13.1 Introduction
This chapter is about the role of narratives in chemistry. Recent studies by historians and philosophers of science have argued that narratives play an important part in shaping scientific explanations; narratives are not, according to this view, only concerned with rhetoric or communication, and not an added extra, but integral to the work of social and natural sciences. In Mary Morgan’s concise definition, ‘what narratives do above all else is create a productive order amongst materials with the purpose to answer why and how questions’ (Reference MorganMorgan 2017: 86).
Notions of narrative are not alien to existing discussions of chemistry: most notably, the Nobel Prize-winning organic chemist Roald Hoffmann has argued that chemical findings should be given narrative form, and similar arguments are present (or at least implicit) in some chemical publications, process ontologies of chemistry and historians’ and social scientists’ critical accounts of chemistry. Despite their differences, these claims are based on a shared understanding of the purpose of narrative which goes beyond attention to productive order: they suggest that narratives should be used to challenge the conventional demarcations of chemical accounts and ‘let the world back in’ by incorporating contingencies, aspects of decision-making, social dynamics and the interactions between humans and chemical substances which are not usually included within the chemical literature. All continue to bring materials together, to answer questions – they are thus still narratives in Morgan’s sense – but they also proceed contrastively, by trying to offer something beyond the conventions of writing in chemistry. These more capacious narratives contrast with the extremely terse form usually adopted by chemical publications. I will call the conventional presentation of chemical findings, ‘thin narratives’, and the more capacious ones recommended by some chemists, philosophers and historians, ‘thick narratives’.
My distinction between the thick and the thin is modelled on the anthropologist Clifford Reference GeertzGeertz’s (1973) celebrated discussion of ‘thick description’. Geertz gave the example of describing someone who was winking, first developed by the philosopher Gilbert Ryle. We could describe a wink in physiological terms – through a very specific sequence of muscle contractions, or more simply in terms of what we observe directly. Or we could say something like, the man winked conspiratorially, according to a cue we had agreed beforehand, and I was delighted. The former confines its description to a single plane: that of observable physiological phenomena – Reference RyleRyle (1947) called it a ‘thin’ description. The latter incorporates context and intentionality, which cannot just be read directly, but require additional elucidation and the incorporation of considerations behind the immediately observable. It is a ‘thick’ description. By extension, a thin narrative is a sequence or productive order, all of whose materials are presented as closely interrelated and conducing to the same purpose, and which can readily be transferred from one situation to another.Footnote 1 The thin narrative may also be presented in a formal language, which encodes relations and interactions between the entities involved in the narrative. A thick narrative, by contrast, is one which incorporates more context and considerations which may not be directly related to the explanatory task at hand, and which may be more difficult to move around.
The distinction between thin and thick descriptions carries normative implications. Geertz thought that anthropology needed thick descriptions; that its accounts would be incomplete and misleading without them. Similarly, the chemists and writers in chemistry who have called for the use of narrative form argue that understanding of chemical processes and chemists’ decision-making will be impoverished without the incorporation of elements which are usually not found in works of chemistry. But the difference between the thick and the thin has been understood in a much wider sense as well. The historian Ted Reference PorterPorter (2012) argues that the institutional and bureaucratic structures of modernity tend to privilege thin descriptions and to denigrate thick ones, and that natural sciences have been justified through an appeal to thinness, sometimes even changing their own thickets of practices and overlooking the persistence of skilled judgement in response to the pressure to offer thin descriptions.
I think that Porter is right to claim that thin descriptions (and thin narratives) are characteristic products of modernity, and that it has often been a chief aim of historical and sociological analysis to restore a measure of thickness. The views of chemistry discussed in this chapter are examples of arguments which have exactly this goal in mind. Nevertheless, Porter’s view requires two qualifications. First, we should not give the impression that thin descriptions and narratives are impoverished, because this risks overlooking the functions which they serve, such as providing a condensed, unitary record of chemical reactions, or shared format for planning out new chemical syntheses. Those functions may come with considerable problems, but that does not imply they are unimportant, and indeed they are of considerable utility to working chemists.
Second, thickening can be seen as an end in its own right, an obvious good. But, as the examples discussed in this chapter indicate, different attempts to thicken a thin narrative can have rather divergent aspirations, incorporate details of different kinds, and also make significant omissions. As a result, even thick narratives can look somewhat thin if the goal is to provide a completely comprehensive account. This can be a strength, as long as thickening in itself is not seen as a way to escape the troubles of thinness, or a way to offer the ‘whole story’ which lurks behind the thin surface.
In this chapter, I describe and analyse thin and thick chemical narratives, using the example of synthetic reaction schemes linked to a ‘classic’ synthesis from the history of chemistry: Robert Robinson’s ‘one pot’ production of tropinone, which was accomplished in 1917. In section 13.2, I present a twenty-first-century rendering of the tropinone reaction scheme, as well as its Reference Robinson1917 counterpart, and use work by the chemist-historian Pierre Laszlo to indicate some of the reasons that chemists may prefer to present their findings in such a thin form. Sections 13.3 and 13.4 contrast two kinds of arguments that conventional presentations of chemical results are deficient on the grounds of their thinness – those employed by chemists and those advocated by analysts of the science, respectively – and explore how such attempts played out in repeated retellings of Robinson’s tropinone synthesis. This leads me, finally, to consider some implications of thinking in terms of thick and thin narratives for historical and philosophical writing about chemistry.
Before I do so, however, I want to introduce my historical case study of a thin chemical narrative which has repeatedly been thickened. The example is drawn from the career of celebrated organic chemist Robert Robinson. Born in Derbyshire in 1886, Robinson (d. 1975) would acquire a reputation as one of the foremost organic chemists of the first half of the twentieth century and become President of the Royal Society and an advisor to the British government on a range of chemical topics, including colonial development. In 1917, Robinson achieved a synthesis of the alkaloid tropinone that significantly simplified the previous multi-stage, and therefore highly inefficient, scheme. Robinson’s scientific paper on the synthesis was published in the same year and detailed how he used counter-intuitive chemical starting products to produce tropinone at room temperature, and without any extremes of alkalinity or acidity. Furthermore, the process involved several reactions which led on from one another without requiring further intervention on the part of the chemist. These features of the synthesis led to its becoming one of the foundational works for Robinson’s reputation as a significant synthetic chemist, and to its elevation to the status of a synthetic ‘classic’ – discussed in textbooks and cited as an inspiration by chemists even now (Reference Medley and MovassaghiMedley and Movassaghi 2013). As we will see in section 13.3, Robinson’s tropinone synthesis has been repeatedly retold by chemists, and was the subject of a sustained historical investigation by Robinson’s one-time student, the Australian biochemist Arthur Birch.
13.2 Synthetic Reaction Schemes as Thin Narratives
Reaction schemes are one of the characteristic ways in which organic chemists plan and record their activities; it is therefore not surprising that Robinson’s landmark publication on the one-pot synthesis of tropinone included such a scheme. Drawing on discussions by Robert Meunier (Chapter 12), Line Andersen (Chapter 19), Norton Wise (Chapter 22) and Andrew Hopkins (Chapter 4) from elsewhere in this volume, this section will discuss some of the features which make reaction schemes distinctive as thin narratives, as well as ways in which they are similar to scientific narratives found in other domains.
Figure 13.1 is taken from a 2013 reconsideration of Robinson’s ‘landmark’ synthesis of tropinone and records a reaction scheme for the synthesis according to twenty-first-century conventions. Read in a clockwise direction, starting in the top left, the scheme shows the ways in which two starting products are subjected to various operations – diluted, reacted with other chemical substances, and so on – which change them into a series of intermediate forms, which gradually become more and more similar to the desired final product (tropinone – see molecule labelled 1 in Figure 13.1). The synthesis of complex natural products can involve many hundreds of separate stages, although this version of the tropinone synthesis only involves three intermediate stages. Indeed, from a chemist’s point of view, what is striking about this reaction is that a considerable amount of change happens in only a few stages. Each stage consists of one or several structural formulae: diagrams through which chemists represent both the composition of chemical substances and their spatial arrangement; knowledge of composition and structure helps chemists to construct explanations about how chemical substances will react with one another. Stages in the scheme occur in a particular sequence of reactions, where structural formulae indicate both the protagonists of the synthesis (the chemical substances which play a part in it) and the functions which these chemical substances can play. The transition between the different steps of the synthetic sequence is indicated by straight arrows, while the intermediary reactions are animated, so to speak, by the curved arrows that join together different chemical structures and show the movement of electrons. These curly arrows, which came into widespread use in the second and third decades of the twentieth century, allow the reaction sequence to offer an indication of what is happening at a molecular level to form the desired final chemical substance.
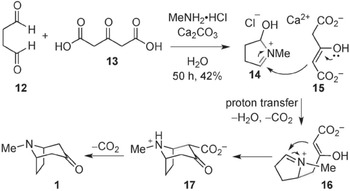
Figure 13.1 Modern representation of Robinson’s ‘landmark’ synthesis of tropinone
If the reaction scheme provides an ordered sequence of chemical events leading to a single goal (the end product), it is also important to note what the scheme does not show. It does not give an indication of what happens to any chemical substances which do not play a role in subsequent stages of the synthesis, and which are treated as waste products. Similarly, the scheme does not give any indication of the process by which the sequence was arrived at. It also presents a series of operations and reactions which may occur within an organism, or in a laboratory, as though they followed on naturally from each other – the role of the human chemist in performing the synthesis does not appear as distinct from the reactions of chemical substances.
Considered in this way, it makes sense to consider chemical reaction schemes as thin narratives: ordered sequences of chemical events conducing to a single, unified end, in which human intervention is flattened onto the same plane as chemical interactions. Moreover, the reaction scheme resembles a ‘narrative of nature’, in Robert Meunier’s sense. As Meunier describes such a narrative (Chapter 12), it ‘relates several events which occur in temporal order and are causally connected’, and which is structured into a beginning, middle and end; like the narratives which Meunier discusses, the reaction scheme ‘does not recount particular events, but rather a type of event happening countless times’. And the sequence appears to be self-evident: it does not foreground the role of a human experimenter or observer. In other ways, however, the sequence is rather unlike the examples which Meunier gives. It is told in a formal visual language (the structural formulae), which requires a chemical training to understand, rather than providing a neat compact set of events that are (potentially) intelligible to non-scientists. It is not that the reaction sequence cannot be paraphrased, or its events presented verbally; instead, a verbal paraphrase of the sequence of chemical events presented in the reaction scheme would be just as terse and technical as the reaction scheme, just as thin a narrative.
Here, for example, is one such verbal description (of a different synthetic reaction), presented by the chemist, historian and philosopher Pierre Laszlo:
L-Proline was esterified (12) by treating it with MeOH and thionyl chloride at 0°C, followed by Boc protection of secondary amine in dry tetrahydrofuran (THF) using triethyl amine as base at rt, furnishing (13), which on LAH reduction at 0°C in dry THF provided alcohol (14).
Unpacking the meaning of this extremely terse sentence, Laszlo argues, relies on the implicit knowledge of the chemist. He attempts two glosses of this piece of ‘chemese’. The first seeks to define the provenance of the chemical substances mentioned in the paper – indicating how they would be obtained – and a description of the verbs, suggesting what is turning into what.
The chemical recipient of this treatment is the amino acid proline, as the (natural) L-enantiomer. It can be bought from suppliers of laboratory chemicals. Its esterification means formation of an ester between its carboxylic COOH group and the simplest of alcohols, methanol (here written as MeOH), another commercial chemical, in the presence of thionyl chloride (SOCl2), also commercial. The reaction scheme bears the instruction ‘0°C-rt, 4 h’, in other words, ‘dissolve proline and thionyl chloride in methanol, held in a cooling bath, made of water with floating ice cubes, at 0°C and let this mixture return to room temperature (rt) over four hours, before extracting the desired product’.
Laszlo goes on to unpack the sentence’s other implicit meanings, in a manner which draws them out towards the laboratory routines of the chemist:
[T]he stated ‘room temperature’ in fact has a meaning more elaborate than ‘the temperature in the laboratory’. It means ‘about 20°C’, hence if the actual room temperature is markedly different, one ought to switch on either heating or air-conditioning.
Laszlo’s commentaries give one perspective from which to unpack the sentence, which works outward from the various materials employed in the experimental process to the routines of the laboratory and the chemist’s view of her workflow and the conditions in which she is working. Different explications could be given. Laszlo’s larger point is that the cognition of chemists involves associative processes, ‘molecular polysemy’, characterized by continually shifting horizons: new chemical discoveries add extra layers of association to the sentence’s existing stock of substances by positing new relations between them. Sentences, such as Laszlo analyses, lack, even as an aspiration, an attempt to fix the meanings of their key terms.
The use of structural formulae and of the terse language of ‘chemese’ are the reasons that I think we should consider chemical syntheses, as typically presented, as thin narratives, even in their verbal form. The powerful and polysemous formal languages of organic chemistry provide a rich but also restrictive vocabulary for describing what has happened or can happen, in chemical terms – for keeping track of how chemical substances change and the reasons for thinking that they may be used to serve chemists’ purposes. Chemists’ use of diagrammatic sequences and of language bring accounts of chemical syntheses into a single plane, with all relevant chemical actions and events describable in the same terms. And structural formulae can be used not only to explain what has happened, to record synthetic achievements or to investigate synthetic pathways in living organisms; the formulae can also be used to plan novel syntheses, with the information encoded in the formulae giving a good idea of what approaches might or might not be workable within the laboratory.Footnote 3 On their own terms, such ‘narratives of nature’ are meant to be self-sufficient, a robust and portable sequence of events which can be unpacked by a skilled chemist.
My attempt to consider such terse and formalized sequences as narratives in their own right, however, also indicates their potential instability – reasons that others might call for them to be ‘thickened’. Other chapters in this book have aligned narrative with the experiential dimension of interpreting a formalized sequence; this is the gist of Line Andersen’s discussion of mathematical proofs (Chapter 19), which Norton Wise (Chapter 22) describes as follows: ‘Reading a proof in experiential terms changes what looks to an outsider like a purely formal structure into a natural narrative for the reader; so too the experiential reading enriches the formal language of rigorous proof with the natural language of narrative, for it calls up meanings that the unaided formal language, lacking background and context, cannot convey’. If the opposition is drawn between formal language, on the one hand, and natural language, on the other, then thin narratives only become narrative when they are interpreted by a skilled reader, who is able to supply context and detail that may be absent from the plane of the formal representation itself. In the absence of a reader who possesses such ‘scripts’, reaction sequences cannot function as narratives. Even so – and I wish to insist on this – organic chemists do not simply animate the dry bones of their thin narratives with their competence, background knowledge and experience; chemists have also argued, explicitly, that the formal languages in which chemical research is presented provide an inadequate account of chemists’ reasoning and the character of the interactions between chemical substances which they employ. I will discuss chemists’ calls for thickening in the next section of this chapter.
For now, I wish to follow Meunier’s lead and ask to what extent these thin chemical narratives might encode their origins in experimental research practices. Meunier (Chapter 12) emphasizes that each part of a narrative of nature ‘can be traced back to an episode of research’, with narratives of nature ‘emerg[ing] gradually from the research literature as facts accepted in a community’, with the experimental aspects of ‘the methods by which the knowledge was achieved […] abandoned like ladders once the new state of knowledge is reached’. Is something similar happening with the narrative of nature provided by the reaction scheme? The answer to this question is a qualified yes: indeed, the narrative of nature is related to past experimental work, but in organic chemical synthesis the experimental narrative retains a stronger presence in an organic chemical reaction scheme than would be the case for the biological narratives which Meunier examines.
We can see this with reference to Figure 13.2, which shows the tropinone reaction scheme as presented by Robinson in his Reference Robinson1917 publication. As before, the scheme bears features of a thin narrative: a sequence of chemical events leading to a single outcome (the tropinone molecule – see bottom right), presented in the formal language of structural formulae, with no explicit indication of the researcher’s interventions or the laboratory context. But, if we compare Figure 13.2 with Figure 13.1, we note an important difference in the way the structural formulae are presented. As Reference Laszlo and KleinLaszlo (2001) remarks, to the eye of the present-day chemist, the structural formulae found in Figure 13.2 and similar publications look like primitive attempts to capture the spatial arrangement of chemical substances. But this is a historical mirage. The way in which late nineteenth- and early twentieth-century chemists used structural formulae, Laszlo argues, was primarily to relate their experimental investigations to the edifice of organic chemistry, to situate new findings in relation to existing work, and to draw the map of relations between chemical substances. The formula ‘spelled out to its proponent a historical account of how it came to be, of how it had been slowly and carefully wrought. A formula was the sum total of the work, of the practical operations, of the inter-relating to already known compounds, which had gone into its elucidation’ (Reference Laszlo and KleinLaszlo 2001: 55). As such, the formula amounted to a kind of ‘condense[d] […] narrative’ (Reference Laszlo and KleinLaszlo 2001: xx), whose history would need to be unpacked by a skilled chemist familiar with the relevant literature.Footnote 4 In other words, the structural formulae of the late nineteenth and early twentieth centuries encoded what Meunier terms ‘research narratives’ – although once again their narrative qualities were not obvious to the non-specialist, and had to be unpacked. It is only on the basis of hindsight, Laszlo says, that present-day chemists might see the structural formulae of the late nineteenth and early twentieth centuries as continuous with those of present-day chemistry. We might even say that these historical research narratives are so thin, bound so tightly into a single plane, that their practically and epistemically significant details cannot easily be recovered by today’s skilled practitioner in synthesis.
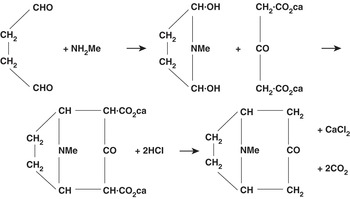
Figure 13.2 Robinson’s original representation of ‘A Synthesis of Tropinone’
Before examining the different styles of thickening, I want to note some of the distinctive uses which a thin narrative could play in the hands of a chemist like Robinson. The same year that Robinson published his laboratory synthesis, he wrote and published a second paper proposing that he might have found a plausible pathway for the formation of alkaloids in living plants. This claim was absent from his first paper, which instead positioned tropinone as a precursor to a number of products of commercial and medical significance. Robinson’s new claim relied on the reaction’s status as a thin narrative. That is, it was a scheme that could be picked up from one context and inserted into another, without changing significantly. Contemporary textbooks show Robinson’s speculations being reported respectfully, and alongside the proposals of other chemists; in the 1910s and 1920s, experimental methods were not available to trace the formation of chemical substances directly. This changed in the early 1930s, with the development of carbon tracing techniques; initially, Robinson’s proposal appeared to have been borne out in practice, although subsequent experimental findings cast doubt on its correctness.
Robinson maintained his distance from experimental attempts to confirm his speculation and was even a little scornful of them. The Australian natural products chemist Arthur Birch, who was at one time Robinson’s student, recalled that Robinson was reluctant to take ‘pedestrian, even if obviously necessary steps beyond initial inspiration’, and would even claim to be disappointed if his findings were confirmed. As a result, ‘if Robinson correctly “conceived and envisaged” a reaction mechanism […] he thought he had “proved” it’ (Reference BirchBirch 1993: 282). For Robinson, the venturesome daring of the thin narratives of organic chemistry was all-important: a way to avoid becoming bogged down in the minutiae of subsequent development.
13.3 The Pot Thickens: Chemists’ Claims
In this section, I will discuss some of the ways in which chemists have sought to thicken the thin narratives described in the previous section, beginning with arguments by the Nobel laureate, poet and playwright Roald Hoffmann. Then I will look at two other sorts of narrative thickening which chemists have employed, which proceed by emphasizing contrastive and contingent aspects of the chemical story.
Roald Reference HoffmannHoffmann (2012: 88) argues that narrative gives a way to ‘construct with ease an aesthetic of the complicated, by adumbrating reasons and causes […] structuring a narrative to make up for the lack of simplicity’. In other words, the interactions between chemical substances which characterize chemical explanations and the decisions of human chemists which impact on chemical research programmes are highly particular, involving contingencies and speculations and evaluations in terms of human interest in order to make sense. Hoffmann aligns scientific narratives with literary ones on three grounds – a shared approach to temporality, causation and human interest – and he particularly emphasizes the greater narrative satisfactions which are often found in oral seminar presentations than in published scientific papers. In drawing his distinction between narrative and information, Hoffman quotes from the philosopher Walter Benjamin: information can only communicate novelty, whereas a story ‘does not expend itself. It preserves and concentrates its strength and is capable of releasing it even after a long time’ (Reference BenjaminBenjamin 1968: 81). In the terms which I am using in this chapter, Hoffmann offers a call for narrative thickening – for getting behind the surface of the conventional chemical article to explain the human dynamics and non-human particularities that have shaped chemical research. In Hoffmann’s view, the role of narratives in chemistry should be taken seriously as a way for chemists to be clearer about how they actually think and work (as opposed to idealizations which would present chemistry as an affair of discovering universally applicable laws). Hoffmann’s position has both descriptive and normative implications. He suggests that if we scratch the surface we will see that chemists do use narratives as a matter of course; but also that if chemists reflect on how they use narratives this will contribute to a better understanding of their work.
‘Classic’ syntheses, like Robinson’s production of tropinone, come to take on the attributes of narratives in Hoffmann’s sense. They are retold for their ingenuity and human interest, to motivate further inquiry, to suggest imitable problem-solving strategies and as part of chemists’ professional memory; some chemists also argue that they are worth revisiting repeatedly to allow new lessons to be drawn. In this sense, they are more like stories than like information, in Hoffmann’s terms. So, for example, the chemists Reference Medley and MovassaghiJonathan Medley and Mohammad Movassaghi (2013: 10775) wrote almost a hundred years after Robinson’s initial synthesis that it had ‘continue[d] to serve as an inspiration for the development of new and efficient strategies for complex molecule synthesis’. The tropinone synthesis has been retold by chemists on a number of different occasions over the past century, and these retellings have drawn out a variety of meanings from the synthesis and related it to subsequent chemical work in a number of different ways. In the process, chemists have used contrasts to emphasize different aspects of the synthesis, or tried to restore contingent historical details or aspects of context which would not be apparent from the elegant, but notably thin, reaction schemes discussed in the previous section.
A similar discontent to the one which Hoffmann expresses with the terseness of conventional chemical publications can be detected in some twentieth-century publications on organic chemical synthesis. Complex, multi-stage syntheses can take many researchers many years to achieve, but the final publication may ignore possible routes which were not taken, or which were successful but proved to be less efficient or in other ways less desirable than the final synthetic pathway. In an article from 1976, the chemist Ivan Ernest explicitly tries to challenge this tendency by reconstructing in some detail the plans which the research group made and the obstacles which led them to give up the approaches which had initially appeared promising. Rather than presenting the final synthesis as an edifice which could only adopt one form, this method of presentation emphasizes the chemists’ decision-making, and the interaction between their plans and what they found in the laboratory. And rather than presenting the structural formulae of the reaction sequence simply as stepping stones towards a pre-determined end, Ernest’s article (1976) emphasizes that each stage of the synthesis should be considered as a node, a moment when several different decisions may be possible. Like Hoffmann’s view of narrative in chemistry, Ernst’s article emphasizes contingency and the human interest of chemical decision-making in the laboratory, giving a more complex and nuanced human story about what this kind of experimentation involves. In other respects, though, it does not diverge significantly from the conventional presentation of thin chemical narratives – it is still presented chiefly in the form of structural formulae, and its presentation is based chiefly on laying different routes alongside each other, giving additional clarity to the decisions made in the final synthesis by comparing it with paths not taken – what could have happened but did not. I call this contrastive thickening because it contributes to the scientific explanation by allowing for a contrast between the final decisions which were made and other paths which could have been taken. Every event in the narrative thus exists in the shadow of some other possibility; what did happen can be compared with what did not.
Beyond telling different ways in which things can happen, chemically, to allow the desired outcome to be reached, contrastive thickening also introduces a different way of thinking about the shape of the whole synthesis and what motivates the relations between its different stages. For example, when Robinson’s reaction scheme for synthesizing tropinone is contrasted with that proposed by German organic chemist Richard Willstätter in 1887, contemporary chemists evoke notions of ‘brute force’ and an ‘old style’ of synthesis to describe Willstätter’s approach. Robinson’s scheme contrasts as a far more efficient experimental methodology, and the first glimpse of a more rational approach towards synthetic planning, which is based on starting with the final form of a molecule and then dividing it up.
Contrastive thickening tries to show that the final form of a chemical synthesis could have turned out differently, but does not make significant changes to the terse manner in which chemical syntheses are presented – Ernst’s article is still narrated primarily in ‘chemese’. Contingent thickening, in contrast, proceeds by fuller narration. For instance, Ernst’s sense that conventional publications on synthesis failed to give the whole story was also cited as inspiration in the first volume of the book series Strategies and Tactics in Organic Synthesis, a collection of papers in which chemists were invited to reflect on the contingencies, human factors and tangled paths of their experimental work. The chapters adopt an avowedly narrative style, and emphasize the prolonged difficulty of synthetic work as well as its eventual achievements. Details include serendipitous discoveries in the chemical literature; and discussions of sequencing syntheses so that their more tricky or untested parts are not attempted at the end, putting previous work into jeopardy. These narrative approaches are intended to stir reflection on problem-solving, and how chemists do not rely on the formal language of structural formulae and planning primarily in their synthetic work. They also share with Hoffmann the goal of keeping chemists motivated and the less codifiable aspects of synthetic knowledge in clear view. The Harvard chemist E. J. Corey writes in his preface to the third volume of the series that
the book conveys much more of the history, trials, tribulations, surprise events (both negative and positive), and excitement of synthesis than can be found in the original publications of the chemical literature. One can even appreciate the personalities and the human elements that have shaped the realities of each story. But, above all, each of these chapters tells a tale of what is required for success when challenging problems are attacked at the frontiers of synthetic science.
In Corey’s view, it is easy to think of synthetic chemistry as ‘mature’ because it has grown more ‘sophisticated and powerful’ over the past two centuries. But the impression of maturity belies the fact ‘that there is still much to be done’ and that the ‘chemistry of today will be viewed as primitive a century from now’. As such, it is important that ‘accurate and clear accounts of the events and ideas of synthetic chemistry’ should be available to the chemists of the future, lest they be misled into thinking that chemistry has become routine. Thickening, in this contingent form, reintroduces research narratives alongside the thin narratives of nature for the benefit of the discipline of chemistry: motivation and inculcation of junior researchers into the culture of synthetic research.
13.4 Analysts’ Narratives: Processual and Contextual Thickening
I now want to discuss two other ways of thickening chemical narratives, which I will call processual and contextual. Whereas the thickenings discussed in section 13.3 have been developed by chemists themselves, accounts of processual and contextual thickening have been developed primarily by analysts of chemistry – philosophers, and historians and social scientists, respectively. The primary goal of these thicker accounts is not to offer a more complete record of laboratory activity in order to assist with chemists’ own activities, but rather to move beyond the plane of the reaction in selecting what requires consideration in recounting chemical processes. Processual and contextual thickenings work to shift the focus of chemical narratives – calling into question the range of humans and non-humans who should be considered as the primary agents of chemistry, the actions and motivations which are held relevant and worthy of discussion, and the locations in which chemistry occurs. These ways of thickening, furthermore, open up the notion of chemical beginnings and endings by raising questions of how some chemical substances come to be available for chemists to study, and of what happens to chemical substances after chemists have finished using them.
To start with processual thickening, then. Some philosophers of chemistry have offered ‘process ontologies’, guided by the view that philosophy should give accounts of processes and the dynamic aspects of being. As the science of transformations of matter, chemistry can be treated in such dynamic terms, which also call into question the seeming fixity of the substances which chemists employ. These arguments proceed from two related claims: first, Gaston Bachelard’s (Reference Bachelard1968: 45) view that the substances that chemistry studies require extensive purification, and hence ‘true chemical substances are the products of technique rather than bodies found in reality’. In this view, the artificiality of chemical substances used in the laboratory circumscribes the types of stuff which are amenable to chemical analysis – samples taken from the messy world are therefore to be understood to the extent that they conform to what chemists can do with their artificial materials. The second claim is that, in the words of A. N. Reference WhiteheadWhitehead (1978: 80), ‘a molecule is a historic route of actual occasions; and such a route is an “event”’. What Whitehead meant was that the chemist’s molecules arise from sequences of specific actions, whether constructed in the laboratory or found outside. So chemistry deals, above all, with processes – which may be occurring on different scales – rather than with fixed substances.
In his metaphysics of chemistry, the chemist Joseph Earley builds on these insights to claim that chemical substances are historically evolved, in the manner of other evolved systems, and have a vast array of potentials, but that in practice these are subject to considerable path dependencies, as ‘[e]very sample has a history (usually unknown and untold) that specifies its current context and limits the range of available futures’ (Reference Earley, Fisher and ScerriEarley 2015: 226). In broad terms, Earley is observing that material history and institutional constraints matter for the definition of chemical substances – and this sounds like he is calling for thicker narratives of chemistry, which take these other factors into account. But, while his philosophical arguments can be read in this way, he also cautions that many of the relevant histories of chemical substances used in the laboratory are ‘unknown and untold’, and that to the extent their origins are unknowable it is not possible to construct narratives about them. This view suggests the need for a measure of caution concerning the extent to which narratives of chemistry can be thickened to incorporate all the relevant contributory historical factors. Although this chemical metaphysics might sound like an abstract warning, it touches on some of the factors which are described in the Strategy and Tactics research narratives – especially the impact on synthesis efforts of which materials happen to be locally obtainable.
I quoted from Birch’s prolonged investigation into Robinson’s synthesis earlier in this chapter; now I want to say a bit more about what he was trying to achieve and how attending to the contingent history of Robinson’s materials helped him to do so. Birch had trained as an organic chemist but made his professional career as a biochemist. He was extremely sensitive to the differences in method and experimental technique between organic chemistry and biochemistry, and suspicious of attempts to claim that practitioners from the two fields could talk straightforwardly to each other, without taking such differences into account. As part of this wider argument, Birch condemned what he characterized as the mythology which had grown up around Robinson’s synthesis – particularly the claim that Robinson had been inspired primarily by an attempt to imitate the natural process by which plants synthesize alkaloids. In an effort to challenge this narrative, Birch interrogated its chronology, drawing on both documentary and material evidence. He noted that Robinson had been interested in a somewhat similar synthesis some years earlier, as a result of a theoretical interest in the structure of alkaloid skeletons which he had developed in discussion with his colleague Arthur Lapworth. Robinson’s initial experimental work for a one-pot type of synthesis, Birch showed, had taken place when he was based in Sydney. Birch even succeeded in tracking down the original bottle of one of the chemical reagents which Robinson had employed in his experiments. As a result, argued Birch, Robinson’s motivations for attempting the tropinone synthesis could not be reduced straightforwardly to an attempt at bio-mimicry, and the synthesis should not be remembered as a precursor to a subsequent unification between organic chemistry and biochemistry. As Birch wrote, ‘the chemist’s natural products […] tend to mark the diversity of organisms by their sporadic occurrences, whereas the biochemist’s materials tend to represent the unity of living matter’; as a result ‘the biochemists in a search for generalities have largely ignored the chemist’s compounds’ (Reference BirchBirch 1976: 224). Digging into Robinson’s legacy, and locating it within the distinct material and processual culture of organic chemistry, gave Birch a way to demonstrate the tensions between different chemical subfields, their different ways of proceeding and the different entities which they considered. Birch also noted that Robinson’s programme in Sydney may have been guided in part by the difficulty of obtaining chemical supplies in the early part of the twentieth century, and chemists’ needs to improvise on the basis of the materials which were locally obtainable. As he noted, most chemical supplies ‘came from abroad and normally might take up to six months to arrive for use’, with the result that ‘[s]ynthetic programmes tended to be organized around what was already in the store,’ and ‘[m]uch early Sydney work was on natural products which grow in the local Bush’ (Reference BirchBirch 1993: 286).
The intention of Birch’s historical narrative is to recover different conceptual and material sources for Robinson’s synthetic work, and to caution against too close an equation between the practices of synthetic organic chemistry and those of biochemistry. He draws attention to the material constraints which bounded some of Robinson’s synthetic decision-making, but which are absent from the published research narrative. In the process, he draws attention to the specificity of the molecular cast of characters involved in organic chemical synthesis. These moves all recall the aspirations of contingent thickening, described above; but they also suggest a wider set of material and conceptual constraints which might need to be incorporated into an account of how chemists make their decisions. These wider questions are consistent with the goals of processual thickening, even though their intent is not philosophical.
Like processual thickening, contextual thickening tries to give chemical narratives depth beyond the laboratory; but it goes beyond material and processual contingencies to explore how chemists’ scientific activities might be informed by social, political, historical and environmental dynamics. This kind of thickening thus often shifts chemists away from the centre of accounts of chemistry in favour of other human users of chemical substances (the farmer who employs pesticides, the sunbather with her suntan lotion), the ways in which chemical substances interact with non-humans, and of the complex, ambivalent meanings associated with relations with chemical substances. In general, the goal of such studies is critical – to look beyond the way chemists think about their materials and the impacts of their activities, and to understand chemical substances not as ‘isolated functional molecules’, but rather in terms of ‘extensive relations’, as the historian Michelle Murphy puts it (Reference Murphy2017: 496). What Murphy means is that chemists’ own evaluations of the impacts of chemical substances are too limited and limiting, and are insufficiently attentive to the myriad roles which such substances play.
Again, some retellings of Robinson’s tropinone synthesis enact a kind of contextual thickening, by showing that his work was not guided solely by scientific considerations, and nor by the material constraints identified by Birch. In Robinson’s own memoir, written late in his life, he talked about what had been happening in his laboratory when he conducted experimental work for the tropinone synthesis at Liverpool University during the First World War. This was a time when the British government had taken a great interest in the utilization of chemical waste products, and the university’s laboratories had been turned over to an effort to make pharmaceuticals from the chemical residues of manufacturing explosives. At Liverpool, they made large quantities of the painkillers beta-eucaine and novocaine by saturating acetone with ammonia; the process was improved by adding calcium chloride. Sludge produced by washing explosives with alcohol was brought from the TNT factory at Silvertown and kept in buckets underneath the laboratory benches. Robinson’s colleague, the Reverend F. H. Gornall, derived useful intermediates from these wastes and analysed their chemical properties. According to Reference RobinsonRobinson (1976: 107), ‘The improvisation of suitable apparatus required for this work, and the necessity for careful operation and control, was found to be a good substitute for the conventional courses’. Robinson was learning too, and by his own account returned to his own earlier experimental work from Sydney. Among the substances which the chemists sought to produce was atropine, an alkaloid which was closely related to tropinone and which was used in the treatment of people who had been exposed to poison gas. As Robinson recounted:
Atropine was in short supply during the First World War and the knowledge of this fact led me to recall that I had contemplated in Sydney a synthesis of psi-pelletierine from glutardialdehyde, methyl-amine and acetone. This idea was a possible extension of pseudo-base condensations and I realised, at Liverpool, that a synthesis of tropinone […] might be effected in a similar manner, starting with succindialdehyde, and tropinone could probably be converted to atropine without difficulty.
There is no evidence that Robinson was able to produce significant quantities of atropine for the British war effort, and his synthetic technique would have been unable to produce large quantities of atropine in any case. But, in this telling, a part of his motivation for returning to this synthesis at this time was that the historical and institutional imperatives brought about by wartime restrictions made the pursuit of a highly efficient synthesis more desirable.
13.5 Conclusion: Unfinished Syntheses
This chapter has drawn a distinction between the use of thin and thick narratives in organic chemical synthesis. Thin narratives allow explanations to be given in a terse form which is portable and not dependent on a particular setting or set of historical circumstances; the four styles of thickening identified here all add depth to the apparent planar self-evidence of thin narratives by exploring the role of unsuccessful lines of research, contingencies, the processes by which substances become available for chemical inquiry, or the relations between chemical syntheses and wider historical, political, environmental and material contexts.
Read alongside the other chapters of this book, I hope that the discussion here clarifies some of the ways in which scientists use narratives. As with geological features, chemists sometimes revisit past synthetic achievements, open them up and unpack their implications. Some of these implications may not have been obvious when a synthesis was first conducted, and for this reason some classic syntheses have the inexhaustible, unfinished quality which Roald Hoffmann associates with stories, in contrast with information. Of course this attitude towards the potentials of past experimental work is not present among all chemists and is not applied to all syntheses. But when chemists do draw upon past experimental achievements or reflect on the ways in which the activities of chemistry ought to be documented, they talk quite often in terms of narrative, and with an explicit awareness of the shortcomings of conventional modes of chemical publication – with the sense that the terse formal languages of chemistry fall short in describing how chemists work and think. Much academic history and public discussion of chemical synthesis has focused on the ways in which synthetic decision-making can be made routine, guided by artificial intelligence and planned using the powerful ‘paper tools’ of chemical nomenclature and structural formulae. Although such an emphasis correctly identifies a major strand in the chemical synthesis of the last 60 years, it has also often been balanced (as in the writings of E. J. Corey, quoted above) with a sense of the abiding complexity and role of contingency which are involved in chemical syntheses. The suggestion here has been that thinking about the difference between thin and thick narratives is a way to preserve a sense of the significance of the two aspects of chemical synthesis.
In Ted Porter’s contrast between thick and thin descriptions, which I quoted in the introduction to this chapter, thickness indicates the complex, contingent, often intractable world, whereas thinness stands for attempts to corral that world into predictable shape. As chemistry deals with processes which are often complex, contingent and intractable, it is perhaps unsurprising that alongside its very robust reaction schemes there should be repeated calls for thickening – ways to put the world back in. It is important to note, however, some of the differences, and possible overlaps, between the different styles of thickening which I have identified. Because processual and contextual thickening emerge chiefly from analysts’ accounts and chemists’ historical writings rather than chemical research publications, it is tempting to see them as offering different forms of narrative to those discussed in sections 13.2 or 13.3. I will first give some reasons that we might want to draw such a division, in terms of the familiar distinction between ‘internalist’ and ‘externalist’ accounts of science, and then indicate the reasons that even though this contrast is suggestive, it is also somewhat misleading. As a first approximation we might associate thin synthetic narratives, and chemists’ own contrastive and contingent thickenings, with internalist accounts of chemistry, which seek to understand the development of the science in its own terms, according to its conceptual and experimental developments, but without reference to a larger historical context. Processual and contextual thickenings, on the other hand, might be aligned with an externalist view of the history of chemistry, which seeks to understand what chemists do and the significance of their narratives as emerging from and as feeding back into a wider array of political, social, material, philosophical and environmental considerations. We might therefore say that such externalist perspectives are proper to the narratives of philosophy, of social science, or of history – in other words, that the problems they seek to solve belong to these different disciplines, rather than to chemistry itself. So the information in Robinson’s memoir might be useful to a historian constructing an account of the relations between wartime production restrictions and innovations in chemical research, but it would be much less likely to be of use to a chemist trying to develop a new synthesis.
Chemists’ use of narratives also has implications for critical analyses of chemistry, especially the work associated with the recent ‘chemical turn’ by social scientists. The goal of these studies, exemplified earlier in this chapter by Michelle Murphy’s work, has been to show the pervasive importance and ambivalent significance of chemical substances for both humans and non-humans, and chemicals’ roles in sustaining ways of life as well as causing harms through pollution, poisoning and addiction. In the process, these studies have shifted focus away from laboratories and towards the myriad settings in which chemicals are found and play an active role. In taking the significance of chemistry away from the chemists, however, and displacing the locus of chemical study from laboratories, such studies may also fail to account for the distinctive terms in which chemists understand their science, and narrate their activities. Given the density and complexity of organic chemists’ language, there are still few historical and social scientific studies which follow their distinctive practices of narrative ordering in significant detail, or which pursue the retellings of a single chemical synthesis, as I have tried to do here. There are not straightforward ways to incorporate the perspectives of the producers and users of thin chemical narratives into thicker accounts of chemistry without attempting to learn to speak ‘chemese’; at the same time, chemists themselves sometimes argue for the need to give a fuller, thicker, account of their activity.Footnote 5





