Background
In 2001, the National Center for Research Resources mandated that the General Clinical Research Centers create a research subject advocate (RSA) role “to enhance the protection of human participants in clinical research studies” [Reference Easa1]. In 2008, the establishment of the Clinical and Translational Science Award (CTSA) consortium shifted the focus from an individual advocate role to programs that safeguard and promote the ethical and safe conduct of clinical research [Reference Winkler2]. The founding members of the New England Research Subject Advocacy Group (NE RSA—Harvard Catalyst [3], Boston University Clinical and Translational Science Institute (CTSI) [4], and Tufts University CTSI [5]) recognized the need to enhance education and understanding of potential research participants and to address poor health communication and inadequate health literacy. The NE RSA group expanded to include representation from the University of Massachusetts CTSA [6] (2010), Dartmouth Synergy [7] (2013), Yale Center for Clinical Investigation [8] (2014), and the Canadian institution Hôpital Montfort (Ottawa) (2015). The group, supported by the participating institutions, engages in inter-institutional and cross-disciplinary collaboration, innovation, community outreach, and research related to participant advocacy. The NE RSA aims to support researchers and participants by increasing awareness and understanding of clinical research and thereby to enhance research capacity, both locally and nationally.
One primary focus of the NE RSA is the collaborative development of materials for community members and prospective research participants, including diverse and underrepresented populations, who may not otherwise have access to clear, high-quality information about clinical research and research participation. The NE RSA first developed materials in partnership with a regional collaboration of CTSA community engagement programs; together the 2 groups created the brochure, “Should I be a Research Subject?” (2011) and a “Research Subject Bill of Rights” (2012). At the request of leadership from Harvard Catalyst’s affiliated human research protection programs (HRPPs) and institutional review boards (IRBs), and recognizing the value of collaboration, shared resources, and cohesive messaging, the NE RSA developed additional materials between 2014 and 2017; these brochures addressed different types of research (e.g., genetic research; social, behavioral, end education research), common research procedures (e.g., blood draws; imaging), and special topics (e.g., incidental findings; surrogate consent for research) for the research community and community at-large. Subsequent topics were and continue to be identified through external input channels (e.g., requests from investigators and research staff, feedback from affiliated HRPPs and community members), and then selected through group discussion and prioritization by Harvard Catalyst RSA Advisory Board and NE RSA.
Iterative and Agile Collaborative Materials Development
The NE RSA identifies potential areas of focus through ad hoc consultations with and requests from researchers, research staff and administrators, CTSA leadership, past research participants, and community members as well as through monthly meetings of regulatory leadership, quarterly meetings of an RSA advisory board, and regular input from leadership across participating institutions. New topics have been identified through feedback received at local presentations to the research community (e.g., a need for information on the use of telemedicine in research), direct requests from investigators working with less common procedures (e.g., use of Transcranial Magnetic Stimulation and Transcranial Electrical Stimulation for research), identification of common research procedures (e.g., MRI, PET, and CT scans for research), and special topics (e.g., surrogate consent for research) requested by research staff, IRBs/HRPPs, and community members. The NE RSA then develops topic-focused brochures through iterative and collaborative drafting and revision in consultation with local content experts (Fig. 1). All resources are reviewed and revised by experts in health literacy and plain language as well as by institutional communications departments. Although the NE RSA materials are informational and not focused on recruitment, all draft documents are provided to participating institutions’ HRPPs for review and comment before being finalized; these HRPPs have determined that, as informational materials, the brochures do not require specific (e.g., per-protocol) IRB review and approval for use and distribution. After content and design are finalized, resources are posted to the NE RSA members’ respective CTSA Web sites in a downloadable format and printed for distribution to affiliated research programs and at community events. Brochures are then professionally translated into over a dozen languages.
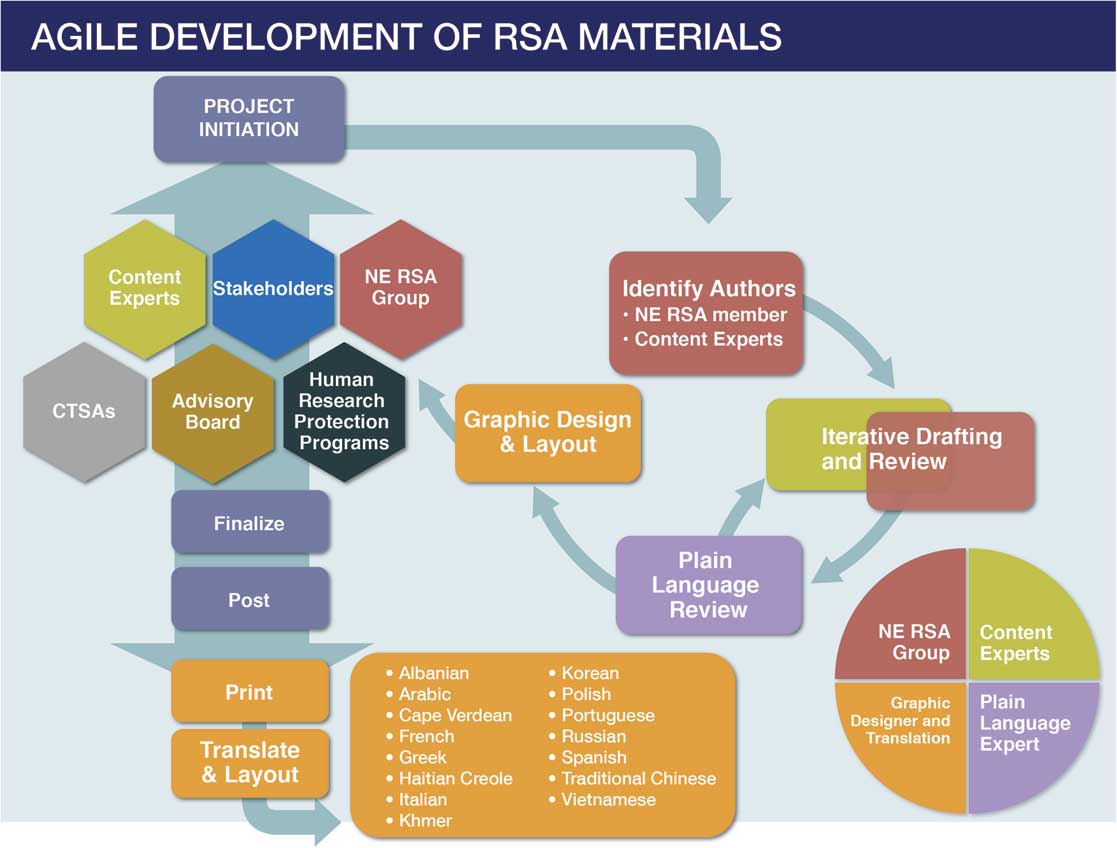
Fig. 1 Agile development of Research Subject Advocacy (RSA) materials. CTSA, clinical and translational science awards; NE RSA, New England Research Subject Advocacy.
The main financial investment for development of these materials is incurred through plain-language review, translation, and graphic design. The NE RSA contracts with experts in health literacy—freelancers, private contractors, and the Center for Information and Study on Clinical Research Participation (CISCRP)—to transform technical language into scientifically accurate, nonpromotional plain language, validated at a 6th–8th-grade reading level [9]. Materials are translated into an additional 15 languages (Table 1) selected by highest need based on the 2008 Massachusetts Department of Public Health report, “Interpreter Services in Massachusetts Acute Care Hospitals” [Reference Simpson and Destine10]. The group will revisit the selected languages when updated publications of this and/or similar reports become available. Translations are contracted to selected companies whose foreign language translation specialists are native speakers and who specialize in translating health-related materials. Whenever possible, the translated brochures are then read by native speakers both from the NE RSA community and abroad. The NE RSA utilizes outside graphic designers to format the layout, branding, and messaging of the materials consistent with health literacy principles. Additionally, each brochure is designed to provide space for research teams to list study-specific contact information. Nonmember institutions that wish to use the materials may contact the NE RSA to request access to the native files so that they may include their logos and/or appropriate contact information.
Table 1 Languages of translation of resource library

The development of this resource library is made possible through the shared support and investment of each participating CTSA. One institution may invest more in design and translation, another in content development, and another in distribution through hosting or attending community events. Members also share local resources and educational opportunities. For example, a series of informational videos for prospective research participants and of training videos for research staff, developed by Harvard Catalyst, are now freely available. Developed by Boston Children’s Hospital, Cincinnati Children’s Hospital Medical Center, and the Children’s Hospital of Philadelphia, a full-color comic book designed to help children understand medical research and the informed consent process can be freely downloaded. The development of the informational materials depends upon the contributions of numerous volunteer content experts from partner institutions and feedback from members of the community.
A Growing Library of Informational Brochures
The materials are designed as informational resources and the anticipated use is as a tool to help empower prospective participants and their families by enhancing their comfort and communication with investigators and research teams before, during, and after the informed consent process. Each brochure provides concise, high-level information specific to the topic and contextualized for research, along with visuals and a series of questions to think through and to ask before deciding whether or not to participate in a study and at any time over the course of a study, giving readers a foundation for conversations with researchers and staff, their doctors, families, and others. Materials emphasize the voluntary nature of research, while also describing the constraints that may occur during the research process. In the drafting and editing of each brochure, language that might introduce or reinforce therapeutic misconception—or otherwise conflate research with healthcare—is carefully avoided. For example, brochures focusing on common procedures focus in particular on the unique aspects of undergoing these procedures in the context of a research study, rather than as part of one’s personal medical care.
To date, the group has developed an open-access library of 23 informational brochures (Table 2), with additional brochures in development. Harvard Catalyst hosts the brochures on its Web site on behalf of the NE RSA institutions (www.catalyst.harvard.edu/services/rsa); participating institutions may then link directly to these files. These materials provide generalizable information about clinical research and research procedures to ensure their utility for a broad audience. If research teams opt to customize or supplement the materials with additional information that addresses more specifically the details of a given study, submission to the cognizant IRB for review and approval should be considered.
Table 2 Download metrics (to end 2017) of currently available brochures
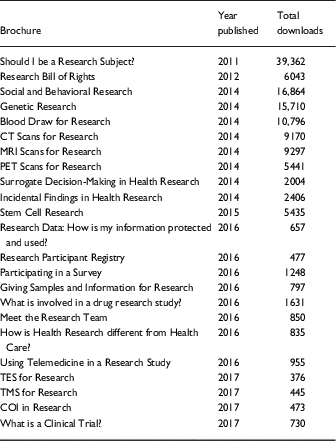
CT, computed tomography; COI, conflict of interest; MRI, magnetic resonance imaging; PET, positron emission tomography; TES, transcranial electric stimulation; TMS, transcranial magnetic stimulation.
Webtrends, an analytics interface, counts transactions by using the weblogs from the Harvard Catalyst webserver. Between 2011 and 2017, the brochures available in the library were downloaded 132,002 times (Table 2). Brochures are posted on a rolling basis as they are finalized. Since inception, the most downloaded brochure has been one of the earliest developed and posted, “Should I Be a Research Subject?,” (2011) followed by the “Social and Behavioral Research” (2014) and “Genetic Research” (2014) brochures. To date, all of the brochures have been translated into an additional 15 languages; the 3 most frequently downloaded languages are Vietnamese, Spanish, and Korean.
Limitations and Next Steps
While written materials provide clear information that participants can take home, review, and share, the NE RSA recognizes that limited literacy and variable learning styles require additional approaches to sharing information. Acknowledging that resources are limited, the group hopes to adapt and develop new content for other media, including video, podcasts, and audio. Our sense is that the visuals embedded in the brochures are helpful, giving potential participants an idea of what an MRI machine looks like or what to expect when undergoing Transcranial Magnetic Stimulation as part of a research study.
We have had positive but limited—and informal—assessments of the utility and impact of these materials on the intended audiences, including research teams and potential participants. A brief evaluative survey, primarily intended to guide future group efforts, was posted for several months on the page through which one downloads the material, but completion of the survey was relatively low as compared with overall downloads, and feedback generally focused on requests for additional resources. We have also solicited feedback at events (e.g., the annual conference of the International Association of Clinical Research Nurses), and plan to engage in more systemic collection of data from investigators and research staff, research participants and their families, and the general public to assess the overall impact of the materials and guide future versions of the brochures. Each brochure is reviewed annually by members of the NE RSA, or more often if indicated, to identify any changes required to retain accuracy or potential updates to improve user comprehension.
The NE RSA team has paid particular attention to the inclusion of diverse caregiver and participant images, but more can be done. Were these brochures adapted and utilized internationally, as we hope that they will be, further representation of local populations may be appropriate, and the materials can be made available for customization of this type. Further, we note that, while translations have been professionally executed, local dialect or use of terms may differ from country to country, as well as from county to county. We welcome feedback on these materials, and invite collaborators to (1) translate any or all of the brochures into additional languages (or correct the versions posted), (2) appropriately modify the pictures that are represented, with permission from the subjects (and photographers) for further distribution, (3) suggest additional topics that may be of service to the research community, (4) test utility and impact of the materials on the intended audiences to enable data-driven improvements in the products, and (5) broadly communicate the availability of these materials to research and participant communities. We request, however, that feedback, modifications, further translations, or similar brochures are shared with us so that we can, in turn, improve the materials and make them widely available.
We believe that these materials are helpful, particularly in light of changing regulations focused on the presentation and provision of information to prospective research subjects, and that their use may enhance basic understanding of clinical research and research procedures, an understanding that is more general than that required of any specific research protocol. We understand research teams often do not have the time to explain, review, and re-review these fundamentals, many of which could be communicated well in advance of potentially recruiting individuals to participate in research. It would be appropriate, for instance, for patients entering a comprehensive cancer center to receive information about clinical research, one’s rights as a research participant, blood draws for research, biobanking, etc., well in advance of requesting participation or any contribution by or from the patient. A thoughtful introduction would allow patients to understand how the determination of safety and efficacy of their own medical regimen rests on the voluntary and generous contributions of others that preceded them. Such an approach would allow patients to become familiar with clinical research at a time when they are simply an observer (and beneficiary), before facing any personal decisions regarding participation. We encourage commitment to plain language, the utilization of the materials that we have generated, and experimentation with these and other modalities of communication for the benefit of participant—and patient—understanding and engagement.
Conclusion
Member CTSAs and their affiliated institutions, investigators and staff, research participants, and the broader community have contributed to and benefitted from these collaborative efforts to provide quality, high-level, and plain-language information about research participation. Importantly, cooperation across the CTSA network, both within and beyond the region, avoids duplication of effort, improves the quality of the final materials, and achieves economies of scale, while providing consistent, cohesive messaging to support, empower, and inform research participants, patients, and the broader community.
Through a process of stakeholder input and collaboration, individual brochures are selected for production by the NE RSA group from which one or more primary authors are identified. Additional content experts from the community, IRB professionals, and others review, modify, and improve the content, after which the draft is reviewed for conformance with plain language and submitted to graphic design. The printed brochure, in English, is deployed and, if necessary, additional changes made before professional translation into an additional 15 languages.
Acknowledgments
The authors thank the community of volunteers who contributed to and guided the selection of topics and the drafting and review process, as well as the numerous content experts at our affiliated institutions, who helped draft and edit the content of the brochures. We specifically thank Amy West of Tufts CTSI who provided graphic design advice; Farrah Belizaire, formerly of Boston University, who worked to develop content and distribute the brochures at local community fairs; Ann Olgetree who provided plain-language review for many of our initial brochures; Susan Kornetsky and collaborators for the generation of the comic book for children; and Maria Cervone and Genoa Polumbo of Harvard Medical School who helped compile the download numbers described in this paper.
Financial Support
This material is the work of the New England Research Subject Advocacy Group, with contributions from member institutions’ affiliated universities and academic healthcare centers. This work was conducted with support from the National Center for Advancing Translational Sciences, National Institutes of Health, through: Boston University Clinical and Translational Science Institute, under award number 1UL1TR001430; the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086; Harvard Catalyst | The Harvard Clinical and Translational Science Center, under award number 8UL1TR000170-05; Tufts Clinical and Translational Science Institute, under award number UL1TR001064; UMass Center for Clinical and Translational Science, under award number UL1-TR001453; and the Yale Center for Clinical Investigation, under award number UL1TR001863. Its contents are solely the responsibility of the authors and do not represent the views of the institutions or the National Institutes of Health.
Disclosures
The authors have no conflicts of interest to declare.







