Introduction
In Serbia, pigs raised in the backyard on individual farms are still a source of Trichinella outbreaks. In most cases, small family outbreaks occur due to the consumption of uninspected Trichinella spiralis infected meat and meat products. The clinical signs of trichinellosis could vary from very mild (almost asymptomatic) to severe forms of the disease, but sometimes the diagnostic process can be complex especially for atypical clinical courses or sporadic cases (when the consumption of small amounts/low infected meat takes place over the time) (Gottstein et al., Reference Gottstein, Pozio and Nöckler2009). A definitive diagnosis of trichinellosis is always based on positive results of highly specific immunodiagnostic tests (Dupouy-Camet & Bruschi, Reference Dupouy-Camet, Bruschi, Dupouy-Camet and Murrell2007). Sometimes, in addition to a positive test for Trichinella, the results of serological tests for other parasites can also be positive, so the final diagnosis of the disease can be delayed. This usually occurs in isolated cases of trichinellosis without typical signs and symptoms, because some of the non-specific symptoms could be common for infections with various parasites, so physicians require serological tests for different parasites to make a definitive diagnosis. A similar case is with Toxocara canis infection. Most infections remain asymptomatic (Macpherson, Reference Macpherson2013), whereas several of them are clinically evident as visceral or ocular larva migrans syndrome. Parasitic infections with T. spiralis or/and T. canis could provoke at least some number of similar symptoms including eosinophilia, fever and myalgia, and consequently both diseases should be considered in the differential diagnosis, especially when the presence and distribution of both parasites in a certain area have been recognized by epidemiological studies (Sofronic-Milosavljevic et al., Reference Sofronic-Milosavljevic, Djordjevic, Plavsic and Grgic2013; Gabrielli et al., Reference Gabrielli, Tasic-Otasevic, Ignjatovic, Fraulo, Trenkic-Bozinovic, Momcilovic and Cancrini2017). An enzyme-linked immunosorbent assay (ELISA) based on excretory–secretory (ES) antigens of T. canis and Western blot (Wb) are most commonly used to diagnose human toxocariasis (Magnaval et al., Reference Magnaval, Glickman, Dorchies and Morassin2001; Iddawela et al., Reference Iddawela, Rajapakse, Perera and Agatsuma2007). Limitation of T. canis testing by ELISA relays in the significant cross-reactivity with other helminths, such as Trichinella, Strongyloides (Jacquier et al., Reference Jacquier, Gottstein, Stingelin and Eckert1991), Fasciola, Ascaris, geohelminthes (Magnaval et al., Reference Magnaval, Fabre, Maurieres, Charlet and de Larrard1991; Romasanta et al., Reference Romasanta, Romero and Arias2003; Mohamad et al., Reference Mohamad, Azmi and Noordin2009) and filariasis (Mohamad et al., Reference Mohamad, Azmi and Noordin2009). However, despite the possible false-positive results, these tests are of clinical importance. A positive ELISA test for T. canis should be checked with confirmatory immunoblot analyses (Wb), especially if the clinical findings are not consistent with a positive test result (Magnaval et al., Reference Magnaval, Fabre, Maurieres, Charlet and de Larrard1991; Fillaux & Magnaval, Reference Fillaux and Magnaval2013). Confirmatory Wb is recommended because it improves sensitivity and specificity of diagnosis (Magnaval et al., Reference Magnaval, Fabre, Maurieres, Charlet and de Larrard1991; Fillaux & Magnaval, Reference Fillaux and Magnaval2013). In Serbia, a small number of laboratories offer ELISA diagnostics of toxocariasis, while the Public Health Institute Nis (PHIN) applies both types of tests. The most often used techniques for Trichinella infection screening are the immunofluorescence antibody assay (IFA) and ELISA (Gamble et al., Reference Gamble, Pozio, Bruschi, Nöckler, Kapel and Gajadhar2004; Dupouy-Camet & Bruschi, Reference Dupouy-Camet, Bruschi, Dupouy-Camet and Murrell2007; Bruschi et al., Reference Bruschi, Gómez-Morales and Hill2019). The simultaneous use of both tests has the added advantage of achieving early detection of trichinellosis (Costantino et al., Reference Costantino, Malmassari, Dalla Fontana, Diamante and Venturiello2001). Since trichinellosis as a health, social and economic problem in Serbia persisted for several decades until recently (Mitic et al., Reference Mitic, Vasilev, Korac, Ilic, Bojic, Gruden-Movsesijan and Sofronic-Milosavljevic2022), the number of diagnostic laboratories that use one of the two mentioned tests has branched out. However, the application of the confirmatory Wb test (in addition to the application of INEP's registered ELISA and IFA tests) is in use only in the National Reference Laboratory for Trichinellosis – INEP (NRLT INEP). In 2014, NRLT INEP in Serbia was contacted by the PHIN, to help the establishment of diagnosis in six patients positive for the anti-T. canis antibodies and to validate the serological findings for patients suspected for trichinellosis during two T. spiralis outbreaks in Serbia. Thus, the aim of this study is to present data regarding 24 patients who were involved in two out of seven outbreaks that occurred in Serbia in 2014. Various serological tests performed at NRLT INEP showed that six Toxocara-positive persons were actually infected with Trichinella and were part of the second outbreak.
Material and methods
Trace back study
In August and September 2014, the Medical Health Center Zaječar (MHCZ), Department of Infectology informed the PHIN about a possible infection of Trichinella spp. in patients. During the examination, all suspected patients in August provided the information that they consumed meat from pig slaughtered on 4 August 2014, and a few days later they ate sausages and dried meat products made from the same meat. All patients in September consumed raw pork sausages bought from a local butcher at the end of August 2014.
Patients
Twenty-four patients with possible trichinellosis were examined in the MHCZ. Ten patients were part of the first outbreak, the onset of which took place at the beginning of August 2014. Fourteen patients were included in the second outbreak. the onset of which started at the end of August 2014. Data on diagnosis and treatment were collected retrospectively from the patients’ medical records.
Laboratory testing
The patients’ blood samples were taken at the MHCZ approximately in the third week after consuming infected pork. Due to the fact that the PHIN is based in the city of Nis, the third largest city in Serbia, and represents an important regional health centre focused on protecting and improving the health of the population of southeastern Serbia, physicians from MHCZ have sent a request to the PHIN for determination of the presence of antibodies to Trichinella and Toxocara in collected sera. After testing, all sera samples were sent to NRLT INEP for analysis. The presence of anti-Trichinella antibodies was performed both by IFA (fluorescein isothiocyanate (FITC) Trichinella spiralis Antibody Detection Kit, INEP, Serbia) and in-house indirect ELISA (Radovic et al., Reference Radovic, Gruden-Movsesijan, Ilic, Mostarica-Stojkovic and Sofronic-Milosavljevic2012). Anti-Trichinella antibody titres ≥1:40 were considered seropositive in IFA. Results exceeding 20% were defined as positive in indirect (i-ELISA) (index of positivity percentage (IP%) is calculated based on optical density (OD) measurements using the following formula: IP% = ((OD samples–OD blank)/(OD positive control–OD blank)) × 100). A confirmatory Wb test was performed when the discrepancies of the results between IFA and i-ELISA were obtained. It was proclaimed as positive if three bands (45, 49 and 53 kDa) are visible indicating that ES infective muscle larva (ES L1) products of parasite reacted with antibodies in tested sera as well as with 7C2C5 monoclonal antibodies (MoAbs) (control). The Wb analyses were done as described previously (Radovic et al., Reference Radovic, Gruden-Movsesijan, Ilic, Mostarica-Stojkovic and Sofronic-Milosavljevic2012). Serum samples were analysed in PHIN by IFA (FITC Trichinella spiralis Antibody Detection Kit, INEP, Serbia), ELISA test (NovaLisa Trichinella spiralis immunoglobulin G (IgG) and NovaLisa Toxocara canis IgG, NovaTec Immunodiagnostica GmbH, Germany) and Immunoblot Assay (Toxocara WB IgG, LDBIO Diagnostics, France). Results above 11 NovaTec Units (NTU) were considered positive in the ELISA test.
Parasitology testing
Samples of pork meat and pork sausages were analysed for the presence of Trichinella spp. muscle larvae in the Veterinary Specialist Institute ‘ Zaječar’ and in NRLT INEP with the magnetic stirrer method (European Commission, 2015). Recovered larvae were collected and the worm burden was expressed as larvae per gram (LPG). Multiplex polymerase chain reaction (PCR) analysis accredited by the European Union Reference Laboratory (EURLP) was used to identify Trichinella at the species level (EURLP, Istituto Superiore di Sanità, Rome, Italy) (Zarlenga et al., Reference Zarlenga, Chute, Martin and Kapel1999; Pozio & La Rosa, Reference Pozio and La Rosa2003). At NRLT INEP, the test was performed with slight modifications (Cvetkovic et al., Reference Cvetkovic, Teodorovic, Marucci, Vasilev, Vasilev, Cirovic and Sofronic-Milosavljevic2011).
Statistical analyses
GraphPad Prism 8.0 (GraphPad Software, California, USA) was used for analysing data and drawing charts. The data are expressed as the mean ± standard deviation. Boxes represent the interquartile range and horizontal lines inside each box represent the median. The vertical lines from the ends of each box encompass the extreme data points.
Results
Trace back study
The investigation determined that the source of the infection in the first outbreak was the consumption of undercooked pork, while the infection in the second outbreak was caused by the consumption of raw pork sausages. Smoked sausages, the source of infection in the second outbreak, were made from domestic swine slaughtered in the village of Štubik (20 kilometres away from the city of Negotin), Bor District, and were bought at the end of August from a local butcher.
The first patient from the first outbreak was registered at a doctor's examination on 14 August 2014, while the last case was discovered on 25 August 2014. In the second outbreak, the first patient went for a medical checkup on 6 September 2014, and the last one on 22 September 2014. Symptoms began to appear after an incubation period of ten to 21 days (average value 17 days) in the first outbreak, while in the second outbreak clinical signs appeared after ten to 18 days (average of 14 days). All patients underwent clinical observation and analyses and were treated with albendazole. Eight patients (six from the first and two from the second outbreak) required hospitalization and treatment with corticosteroids. The period of hospitalization ranged between seven and 18 days (average 13 days). Five patients were in the hospital between 16 and 18 days. All patients were clinically monitored during and after treatment and all recovered completely. The timeline with data on consumption of Trichinella infected meat, incubation period, symptoms, hospitalization and serological analysis data are shown in fig. 1.

Fig. 1. Timeline for the two trichinellosis outbreaks in Serbia, 2014, showing data on consumption of Trichinella infected meat, incubation period, symptoms, hospitalization and serological analysis of 15 patients who fulfilled the trichinellosis case definition. Abbreviations: C, consummation of Trichinella infected meat; →, incubation; /\/\/\/\, symptoms; ——, hospitalization; and *, positive serological test for Trichinella.
As shown by parasitological investigations in the Veterinary Specialist Institute ‘Zaječar’, the source of the first outbreak was pork infected with Trichinella spp. Positive samples of pork meat were sent to NRLT INEP where 2.7 LPG were found and multiplex PCR revealed that recovered larvae belonged to T. spiralis. The raw pork sausages that caused the second outbreak were also infected with Trichinella spp., but were not available for the detection of these species. It is worth pointing out here that both of above-mentioned domestic pigs originated from the same Bor District which has been recognized as an area with a high Trichinella infection rate among swine (Sofronic-Milosavljevic et al., Reference Sofronic-Milosavljevic, Djordjevic, Plavsic and Grgic2013).
Patients
In the first trichinellosis outbreak, this study evaluated ten patients from two districts, Bor District and Zaječar District (seven patients out of ten were with a place of residence in the municipality of Kladovo and three patients from the city of Zaječar), while in the second outbreak 14 patients were from the cities of Zaječar and Negotin (belonging also to the two above-mentioned districts located in eastern Serbia (fig. 2). Data from medical records showed that 20 persons were adults, 12 males with an average age of 41.5, ranging from 18 to 67 years, and eight women with an age of 33 to 74 years (average 51.25 years). Of the four children involved in the outbreaks, two were one year old, one 11 years old and one 13 years old (table 1).
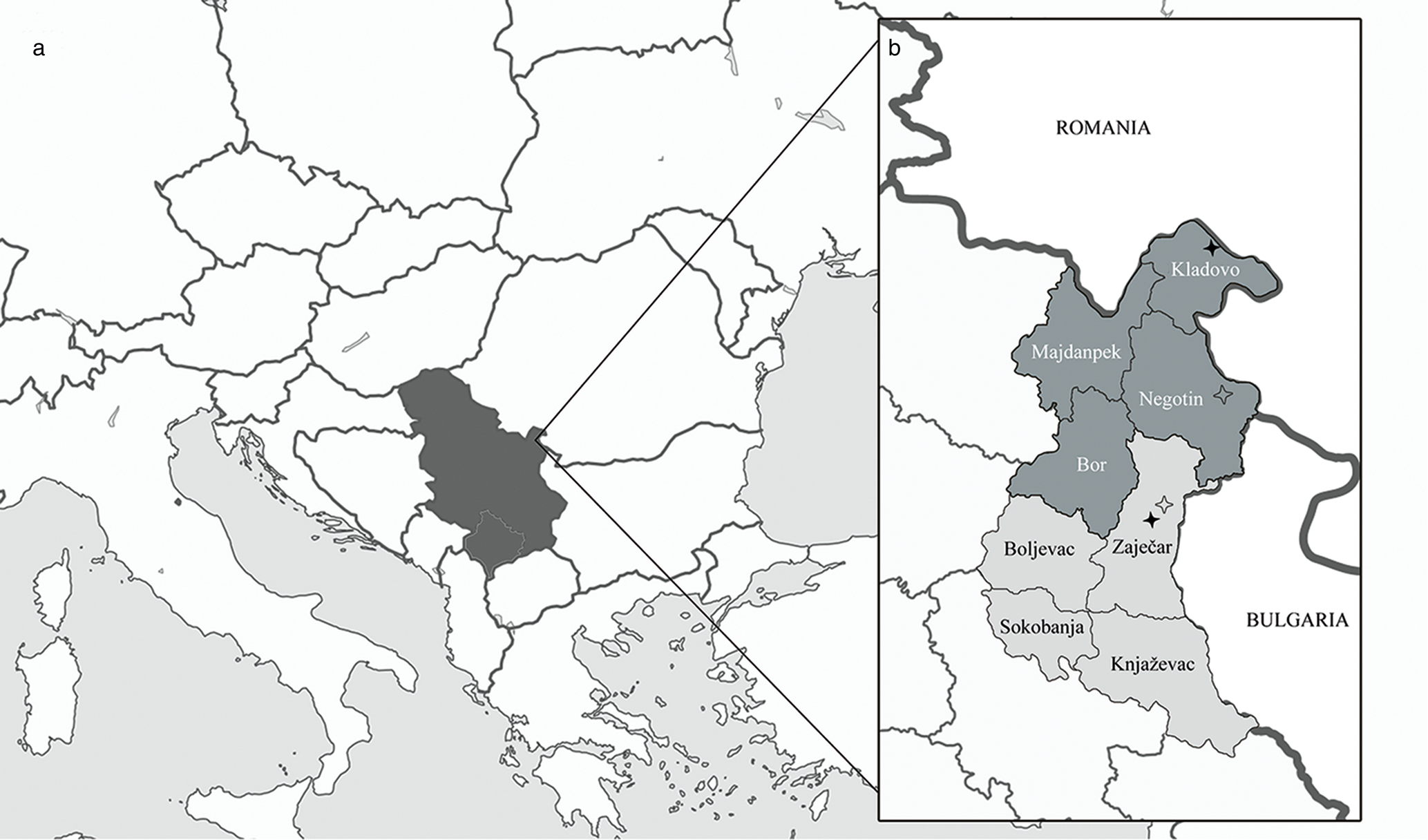
Fig. 2. Geographical presentation of the position of Serbia in Europe and the position of two districts in Serbia where trichinellosis outbreaks occurred in 2014: (a) Serbia in Europe (coloured black, image source: https://commons.wikimedia.org/wiki/File:Serbia_in_Europe_%28-rivers_-mini_map%29.svg); and (b) map of Eastern Serbia. Bor District is in dark grey colour, Zaječar District is given in light grey; black stars indicate hometowns of the patients involved in the first outbreak: Kladovo and Zaječar, empty stars designate hometowns of the patients involved in the second outbreak: Negotin and Zaječar.
Table 1. Patients’ data and immunoserological results for two Trichinella spiralis outbreaks in Serbia, 2014 obtained at the National Reference Laboratory for Trichinellosis – NRLT INEP and the Public Health Institute Nis – PHIN.

Clinical and laboratory findings
Eighteen patients with suspected trichinellosis were examined at the MHCZ because of fever, eyelid oedema and myalgia. However, two patients had non-specific clinical manifestations and high eosinophilia at the beginning of the second outbreak. Examination of possible trichinellosis or toxocariasis was done in the evaluation of patients for helminthic aetiology of eosinophilia. Immunoserological tests showed that the patients were positive for both parasites. The analyses were also performed for members of their families and the sera were reactive to both parasites, that is, the presence of antibodies against both parasites were proven. However, clinical findings did not support Toxocara spp. infection because the fundus examination of the eyes was negative and the chest X-ray was negative.
In the NRLT INEP patients from both outbreaks were diagnosed according to the trichinellosis case definition criteria proposed by Dupouy-Camet & Murrell (Reference Dupouy-Camet, Murrell, Dupoy-Camet and Murrell2007) and according to those criteria 15 patients had specific signs and symptoms. One of the most dominant symptoms was fever with a high temperature that reached 39.5–40°C, which was registered in 66.7% of patients (ten/15). Eight patients (53.3%) had eyelid oedema, while 20% of patients had weakness and nausea. Diarrhoea was present in four patients (26.7%). The largest number of persons complained of myalgia (11 of them, that is, 73.3%). Three patients from the second outbreak (20%) had no symptoms.
Haematological and biochemical results such as eosinophilia and elevated creatine kinase (CK), lactate dehydrogenase (LDH) and immunoglobulin E (IgE) indicated trichinellosis. Fifteen patients had severe hypereosinophilia (peripheral blood absolute eosinophil count >1.5 × 109/l) and elevated eosinophil percentage above 12%. Absolute eosinophil count was 3.92 ± 2.05 × 109/l (range 1.9–8.2 × 109/l). The percentage of eosinophils was between 12.4 and 43.2 with an average of 25.34 ± 9.64. Elevated CK (ref. range <200 U/l) with a mean level of 871 ± 413.54 was detected in eight patients (53.3%). Ten patients (66.7%) had raised LDH (ref. range <227 U/l) with a mean level of 725.50 ± 226.94. Six patients (40%) had an increased IgE level (ref. range 1.3–165.3 IU/ml) ranging from 186.7 to 2204.4. Clinical findings and haematological and biochemical results are shown in online Supplementary table S3.
Immunoserological findings
The results in PHIN were positive for anti-Trichinella antibodies in IFA and ELISA tests for 13 patients (54%). Eight patients had a low positive result (1:40) in the IFA test while in nine cases the antibody titre ranged from 1:80 to 1:160. So, 71% (17/24) of patients tested positive with a mean IFA titre of 94.12 ± 58.21. Positive ELISA results (13/24) varied from 12 to 67 NTU. Mean serum levels of positive anti-Trichinella IgG antibodies were 38.90 ± 17.44. Also, PHIN analysed 12 serum samples for the presence of anti-T. canis antibodies for a differential diagnosis with trichinellosis. Analysis revealed that six patients had anti-T. canis IgG antibodies in the ELISA test and results were confirmed by specific and confirmatory Wb test (fig. 3). Serological results obtained in PHIN are shown in table 1, fig. 4 and online Supplementary tables S1 and S2.

Fig. 3. Patterns of reactivity of Toxocara canis and Trichinella spiralis antigens with representative serum samples from patients with positive serology to both parasites by Western blot (Wb): (a) representative Wb profile with specific bands of low molecular weight 24–35 kDa in human sera infected with T. canis: Line (A1) positive control; line (B1) serum sample from patient 13; Line (C1) serum sample from patient 15; Line (D1) serum sample from patient 16; and (b) representative Wb profile with a characteristic triad of bands of molecular weight 45, 49, 53 kDa in human sera infected with T. spiralis: Line (A2) 7C2C5 Mo At (positive control); Line (B2) serum sample from patient 13; Line (C2) serum sample from patient 15; Line (D2) serum sample from patient 16.

Fig. 4. Summary of immunofluorescence antibody assay (IFA) and enzyme-linked immunosorbent assay (ELISA) results for anti-Trichinella antibodies in 24 patients from two Trichinella spiralis outbreaks in Serbia, 2014: (a) IFA titre; and (b) ELISA immunoglobulin G index (National Reference Laboratory for Trichinellosis – NRLT INEP) and units (Public Health Institute Nis – PHIN).
Suspected Trichinella infection was serologically detected in 87.5% of patients in the NRLT INEP. The Wb analyses confirmed the diagnosis in five cases, of which four patients were positive for T. canis (fig. 3). Seven patients had a low positive result (1:40 or 1:80) in the IFA test while in nine cases the antibody titre ranged from 1:160 to 1:320. High titres of 1:640 and 1:1280 were observed in two patients. So, 75% (18/24) of patients tested positive with a mean IFA titre of 262.22 ± 299.72. Positive ELISA results (19/24) varied from 21% to 98% IP. Mean serum levels of positive anti-Trichinella IgG antibodies expressed through index of positivity value were 48.49 ± 25.41. Serological results obtained at NRLT INEP are shown in table 1, fig. 4 and online Supplementary tables S1 and S2.
Discussion
Parasitic infections with T. spiralis and T. canis have some of the similar non-specific signs and symptoms (e.g. leucocytosis with eosinophilia, fever, weakness and myalgia). These infections are characterized by muscle involvement by Trichinella nurse cell formation or due to Toxocara larvae migrans presence. Also, the treatment of both infections is the same and includes anthelmintic and anti-inflammatory drugs. Therefore, both diseases were considered as possible diagnoses in 12 persons in the second outbreak because of symptoms such as fever and myalgia accompanied by eosinophilia. An analysis performed in PHIN determined that six patients were positive for anti-Toxocara antibodies. However, positive serological results for Trichinella infection in NRLT INEP for the same six patients indicated that these persons actually did have trichinellosis. A total of 21 persons tested in INEP and PHIN were positive for the presence of anti-Trichinella antibodies. However, serological findings showed a level of discrepancy of 25% between the ELISA performed at INEP and PHIN. The disagreement between the indexes of positivity obtained by ELISA is probably due to the use of tests that had different performances, since one is in-house and the other is commercial. Using the same IFA test, the level of disagreement was lower (4%). The discrepancy between the positive titres obtained by IFA probably arose from the subjective evaluation of the results by the persons reading the results. However, in the case of Trichinella infection, the titre level is not of crucial importance for the diagnosis of patients – it is much more important whether the result is correct in the terms of positive/negative. Thus, the interpreted results of INEP and PHIN did not differ in determining whether anti-Trichinella antibodies were present or not, except in one patient. It is recommended that trichinellosis with an atypical clinical course with eosinophilia and fever should be differentiated from fascioliasis, toxocariasis or invasive schistosomiasis (Dupouy-Camet & Murrell, Reference Dupouy-Camet, Murrell, Dupoy-Camet and Murrell2007). Thus, the presence of antibodies specific for the above-mentioned two parasites in this study could indicate either the presence of co-infection or cross-reactivity between antigens applied in the serological tests and used for antibody detection against these parasites. Toxocariasis has been reported in numerous cases in humans like co-infection with other parasites such as Toxoplasma gondii, Plasmodium falciparum and Echinococcus granulosus (Jones et al., Reference Jones, Kruszon-Moran, Won, Wilson and Schantz2008; Macpherson, Reference Macpherson2013; Kamuyu et al., Reference Kamuyu, Bottomley and Mageto2014). In this case, based on ELISA and Wb test-positivity for the presence of anti-Toxocara antibodies, it was not possible to conclude whether it was a current or a past infection. In toxocariasis, the determination of different classes and subclasses of immunoglobulins (immunoglobulin M (IgM), immunoglobulin A (IgA) and immunoglobulin G4A) cannot distinguish whether it is an acute or chronic infection (Fillaux & Magnaval, Reference Fillaux and Magnaval2013). In many parasitic infections, the presence of specific IgM and IgA is characteristic of the early phase of the infection, while in toxocariasis they are also detected in the acute and in the chronic stage of the disease (Smith, Reference Smith, Lewis and Maizels1993; Rubinsky-Elefant et al., Reference Rubinsky-Elefant, Shimizu, Sanchez, Jacob and Ferreira2006). As asymptomatic or with non-specific symptoms, toxocariasis remains undiagnosed in a large number of patients, but still leaves residual specific antibodies. Thus, IgG antibodies persisted for four years in children after infection and thiabendazole therapy in a follow-up study of Rubinsky-Elefant et al. (Reference Rubinsky-Elefant, Shimizu, Sanchez, Jacob and Ferreira2006). Eosinophilia and increased IgE immunoglobulin are parameters that contribute to the diagnosis of acute infection. But, apart from increased eosinophils in our group of patients, the clinical findings such as fundus eye examination and chest X-ray did not support toxocariasis. So, the positive test for T. canis in this study may have been obtained due to cross-reactivity and possible lack of the applied test, but also possible co-infection and/or the existence of residual antibodies to T. canis. Yera et al. (Reference Yera, Andiva, Perret, Limonne, Boireau and Dupouy-Camet2003) reported cross-reactions of T. spiralis crude antigens with serum samples from patients with toxocariasis. Also, cross-reactions of Toxocara ES antigens with sera from patients with trichinellosis and strongyloidiasis in routine immunodiagnosis with the ELISA test were observed by Jacquier et al. (Reference Jacquier, Gottstein, Stingelin and Eckert1991). Common antigenic components on helminths may be the cause of these cross-reactions that reduced the specificity of immunoenzymatic tests based on Toxocara ES antigens (Fillaux & Magnaval, Reference Fillaux and Magnaval2013). In the current study, in addition to the screening ELISA test in the diagnosis of toxocariasis, a confirmatory Wb test was used, which enables the differentiation between specific and non-specific anti-Toxocara antibodies according to ES antigens. Magnaval et al. (Reference Magnaval, Fabre, Maurieres, Charlet and de Larrard1991) showed that positive result of the ELISA test for anti-Toxocara antibodies can be confirmed by Wb because it is as sensitive as ELISA, but also quite specific when bands of lower molecular weight are taken into account, from 24 to 35 kDa. In all positive Wb test results in our patients, the presence of specific bands of low molecular weight (24–35 kDa) indicating the presence of specific anti-Toxocara IgG in the serum samples was observed. Although the vast majority of those infected with Toxocara have nonspecific symptoms and remain undiagnosed, the infection leaves residual specific antibodies that are the cause of a positive serodiagnosis, so it is possible that the patients in our study were also infected in the past and therefore have specific antibodies.
Infection with Toxocara, a roundworm of dogs and cats, is one of the most common zoonotic helminth infections worldwide (Rubinsky-Elefant et al., Reference Rubinsky-Elefant, Hirata, Yamamoto and Ferreira2010). The worldwide seroprevalence rate of human toxocariasis is estimated at 19% (Rostami et al., Reference Rostami, Riahi, Holland, Taghipour, Khalili-Fomeshi, Fakhri, Omrani, Hotez and Gasser2019). Toxocariasis in humans is primarily a soil-transmitted zoonosis (Magnaval et al., Reference Magnaval, Glickman, Dorchies and Morassin2001). After accidental ingestion of embryonated eggs from contaminated soil and food or raw meat, humans become infected (Despommier, Reference Despommier2003; Overgaauw & van Knapen, Reference Overgaauw and van Knapen2013). Thus, in this study, patients positive for Toxocara were most likely exposed to an infection that went asymptomatically and unnoticed, so they did not even know it, but they had antibodies against that helminth. Namely, the first study on the asymptomatic toxocariasis seroprevalence in Serbia, conducted in 2015, revealed the real exposure to this infection and its prevalence in the population (Gabrielli et al., Reference Gabrielli, Tasic-Otasevic, Ignjatovic, Fraulo, Trenkic-Bozinovic, Momcilovic and Cancrini2017). The results showed overall seroprevalence of 23.5%, while looking at the regions of county seroprevalence in East Serbia was 18.2%, in the North 15.5% and in Southeast Serbia 9.5%. These results classified the country among the hyperendemic regions. Also, Serbia is hyperendemic for toxocariasis in dogs with high T. canis infection rates of 35.27% in the area of Belgrade (Kulisic et al., Reference Kulisic, Pavlovic, Milutinovic and Aleksic-Bakrac1998). The prevalence of toxocariasis infection in dogs has not changed much in ten years, as the study in Belgrade in 2008 detected Toxocara eggs in 35.3% of dogs (Nikolic et al., Reference Nikolic, Dimitrijevic, Katic-Radivojevic, Klun, Bobic and Djurkovic-Djakovic2008). Research on the prevalence of intestinal parasites in dogs from six public dog shelters in the Republic of Serbia in 2017 and 2018 showed an overall prevalence of T. canis infection of 18.5%, while in young dogs it was the most common parasite (33.5%) (Ilic et al., Reference Ilic, Nisavic, Gajic, Nenadovic, Ristic, Stanojevic and Dimitrijevic2021). These prevalence data indicated ongoing human exposure to infection through soil containing Toxocara eggs, as confirmed by the results of a study conducted in five public parks in Belgrade (Colovic-Calovski et al., Reference Colovic-Calovski, Jekic, Stevanovic, Dubljanin, Kulisic and Dzamic2014). Also, in 2019, research conducted in the area of public parks of the city of Nis, in southeast Serbia, determined the degree of contamination with the ascarid T. canis (14–22%) in soil samples, while in sand samples the degree of contamination with the helminth T. canis was 26% (Ristic et al., Reference Ristic, Miladinovic-Tasic, Dimitrijevic, Nenadovic, Bogunovic, Stepanovic and Ilic2020). Thus, pets that excreted eggs into the human environment without the involvement of vectors or intermediate hosts could play an important role in the transmission of toxocariasis (Overgaauw & van Knapen, Reference Overgaauw and van Knapen2013).
The predominant symptoms in patients included in this study were fever, eyelid oedema, muscle pain, weakness and diarrhoea which indicated possible trichinellosis according to an algorithm for diagnosing of acute Trichinella infection (Dupouy-Camet et al., Reference Dupouy-Camet, Kociecka, Bruschi, Bolas-Fernandez and Pozio2002). Periorbital and facial oedema are typical allergy-like signs of trichinellosis (Watanabe et al., Reference Watanabe, Bruschi and Korenaga2005). Due to the difficult diagnosis, the patient's medical history should be always collected as additional information. An epidemiological link such as exposure to a common source or contaminated meat is an important factor in considering trichinellosis in the differential diagnosis of the disease (Dupouy-Camet et al., Reference Dupouy-Camet, Kociecka, Bruschi, Bolas-Fernandez and Pozio2002). On the one hand, anamnestic data on the consumption of meat from pigs slaughtered a month earlier, in August 2014, indicated Trichinella infection, while on the other hand a questionnaire performed by MHCZ did not result in responses indicating that people were aware of possible exposure to the infection with Toxocara spp. Toxocariasis could not then be excluded and/or confirmed from the differential diagnosis based on socio-epidemiological history. In this case, a well-taken history of patients regarding risk factors established for a diagnosis of trichinellosis was confirmed in NRLT INEP. The anamnestic data obtained from the patients coincided with data of NRLT INEP obtained from the systemic epizootilogical investigations of T. spiralis infection in pigs, carried out in the municipality of Kladovo, which classified this region as one of the endemic districts of Serbia for trichinellosis (Cuperlovic, Reference Cuperlovic1991; Cuperlovic et al., Reference Cuperlovic, Djordjevic and Pavlovic2005). Obviously, there were two outbreaks, territorially overlapping and temporally close, probably due to the high Trichinella infection rate in swine in this region of Serbia and the bad habits of farmers to neglect examination of the meat samples for the presence of Trichinella larvae.
The applied diagnostic tests showed that a total of 67% (16/24) patients had trichinellosis proven by IFA and ELISA tests. The application of the confirmatory test by the Wb technique was necessary in five more cases when there was a discrepancy between the results obtained by IFA and ELISA tests. The Wb as a specific and sensitive method allows the detection of specific anti-Trichinella antibodies and differentiation of cross-reactive antibodies in patients with toxocariasis, filariasis, anisakiasis and other parasitic infections (Gómez-Morales et al., Reference Gómez-Morales, Ludovisi, Amati, Cherchi, Pezzotti and Pozio2008). Simultaneous application of several immunoserological tests which use different Trichinella antigens enables earlier detection of anti-Trichinella antibodies (Costantino et al., Reference Costantino, Malmassari, Dalla Fontana, Diamante and Venturiello2001; Gamble et al., Reference Gamble, Pozio, Bruschi, Nöckler, Kapel and Gajadhar2004; Dupouy-Camet & Bruschi, Reference Dupouy-Camet, Bruschi, Dupouy-Camet and Murrell2007; Bruschi et al., Reference Bruschi, Gómez-Morales and Hill2019). Thus, in NRLT INEP, all sera samples were tested by the IFA test on slides with whole Trichinella larvae sections, an in-house i-ELISA test with microtitre plates coated with ES L1 antigens, and in a Wb test with 7C2C5 MoAb. The 7C2C5 MoAb recognizes an immunodominant epitope unique to the muscle larvae stage of the genus Trichinella in three ES L1 components of 45 kDa, 49 kDa and 53 kDa (Gamble & Graham, Reference Gamble and Graham1984). Sera samples that had specific anti-Trichinella antibodies which recognized the Trichinella-specific triad in the Wb (same as 7C2C5 MoAb) provided the final proof and enabled confirmation of the infection with T. spiralis. Three patients (12.5%) were negative for the presence of anti-Trichinella antibodies in both applied tests. The reason for negative serological tests may be early sampling because the titre of anti-Trichinella antibodies was not yet at the level of detection. This means that a total of 87.5% (21/24) people were infected with T. spiralis in these two outbreaks. In the second outbreak, serum reactivity to T. canis was determined in six patients.
Here, presented cooperation between experts in the helminthology field provided additional value in the search for the final diagnosis of trichinellosis. The Reference Laboratory performs the health activity of providing advice and consultations to other laboratories and health professionals regarding the diagnosis, prophylaxis and treatment of the disease. The dilemma about the existence of co-infection with T. canis remained open mainly due to the lack of a highly specific test.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X22000712.
Acknowledgement
We are very grateful to Dr Ray H. Gamble for providing us with the 7C2C5 hybridoma cell line.
Financial support
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant number 173047 and Contract No. 451-03-68/2022-14/ 200019).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients prior to collection and analysis of serum samples.









