, Sen, Gaikwad, Gowda and Dikshit2017).
The discrimination power of the SSR markers was determined based on PICs. All the primers used seem to be reasonably informative because they had PIC and MI values greater than 0.5, indicating highly informative SSR marker for diversity assessment (Kumar et al., Reference Kumar, Hash, Singh, Basava and Srivastava2020). A high MI of the marker indicates the better ability of their combination to detect the differences between larger populations of foxtail millet (Gilbert et al., Reference Gilbert, Lewis, Wilkinson and Caligari1999). In this investigation the number of alleles was found to have a positive correlation (r 2 = 0.86, P = 0.000) with PIC values, implying that the alleles number amplified could be used indirectly to assess PIC of the SSR primers in the foxtail millet genotypes. Association of allele number and PIC value was a result of microsatellite marker genotyping (Lebedev et al., Reference Lebedev, Subbotina, Maluchenko, Lebedeva, Krutovsky and Shestibratov2020).
The extent of genetic variation in among the Setaria genotypes selected from diverse geographical locations around the world could be one of the reasons of high polymorphism for these primers. Highly polymorphic and effective SSRs with high alleles ranged from 2 to 16 were obtained using genome-wide microsatellite variant analysis of S. italica L. (Yang et al., Reference Yang, Yan, Shah, Warburton, Li, Li, Gao, Chai, Fu and Zhou2010; Zhang et al., Reference Zhang, Tang, Zhao, Li, Yang, Qie, Fan, Li, Zhang and Zhao2014) and number of targeted loci sited by SSR markers (Hayden and Sharp, Reference Hayden and Sharp2001). Detection rate of the genotypes by SSRs depends on the length of primers. Those with more than 20 bp could able to detect the variation between genotypes more efficiently compared to shorter primers (Wang et al., Reference Wang, Yang, Jin, Zhou, Wang, Yu and Yang2015), which is the case for most of the primers used here. In addition, these primers could have better detection for the heterogeneous alleles, especially in the related varieties (Ho et al., Reference Ho, Wu, Hsu, Huang, Huang and Chiang2011).
The biplot diagram for structuring genetic distance indicates a wide distribution of the genotypes on the plate using SSR alleles' data. For ISe 869 and ISe 1851 originated from India are in close relation to yellow spike. These genotypes were identified to have similar potential power to tolerate the drought stress induced by PEG-6000. Response of ISe 1851 to abiotic stress such as salinity was identified previously (Upadhyaya et al., Reference Upadhyaya, Vetriventhan, Deshpande, Sivasubramani, Wallace, Buckler, Hash and Ramu2015). The two green foxtail millet genotypes (PI230134 and PI223677) positioned close to each other for indicating their similarity. They both are weedy relative to foxtail millet and integrating them in the study would suitably attribute to the genetic analysis of foxtail millet characteristics (Li and Brutnell, Reference Li and Brutnell2011). The genotypes SiA 2644 and SiA 1244 were genetically close to local genotype based on the SSR alleles, which is useful for transferring superior genes via hybridization programme to the local, or vice versa. High relatedness of both lines P-kf11 and P-kf13 are also indicated in this diagram. Other genotypes are distributed with variable distance on the plot. Similar patterns of clustering groups for the genotypes were obtained for the morphological data. The relationship between SiA 2644 and SiA 1244, and between both lines P-kf11 and P-kf13 based on the induced stress experiment and the DNA marker data is likely to confirm the value of relying on morphological and molecular data. It is expected that the SSR markers applied on the foxtail genotypes might position close to alleles responsible for stress tolerance in the foxtail millet genome.
Foxtail genotypes sourced from India were distributed in four groups. This clustering format might be supported with prehistorical evidence (Stevens and Fuller, Reference Stevens and Fuller2017; Martin et al., Reference Martin, Messager, Bedianashvili, Rusishvili, Lebedeva, Longford, Hovsepyan, Bitadze, Chkadua and Vanishvili2021), as foxtail millet is originated somewhere in Asia, then they were further diversified and spread around the world. Ungrouping the genotypes from the same region in the current study indicating that foxtail millet possesses a wide genetic base (Diao et al., Reference Diao, Schnable, Bennetzen and Li2014; Sapkota et al., Reference Sapkota, Pandey, Thapa, Yadav, Ghimire and Timalsina2016) and diverse germplasm collection from diverging areas of Eurasia with different genetic background might be introduced and cultivated in suitable environments of the same region (Motuzaite-Matuzeviciute et al., Reference Motuzaite-Matuzeviciute, Colledge and Jones2008). This could support the idea that such clustering did not appear to be a result of recent hybridization events (Hirano et al., Reference Hirano, Naito, Fukunaga, Watanabe, Ohsawa and Kawase2011). However, some evidence suggesting that the genetic clustering across the foxtail species coincides with the geographical areas more than taxonomy (Lin, Reference Lin2012), combination of foxtail genotypes from Australia, Russia, India and Iran in the first group indicates their genetic relation independent of their geographical distribution (Ali et al., Reference Ali, Choi, Do, Lee, Oh, Park, Cho and Lee2016), as they might have the ancestor relation (Wei et al., Reference Wei, Hui, Wang, Li and Diaw2012). The results obtained here, to cluster foxtail genotypes from diverse geographical regions in one group, are in accordance with what was investigated by other researchers (Lin et al., Reference Lin, Chiang, Chang and Kuoh2011; Gupta et al., Reference Gupta, Kumari, Sahu, Vidapu and Prasad2012; Kim et al., Reference Kim, Sa, Park and Lee2012), to find out that geographical distribution of the foxtail millet is not able to frame their genetic relationship. Wide growing of foxtail millet in semi-arid areas of the Middle East and large areas of India (Krishna, Reference Krishna2013) belonging to eastern complex (race Maxima) would indicate their genetic similarity between the accessions from different countries there. The current diversion of foxtail genotypes will help the breeder in tagging the right foxtail genotypes for development programmes, especially in crossing programmes to guarantee the production of unique offspring from diverse genotypes.
The accessions of green foxtail millet (PI230134 and PI223677) were shown to locate within the same group. Green foxtail millet identified to be the ancestor of foxtail millet (Hsieh et al., Reference Hsieh, Chen, Liao, Lin and Chen2021). Indeed, repeated genetic introgression has likely resulted from the interspecific hybridization of green foxtail and foxtail millet, due to cross-compatibility of S. italica and S. viridis (Li and Brutnell, Reference Li and Brutnell2011), and established close genetic architecture for both species, as foxtail millet maintained 45% of its wild diversity (Wang et al., Reference Wang, Chen, Zhi, Yang, Li, Wang, Li, Zhao, Chen and Diao2010). This fact will facilitate the introgression of desirable genes from the wild ancestor (green foxtail) into the foxtail cultivars easily, and reduce the risk of genetic drift of elite varieties due to continued inbreeding of foxtail genotypes (Bhandari et al., Reference Bhandari, Nishant Bhanu, Srivastava, Singh and Shreya2017; Allaby et al., Reference Allaby, Ware and Kistler2019). Krishna (Reference Krishna2013) has stated that foxtail millet is grown widely in semi-arid areas in Turkey, Syria, northern Iran, Iraq and large areas of India. The highest values of genetic similarity were found between some accessions of these areas; as they might belong to eastern Asia and European complexes (races Maxima and Moharia). While the Australian genotypes (yellow spike) and two Indian genotypes ISe 869 and ISe 1851 show high deviation from other genotypes, which allows them to be structured in a different cluster. This combination between yellow spike from Australia and two Indian genotypes (ISe 869 and ISe 1851) could be referring to their origin from another complex (race Indica) in spite of their geographical distance (Donnelly et al., Reference Donnelly, Adams and Dekker2014; Upadhyaya et al., Reference Upadhyaya, Vetriventhan, Deshpande, Sivasubramani, Wallace, Buckler, Hash and Ramu2015).
Depending on the above analysis the genotypes used here were found to be divergent, and they could be utilized successfully in a hybridization programme for the genetic improvement of drought resistance in foxtail millet. High genetic diversity in foxtail millet was also found by other researchers (Yadav et al., Reference Yadav, Adhikari, Gautam, Ghimire and Dhakal2018; Zhang et al., Reference Zhang, Liu, Zhang, Zheng, Xing and Jia2019). The direct relationship of this crop to humans from ancient cultures as a food and feed had a big influence on its distribution. Foxtail millet was distributed around the world through domestication and new introduction, to become a worldwide crop (Diao and Jia, Reference Diao and Jia2017). Due to its adaptability, this crop should be involved in cereal developing programme for yield, offering better promising to cope with the abiotic stresses.
Conclusion
The foxtail millet genotypes were screened for their resistance under induced drought stress using PEG-6000 at germination and seedling stage. Variable distances were identified between the genotypes based on the morphological traits. ISe 869 and ISe 1851, followed by yellow spike are highly associated with tolerance to drought stress. The analysis of phenotypic data was likely in accordance with the results from the SSR allele data to cluster the genotypes in different groups. Green foxtail millet genotypes clustered together for both morphological and SSR data set. ISe 1851, ISe 809 and yellow spike were clustered together in a group for both morphological and SSR data analysis. Close position of both DNA markers and alleles of drought tolerance in this crop could answer similar clustering of both data set analyses.
Extra insight into the genetic resources can be achieved through this investigation, to facilitate their use in future genetic improvement programmes. Indeed, based on the in vitro experiment, the three most tolerant genotypes ISe 869, ISe 1851 and yellow spike are recommended to be cultivated under drought conditions in Iraq and similar localities subject to drought stress around the world. Further investigations including of more millet germplasm, greater number of SSRs and other DNA markers parallel to field trials would be useful to clarify their genetic relationship, origins and their genetic background for the rapid development of this crop under different rainfed conditions in the world.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262123000151
Acknowledgements
The authors are grateful to the College of Agricultural Engineering Sciences, the University of Sulaimani, Kurdistan-Iraq for their permission and aids to work in their laboratories. The authors also acknowledge the Tikrit University, College of Sciences for their financial support for the project. The authors also appreciate the efforts of Dr Heydar Azizi at Guilan University in Iran, and Oral Muhammad Musa at Sulaimani Agriculture Research Center in Sulaimani-Iraq to facilitate the provision of some of the foxtail millet genotypes. The effort of Dr Persidor Kendabie, at the University of Nottingham, UK, is also highly appreciated for editing the manuscript.








