Introduction
Insomnia disorder is a sleep condition that means difficulties in falling asleep, maintaining sleep, or waking up too early in the morning and associated daytime impairment (American Psychiatric Association, 2013). Insomnia disorder is a prevalent condition, affecting approximately 10% of the general population (Morin et al., Reference Morin, Drake, Harvey, Krystal, Manber, Riemann and Spiegelhalder2015), and is often co-morbid with psychiatric diagnoses (Ford and Kamerow, Reference Ford and Kamerow1989; Kim et al., Reference Kim, Jeon, Hong, Bae, Lee, Chang and Cho2012), particularly so with anxiety and mood disorders. When insomnia is co-morbid, it has been related to more severe psychiatric symptomatology (McCall et al., Reference McCall, Blocker, D’Agostino, Kimball, Boggs, Lasater and Rosenquist2010; Sunderajan et al., Reference Sunderajan, Gaynes, Wisniewski, Miyahara, Fava, Akingbala and Trivedi2010; Taylor et al., Reference Taylor, Lichstein, Durrence, Reidel and Bush2005) and higher risk for new psychiatric episodes (Sylvia et al., Reference Sylvia, Dupuy, Ostacher, Cowperthwait, Hay, Sachs and Perlis2012; Troxel et al., Reference Troxel, Kupfer, Reynolds, Frank, Thase, Miewald and Buysse2012). As co-morbid insomnia is regarded as an independent health problem that warrants treatment in its own right (American Psychiatric Association, 2013), research attempts have been made during the past decade to explore the efficacy of various treatments for co-morbid insomnia.
Cognitive behavioural therapy for insomnia (CBT-I) is a multi-component and evidence-based treatment (Morin et al., Reference Morin, Drake, Harvey, Krystal, Manber, Riemann and Spiegelhalder2015). CBT-I aims to alter sleep-related misconceptions and thought patterns, to change maladaptive sleep habits, and to lower sleep-disrupting arousal. Recent guidelines have underscored that CBT-I should be considered the treatment of first choice for patients with insomnia disorder, either delivered face-to-face or via the internet (Qaseem et al., Reference Qaseem, Kansagara, Forciea, Cooke and Denberg2016; Riemann et al., Reference Riemann, Baglioni, Bassetti, Bjorvatn, Dolenc Groselj, Ellis and Gonçalves2017; Zachariae et al., Reference Zachariae, Lyby, Ritterband and O’Toole2016). Also, several meta-analyses and reviews have demonstrated the efficacy of CBT-I in the context of other mental disorders, with effect sizes for key insomnia symptoms ranging from 0.68 to 0.91 (Geiger-Brown et al., Reference Geiger-Brown, Rogers, Liu, Ludeman, Downton and Diaz-Abad2015; Jansson-Fröjmark and Norell-Clarke, Reference Jansson-Fröjmark and Norell-Clarke2016; Wu et al., Reference Wu, Appleman, Salazar and Ong2015). However, relatively few studies have been carried out exploring CBT-I for insomnia co-morbid with other mental disorders, and there has been a limited number of mental disorders included in trials, predominantly insomnia co-morbid with alcohol dependence, post-traumatic stress disorder (PTSD) and major depression (Geiger-Brown et al., Reference Geiger-Brown, Rogers, Liu, Ludeman, Downton and Diaz-Abad2015; Wu et al., Reference Wu, Appleman, Salazar and Ong2015).
One of the many psychiatric co-morbidities that has received little attention in treatment studies is generalized anxiety disorder (GAD). There are several reasons why treatment research is needed for those with insomnia disorder co-morbid with GAD. First, the sleep difficulties among GAD patients are characteristic insomnia symptoms, including difficulty falling and staying asleep, as well as restless sleep (American Psychiatric Association, 2013). Second, insomnia disorder and GAD very often co-occur. Among adult patient samples, 85–90% of GAD patients report dissatisfaction with their sleep, and 52–68% can be classified as having moderate or severe insomnia (Bélanger et al., Reference Bélanger, Morin, Langlois and Ladouceur2004; Brenes et al., Reference Brenes, Miller, Stanley, Williamson, Knudson and McCall2009; Ferre Navarrete et al., Reference Ferre Navarrete, Perez Paramo, Fermin Ordono and Lopez Gomez2017). Using structured interviews for the assessment of insomnia disorder and GAD, 9–13% of insomnia participants meet criteria for GAD (Breslau et al., Reference Breslau, Roth, Rosenthal and Andreski1996; Mellinger et al., Reference Mellinger, Balter and Uhlenhuth1985). Polysomnographic findings also suggest that GAD patients, relative to individuals without GAD, in objective terms display increased wake time as well as decreased sleep efficiency and total sleep time (Monti and Monti, Reference Monti and Monti2000).
Two additional reasons should be pointed out as to why clinical research is warranted for those with insomnia disorder co-morbid with GAD. Research shows that, among GAD patients, worry and poor sleep interact over time. In one study using ecological momentary assessment, worry and sleep quality formed a bidirectional relationship across one week (Thielsch et al., Reference Thielsch, Ehring, Nestler, Wolters, Kopei, Rist and Andor2015). In another study, it was more common for anxiety disorders to develop before insomnia disorder when co-morbid cases were investigated (Johnson et al., Reference Johnson, Roth and Breslau2006). At the same time, other investigations have reported results consistent with the notion that insomnia might increase the risk of anxiety (Breslau et al., Reference Breslau, Roth, Rosenthal and Andreski1996; Gehrman et al., Reference Gehrman, Seelig, Jacobson, Boyko, Hooper, Gackstetter and Team2013; Jansson-Fröjmark and Lindblom, Reference Jansson-Fröjmark and Lindblom2008; Neckelmann et al., Reference Neckelmann, Mykletun and Dahl2007; Sivertsen et al., Reference Sivertsen, Lallukka, Salo, Pallesen, Hysing, Krokstad and Øverland2014). Furthermore, examining the aetiologicol overlap between the genetic and environmental influences on insomnia disorder and GAD implies the same genetic vulnerability for both conditions, and significant overlap in environmental influences (Lind et al., Reference Lind, Hawn, Sheerin, Aggen, Kirkpatrick, Kendler and Amstadter2017). The latter finding suggests that insomnia disorder and GAD may share environmental influences, such as acute stressors, which may be valuable when considering the development and treatment of both disorders. As insomnia disorder and GAD often occur together and form a reciprocal relationship, a vital area for research is to examine treatment alternatives for patients with insomnia disorder co-morbid with GAD.
To our knowledge, only one previous treatment study has explored the efficacy of CBT-I for patients with insomnia disorder co-morbid with GAD. In a single-subject design, 10 patients were exposed to CBT-I and CBT for GAD in a sequenced manner (Belleville et al., Reference Belleville, Ivers, Belanger, Blais and Morin2016). The 10 participants were randomized to receive either CBT-I first (followed by CBT for GAD) or CBT for GAD first (followed by CBT-I). While CBT-I resulted in improvements in sleep quality and insomnia symptoms, there were no indications that CBT-I decreased worry and anxiety symptoms. CBT for GAD produced reductions in time worrying and sleep efficiency in most patients. CBT for GAD did not improve insomnia symptoms. There are other indications suggesting that worry does not hamper the efficacy of CBT-I. For example, one trial showed that low and high worriers achieved similar sleep outcomes after CBT-I (Hamoen et al., Reference Hamoen, Redlich and de Weerd2014).
The aim of the present study was to examine the efficacy of CBT-I among patients with insomnia disorder co-morbid with GAD. The investigation was based on theoretical notions as well as on a limited treatment research focusing on insomnia patients with co-morbid GAD. Based on the empirical literature, we reasoned that reduced insomnia symptoms could be expected to result in decreased anxiety and worry (e.g. Thielsch et al., Reference Thielsch, Ehring, Nestler, Wolters, Kopei, Rist and Andor2015). One theoretical model, the Triple-R model (Maurer et al., Reference Maurer, Espie and Kyle2018), proposes that treatment elements in CBT-I could have a profound impact on cognitive processes, such as reducing unhelpful beliefs and worry. As a result, we reasoned that CBT-I could decrease core symptoms in GAD. Turning to the limited treatment research on insomnia co-morbid with GAD, we decided to use a CBT-I manual that has an enhanced focus on both behavioural and cognitive maintaining processes and that has been shown to outperform therapies with a narrower focus (Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014). Also, the current study aimed to assess outcome domains not indexed in the former trial (Belleville et al., Reference Belleville, Ivers, Belanger, Blais and Morin2016), i.e. functional impairment and quality of life, as these are vitally affected in patients with insomnia disorder and GAD. Finally, we aimed to examine putative mechanisms for CBT-I, based on the importance of such a focus in the treatment research area on both insomnia disorder and GAD. In this study, the putative mechanisms were chosen based on prominent behavioural and cognitive models of insomnia disorder (Harvey, Reference Harvey2002; Schwartz and Carney, Reference Schwartz and Carney2012). These models build on the notion that homeostatic sleep pressure, the regulation of the sleep–wake cycle through circadian rhythms, and cognitive processes maintain insomnia disorder. As a result, behavioural (e.g. bedtime variability and time in bed) and cognitive processes (e.g. unhelpful beliefs and safety behaviours) were assessed in the current study.
Due to the limited extant research base, an open trial design was chosen. The efficacy of CBT-I was evaluated primarily on insomnia-related outcomes but also on anxiety, worry, depression, functional impairment, quality of life and adverse events. Also, measures indexing putative mechanisms for CBT-I were added. Based on theoretical notions, we hypothesized that, following CBT-I, symptoms of insomnia, anxiety and worry would be reduced, and that insomnia-related putative mechanisms would be reversed. For the remaining outcomes, the analyses were conducted mainly from an exploratory viewpoint due to limited prior evidence.
Method
Overview of the study
Patients were screened in three steps (screening questions, telephone interview and sleep diary) and then treated with CBT-I for 10 weeks. The patients were assessed at pre-treatment, bi-weekly during treatment, at post-treatment, and at 6-month follow-up. The study was reviewed and approved by the Regional Ethical Board in Stockholm, Sweden (reference number 2016/856–31). Based on recommendations by Whitehead and colleagues (Reference Whitehead, Julious, Cooper and Campbell2016), the aim was to recruit at least 15 participants to the study, with an estimated power of 90% and an expected medium difference between CBT-I and an active control condition for the primary outcome in a later trial.
Recruitment, inclusion and exclusion criteria, and flow of participants
A summary of the flow of participants through the study is presented in Fig. 1. The inclusion and exclusion criteria used during screening in three phases (i.e. web-administered screening questions, telephone interview, and sleep diary) were based on expert recommendation for a standard research assessment of insomnia disorder and The Diagnostic and Statistical Manual of Mental Disorders-5 (American Psychiatric Association, 2013; Buysse et al., Reference Buysse, Ancoli-Israel, Edinger, Lichstein and Morin2006; Edinger et al., Reference Edinger, Bonnet, Bootzin, Doghramji, Dorsey, Espie and Stepanski2004; Lichstein et al., Reference Lichstein, Durrence, Taylor, Bush and Riedel2003). Potential applicants were recruited via a newspaper advertisement in Stockholm (Sweden) and invited to receive information on a study-specific web platform and via telephone with one of the study authors. Applicants were then asked to sign a consent form and answer screening questions on the web platform. During the first stage of screening, applicants reported their sociodemographic parameters (age, gender, civil status, occupational status, educational level and country of birth), insomnia [the Insomnia Severity Index (ISI); Bastien et al., Reference Bastien, Vallières and Morin2001], frequency and duration of insomnia symptoms, worry [the Penn State Worry Questionnaire (PSWQ); Meyer et al., Reference Meyer, Miller, Metzger and Borkovec1990], depressive symptoms [the Patient Health Questionnaire (PHQ-9); Kroenke et al., Reference Kroenke, Spitzer and Williams2001], and suicide risk (item 9 in the PHQ-9). Those who were: (1) 18 years or older, (2) exceeding the ISI cut-off at 10 points (Morin et al., Reference Morin, Belleville, Bélanger and Ivers2011), (3) reporting clinical nighttime symptoms (i.e. 2 points or more on at least one of the three initial ISI questions), (4) stating clinical daytime symptoms (i.e. 2 points or more on ISI item 5 and/or item 7), (5) reporting occurrence of sleeping difficulties three nights or more per week over at least 3 months despite adequate opportunities for sleep, (6) exceeding the PSWQ cut-off at 45 points (Behar et al., Reference Behar, Alcaine, Zuellig and Borkovec2003; Startup and Erickson, Reference Startup and Erickson2006), and (7) willing to participate in therapy for 10 weeks were eligible for the second round of screening. Applicants were also asked about their depression symptoms severity and suicidal ideation. Those who were severely depressed (more than 20 points on the PHQ-9; Kroenke et al., Reference Kroenke, Spitzer and Williams2001) and/or reporting high suicidal ideation (i.e. better off dead or hurting oneself) but without plans or intentions (1 point or more on item 9 in the PHQ-9) were excluded from the trial. Applicants reporting severe depression and/or high suicidal ideation were referred to psychiatric care.
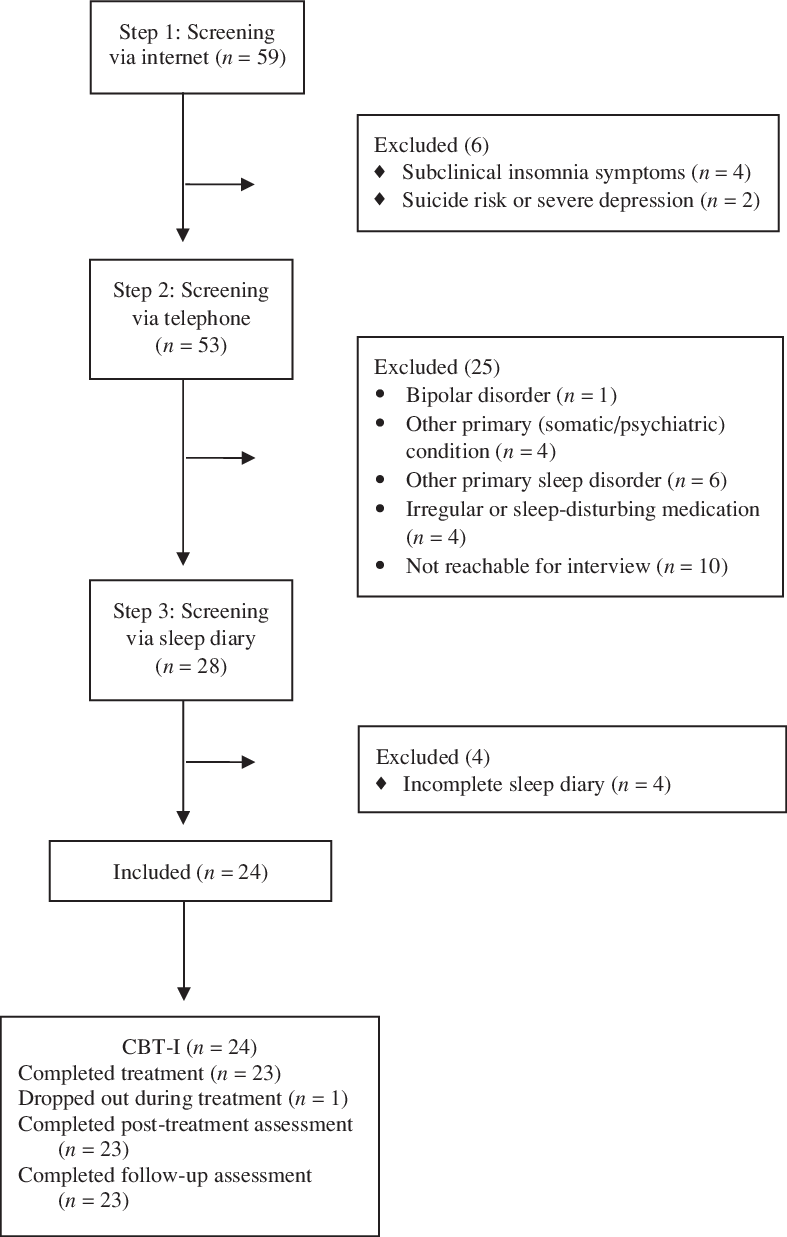
Figure 1. Flow diagram of study participants from screening to follow-up assessment.
During the second screening phase, applicants were contacted for a semi-structured telephone interview based on the Duke Structured Interview for Sleep Disorders (DSISD) (Edinger et al., Reference Edinger, Wyatt, Stepanski, Olsen, Stechuchak, Carney and Means2011) and the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Janavs, Weiller, Keskiner and Dunbar1997) to assess sleep and mental disorders. Also, these interviews aimed to identify and exclude applicants if sleeping problems were due to evident environmental conditions (e.g. pregnancy), night or rotating shift work (>3 shifts a week), at least moderate intake of alcohol (>2 standard drinks a day; Ebrahim et al., Reference Ebrahim, Shapiro, Williams and Fenwick2013) or caffeine (>4 beverages a day or >2 after 18.00 h), or if participation in CBT-I had occurred within the past 5 years. Further criteria required that insomnia disorder and GAD were disabling and distressing conditions, that reported somatic conditions were stable and/or that the applicant was receiving treatment for the condition, and that insomnia disorder and GAD were still present despite treatment for any somatic or psychiatric co-morbidity if co-morbidities were present. For medication, the following criteria were used: (a) if sleep medication was reported, it had to be relatively stable for the last 3 months, (b) if selective serotonin reuptake inhibitor (SSRI) use was reported, the onset of the medication, or the last change of dosage, should be at least 3 months prior to the telephone interview, and (c) if applicants were regularly consuming sleep-disturbing medications (e.g. benzodiazepines, pain medicines, and anti-hypertensives), they were excluded. Further criteria for exclusion were applicants with a history of psychotic or bipolar disorders or other current primary sleep disorders than insomnia disorder (sleep apnoea, restless legs syndrome, periodic limb movement disorder, circadian rhythm disorder, and parasomnias).
During the third screening phase, applicants completed a 7-day sleep diary (for details, see ‘Insomnia symptoms’ section below). The inclusion criterion at the third phase was to report at least 3 days of sleep difficulties in the sleep diary, i.e. sleep initiation, sleep maintenance or waking up too early, defined as 30 min or more for each occasion.
Measures and procedure
The ISI was used as the primary measure, and the remaining clinical outcomes as secondary measures. The self-report scales were administered at pre-treatment, post-treatment and 6-months follow-up. One of the self-report instruments, the ISI, was also completed by the participants bi-weekly during treatment (end of weeks 2, 4, 6 and 8). The sleep diary variables were filled out on paper daily from pre-treatment to post-treatment. At pre-treatment, biweekly (weeks 2, 4, 6 and 8), and at post-treatment, the participants transferred their sleep diary data to the digital platform. The putative mechanisms measures were administered at pre-treatment, biweekly and post-treatment. Adverse events and treatment satisfaction were only completed at post-treatment. Diagnostic measures as well as treatment credibility and expectancy were assessed with the study therapists, via telephone at pre-treatment or the first treatment session. The remaining measures were administered through an online, secure platform. Three automatic email reminders were sent out if an assessment had not been completed.
Diagnostic measures
To assess the diagnostic criteria for insomnia disorder, the DSISD was used (Edinger et al., Reference Edinger, Wyatt, Stepanski, Olsen, Stechuchak, Carney and Means2011). The DSISD is a semi-structured interview with acceptable reliability (inter-rater reliability of 0.59 for insomnia related to a mental disorder) and validity. To identify GAD, the MINI 6.0.0 was used. The MINI is a psychometrically sound semi-structured clinical interview with acceptable reliability and validity (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Janavs, Weiller, Keskiner and Dunbar1997), although there are indications that the MINI has limitations regarding the assessment of GAD (Verhoeven et al., Reference Verhoeven, Swaab, Carlier, van Hemert, Zitman, Ruhe and Giltay2017). The structured interviews were conducted by a trained and supervised masters student at the end of her clinical training.
Treatment credibility, expectancy, completion and satisfaction
To assess treatment credibility and expectancy of CBT-I, the Credibility/Expectancy Questionnaire (CEQ) (Devilly and Borkovec, Reference Devilly and Borkovec2000) was administered. CEQ is a 6-item questionnaire with demonstrated acceptable psychometric characteristics (α = .86 in this sample) and has indicated the ability to predict outcome. The number of attended sessions and completed homework assignments were assessed by the study therapists. To determine treatment satisfaction, the Client Satisfaction Questionnaire (CSQ-8) was used (Attkisson and Zwick, Reference Attkisson and Zwick1982).
Insomnia symptoms
The Insomnia Severity Index (ISI) was used to assess participants’ overall perception of insomnia severity (Bastien et al., Reference Bastien, Vallières and Morin2001; Morin et al., Reference Morin, Belleville, Bélanger and Ivers2011). The 7-item questionnaire is rated on a 5-point scale (0–4) with a total score of 0–28 and assesses both night- and daytime symptoms. The ISI was also used as a screening tool (employing the validated cut-off at 10 points; Morin et al., Reference Morin, Belleville, Bélanger and Ivers2011) and to categorize the number of responders (achieving a change of 8 points or more) and remitters (final score below 8) (Morin et al., Reference Morin, Belleville, Bélanger and Ivers2011). In the current sample, the internal consistency (Cronbach’s alpha) was acceptable at α = .81.
To assess night-time symptoms, a 7-day sleep diary was administered (Carney et al., Reference Carney, Buysse, Ancoli-Israel, Edinger, Krystal, Lichstein and Morin2012). The diary assessed bedtime, lights-out time, sleep onset latency (SOL), wake time after sleep onset (WASO), early morning awakening (EMA) and rise-time. From these measures, the online diary automatically calculated total sleep time (TST), time in bed (TIB) and sleep efficiency (SE). Outcome measures were calculated as weekly means of SOL, WASO, EMA and TST. The sleep diary is viewed as the gold standard subjective measure of sleep (Buysse et al., Reference Buysse, Ancoli-Israel, Edinger, Lichstein and Morin2006).
Anxiety, worry, functional impairment, depression and quality of life
To measure anxiety, worry, functional impairment, depression and quality of life, five psychometrically validated self-report measures were used. In the current sample, the internal consistency (Cronbach’s alpha) was between .78 and .93 for the five scales. The Generalized Anxiety Disorder Screener (GAD-7) was employed to assess anxiety-related symptoms (Spitzer et al., Reference Spitzer, Kroenke, Williams and Löwe2006). The seven items are rated on a 4-point scale ranging from 1 (not at all) to 4 (nearly every day); higher scores indicate elevated anxiety symptoms. In the current study, a cut-off at ≥10 points on the GAD-7 was used to estimate probable GAD-diagnosis (Spitzer et al., Reference Spitzer, Kroenke, Williams and Löwe2006). The PSWQ was used as an index of worry (Meyer et al., Reference Meyer, Miller, Metzger and Borkovec1990). The 16 items are rated from 1 (not at all typical of me) to 5 (very typical of me); higher scores reflect elevated worry. To estimate pathological versus non-pathological worry, a cut-off at 45 points was used in the current study (Behar et al., Reference Behar, Alcaine, Zuellig and Borkovec2003; Startup and Erickson, Reference Startup and Erickson2006). The Work and Social Adjustment Scale (WSAS) was used as an index of functional impairment (Jansson-Fröjmark, Reference Jansson-Fröjmark2014; Mundt et al., Reference Mundt, Marks, Shear and Greist2002). The five-item questionnaire is rated on a 9-point scale (0–8) with a total score of 0–40; higher scores indicate elevated functional impairment. The Patient Health Questionnaire (PHQ-9) was employed to determine depression symptoms (Kroenke et al., Reference Kroenke, Spitzer and Williams2001). The nine items are rated on a 4-point scale (0 = not at all; 3 = nearly every day) corresponding to the DSM-IV criteria for depression. The Brunnsviken Brief Quality of Life (BBQ) was used to evaluate quality of life (Lindner et al., Reference Lindner, Frykheden, Forsström, Andersson, Ljótsson, Hedman and Carlbring2016). The BBQ consists of 12 items assessing quality of life in six areas of life; higher scores indicate elevated quality of life.
Adverse events
Adverse events were assessed based on a checklist of 14 somatic and psychological events used in prior research (Kyle et al., Reference Kyle, Morgan, Spiegelhalder and Espie2011). The participants were asked to rate if any of the 14 adverse events (e.g. bodily pain, dizziness, low mood, and fatigue/exhaustion) had occurred as a result of treatment.
Putative mechanisms
Eight putative mechanisms were assessed. In this sample, the internal consistency (Cronbach’s alpha) was between .73 and .94 for the eight measures. Four validated self-report scales were used to assess cognitive mechanisms. First, the Anxiety and Preoccupation about Sleep Questionnaire-2 (APSQ-2) was used to determine insomnia-related worry (Jansson-Fröjmark and Sunnhed, Reference Jansson-Fröjmark and Sunnhed2019). The two items in the APSQ-2 are scored from 1 (strongly disagree) to 10 (strongly agree); a higher score indicates elevated insomnia-related worry. Second, the Dysfunctional Beliefs about Sleep scale (DBAS-16) was employed to assess unhelpful beliefs about sleep (Morin et al., Reference Morin, Vallières and Ivers2007). The response alternatives for the 16 DBAS items are from 0 (strongly disagree) to 10 (strongly agree); a higher score is suggestive of stronger unhelpful beliefs. Third, the Sleep Associated Monitoring Index-8 (SAMI-8) was used to determine attention for and monitoring of sleep-related threat (Jansson-Fröjmark and Sunnhed, Reference Jansson-Fröjmark and Sunnhed2019). The eight items in the SAMI-8 are scored from 1 (not at all) to 5 (all the time) with a total score range from 8 to 40; a higher score indicates elevated attention and monitoring. Fourth, the Sleep-Related Behaviours Questionnaire (SRBQ) was employed to assess insomnia-related safety behaviors (Ree and Harvey, Reference Ree and Harvey2004). The response alternatives for the 32 items are from 0 (almost never) to 4 (almost always); a higher score suggests elevated safety behaviours. To assess behavioural mechanisms, three items were calculated from the daily sleep diaries: bedtime variability, rise-time variability, and time in bed. Bedtime variability and rise-time variability were operationalized as the within-subject standard deviation for each participant (Edinger et al., Reference Edinger, Wohlgemuth, Radtke, Marsh and Quillian2001; Edinger et al., Reference Edinger, Olsen, Stechuchak, Means, Lineberger, Kirby and Carney2009). The two variability items were calculated by estimating the standard deviation across one week for each assessment point. Time in bed was estimated by calculating the time period between bedtime and rise-time for each night during a week (Edinger et al., Reference Edinger, Wohlgemuth, Radtke, Marsh and Quillian2001; Edinger et al., Reference Edinger, Wohlgemuth, Radtke, Coffman and Carney2007; Edinger et al., Reference Edinger, Olsen, Stechuchak, Means, Lineberger, Kirby and Carney2009; Krystal and Edinger, Reference Krystal and Edinger2010; Lichstein et al., Reference Lichstein, Riedel, Wilson, Lester and Aguillard2001; Rybarczyk et al., Reference Rybarczyk, Lopez, Benson, Alsten and Stepanski2002; Rybarczyk et al., Reference Rybarczyk, Stepanski, Fogg, Lopez, Barry and Davis2005). The daily calculations for time in bed were then averaged across the week.
CBT-I
Cognitive Behavior Therapy for Insomnia (CBT-I) consisted of 10 weekly individual face-to-face therapy sessions lasting 60–75 minutes. The therapy followed a manual (Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014). The number of weeks was determined based on previous research (Harvey et al., Reference Harvey, Sharpley, Ree, Stinson and Clark2007; Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014). All sessions followed a structured agenda including (a) review of homework assignments, e.g. sleep diaries, (b) compliance issues and problem-solving centering around homework assignments, (c) rationale, skills training, and implementation of treatment components, and (d) homework assignments. The treatment was administered by two licensed clinical psychologists who had received training and supervision. To ensure treatment implementation, treatment delivery, receipt and enactment were monitored across all sessions, e.g. through documentation of all sessions by the therapist, patient hand-outs and using homework worksheets (Lichstein et al., Reference Lichstein, Riedel and Grieve1994).
CBT-I contained a combination of a behaviour therapy (BT) and cognitive therapy (CT) approach. As in a previous trial on insomnia disorder (Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014), case formulation (Harvey, Reference Harvey2005) was used to assess likely maintaining factors for each patient to determine the relative time and ordering of treatment components. The formulation was guided by the symptoms that were present and the patients’ responses to questions, sleep diaries and questionnaires during the first sessions. While the first session was more generic [presenting the CBT approach, psychoeducation, introducing the 3 P Model of Insomnia (Spielman et al., Reference Spielman, Caruso and Glovinsky1987a), keeping a sleep diary, and setting treatment goals], the remaining sessions were more devoted to implementing treatment components.
In the current study, three BT components were used across patients. First, sleep restriction (Spielman et al., Reference Spielman, Saskin and Thorpy1987b) was implemented when the patient displayed excessive time in bed and low sleep efficiency. Sleep diaries were used to prescribe a sleep window for the coming week, which was then reviewed the following week. The sleep window was then increased or decreased depending on the sleep efficiency percentage (below or above 85%). The second BT intervention, stimulus control (Bootzin, Reference Bootzin1972), was used when there were indications that the patient engaged in sleep-incompatible behaviours within the bedroom environment, e.g. going to bed without signs of sleepiness, tossing and turning in bed, and displaying variability in rise-times. Third, sleep hygiene procedures were implemented when there were signs that the patient had bedroom circumstances and/or lifestyle factors perpetuating insomnia disorder, e.g. light exposure in bed and consuming caffeinated beverages in the hours before bedtime.
Building on a cognitive model of insomnia disorder (Harvey, Reference Harvey2002), treatment components were used to influence the proposed cognitive maintaining mechanisms: (a) unhelpful beliefs about sleep (Morin et al., Reference Morin, Blais and Savard2002), (b) sleep-related or sleep-interfering worry (Tang and Harvey, Reference Tang and Harvey2004), (c) attentional bias and monitoring for sleep-related threat (Semler and Harvey, Reference Semler and Harvey2004), and (d) misperception of sleep (Harvey and Tang, Reference Harvey and Tang2012). Two core CT strategies were used to reverse the cognitive maintaining mechanisms: verbal, Socratic challenging (e.g. challenging an unhelpful, sleep-related belief or discussing the disadvantages of monitoring for internal or external threats) and behavioural experiments. As in a previous trial (Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014), a minimum of four experiments was used for each patient: a monitoring/attentional bias experiment, the sleep survey experiment, the energy-generating experiment, and the fear of poor sleep experiment (Harvey et al., Reference Harvey, Sharpley, Ree, Stinson and Clark2007). Following the cognitive model of insomnia disorder, attention and homework assignments were split between reversing the cognitive maintaining mechanisms during the daytime and the night-time (Harvey, Reference Harvey2002).
Statistical analysis
Treatment effects were examined using intention-to-treat linear mixed models. These models were used because, in the analysis of longitudinal data, repeated observations for the same individual are correlated, which violates the assumption of independence necessary for more traditional, repeated measure analysis and leads to bias in regression parameters. Furthermore, mixed-effect models can accommodate missing data by using all available data and the integration of time-varying factors, which are issues in the present study. Estimated parameters were obtained using a mixed-models approach employing a compound symmetry covariance structure because it provided the best model in an information criteria comparison. An alpha level of .05 was used as the criterion for statistical significance.
Estimated marginal means and standard deviations from mixed models were used to calculate effect sizes (ES). Within-group effect sizes (Cohen’s d) were calculated [(pretreatment minus post-treatment or 6-month follow-up)/pooled standard deviation] to gain an impression of the magnitude of improvement associated with CBT-I. A threefold classification of effect sizes has been proposed (Cohen, Reference Cohen1988): small (0.20–0.49), medium (0.50–0.79) and large (0.80 and above). The same effect size analyses were conducted using observed data and the results were nearly identical and did not change our interpretation (data not shown).
Results
Participant characteristics
In total, 24 participants diagnosed with insomnia disorder and GAD were included in the study. A description of the participants is presented in Table 1.
Table 1. Descriptive statistics of the 24 study participants

a Headache (5 patients), respiratory diseases (2), heart diseases (1), gastric diseases (1) and chronic pain (1).
b Social anxiety disorder (4 patients), major depression (3) and panic disorder (1).
c For heart diseases (5 patients), anti-depressants (4), headache (3), tranquilizers (3), for asthma (2), allergy (1), and gastric issues (1).
Treatment credibility, expectancy, completion and satisfaction
According to the CEQ completed at session 1, treatment credibility [mean = 19.3 (SD = 3.7)] and treatment expectancy [mean = 18.3 (SD = 4.6)] were high. Session attendance for all 24 patients was on average at 9.1 sessions (SD = 2.4). One participant dropped out of the study before commencing CBT-I, and four patients attended less than the planned 10 sessions (8 sessions: n = 3, 9 sessions: n = 1) due to time constraints and/or illness. Homework completion was, on average for all 24 patients, 88.2% (SD = 12.9). According to the CSQ completed at post-treatment, treatment satisfaction was high [mean = 25.7 (SD = 5.1)].
Effects of CBT-I on clinical outcomes
The descriptive statistics for the clinical outcomes are displayed in Table 2. First, analyses were executed to explore changes in the ISI. Mixed-effect models showed a significant effect of time on the ISI across the seven assessment points (F = 35.49, p < .001), with significant change occurring from pre-treatment to week 6. The within-group effect sizes were large (d = 2.5–2.8). As can be seen in Fig. 2, 60.9% of the participants were treatment responders at both post-treatment and follow-up when using the ISI cut-offs. Also, 47.8% of the patients at post-treatment and 26.1% at follow-up met the criterion for remission.
Table 2. Estimated marginal means, standard deviations and effect sizes for the clinical outcomes – from pre-treatment to the 6-month assessment

BBQ, Brunnsviken Brief Quality of Life; d, Cohen’s d; EMA, early morning awakening; FU, 6-month follow-up; GAD-7, Generalized Anxiety Disorder Screener-7; ISI, Insomnia Severity Index; PHQ-9, Patient Health Questionnaire-9; Pre, pre-treatment; Post, post-treatment; PSWQ, Penn State Worry Questionnaire; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset; WSAS, Work and Social Adjustment Scale.
a Expressed in minutes.
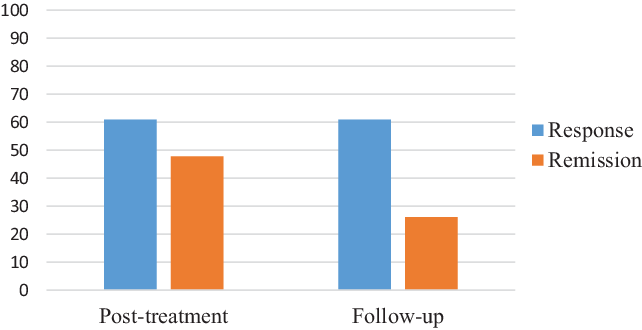
Figure 2. Response and remission according to ISI cut-offs. Response: improvement of 8 points or more on the ISI from pre-treatment to post-treatment or follow-up; remission: score of less than 8 points on the ISI at post-treatment or follow-up.
Analyses on the four sleep diary outcomes were then executed. Mixed-effect models showed a significant effect of time on SOL (F = 13.53, p < .001), WASO (F = 10.09, p < .001), EMA (F = 7.31, p < .001) and TST (F = 9.38, p < .001). The significant changes emerged between pre-treatment and week 6 for SOL, WASO and TST, and from pre-treatment to week 2 for EMA. The within-group effect sizes on the four sleep diary parameters were large (d = 0.8–1.3).
The effects of CBT-I on the additional self-report measures (GAD-7, PSWQ, PHQ-9, WSAS and BBQ) were then analysed. The analyses showed a significant effect of time on GAD-7 (F = 22.34, p < .001), PSWQ (F = 16.80, p < .001), PHQ-9 (F = 7.79, p = .001), WSAS (F = 49.16, p < .001) and BBQ (F = 7.84, p = .001). The significant changes occurred from pre- to post-treatment for GAD-7, PHQ-9, WSAS and BBQ, and from pre-treatment to follow-up for PSWQ. The within-group effect sizes on the four self-report scales were between medium and large (d = 0.5–1.7). Using the two GAD outcomes (PSWQ and GAD-7) to estimate the percentage of patients falling below validated cut-offs following CBT-I, 13.0% scored below the PSWQ cut-off of 45 points at post-treatment and 26.1% at follow-up, and 33.3% had a total score below 10 points on the GAD-7 at post-treatment and 61.9% at follow-up.
Adverse events
Of the 23 patients that started CBT-I, seven (30.4%) of the participants reported an adverse event at post-treatment. The reported events were in descending order (with the number of reports in parentheses): fatigue/exhaustion (5), extreme sleepiness (4), reduced motivation/energy (2), low mood (1), and dizziness (1).
Effects of CBT-I on putative mechanisms
The descriptive statistics for the putative mechanisms are displayed in Table 3. Mixed-effect models showed a significant effect of time on the four cognitive mechanisms: APSQ-2 (F = 16.39, p < .001), DBAS-16 (F = 15.23, p < .001), SAMI-8 (F = 11.41, p < .001) and SRBQ (F = 14.35, p < .001). The significant changes emerged between pre-treatment and week 6 for DBAS-16, SAMI-8 and SRBQ, and from pre-treatment to week 4 for APSQ-2. The within-group effect sizes for the cognitive mechanisms were large (d = 0.8–1.6). Furthermore, mixed-effect models demonstrated a significant effect on one of the three behavioural mechanisms with a large within-group effect size: time in bed (F = 9.42, p < .001). The significant changes occurred from pre-treatment to week 2 for time in bed. The two remaining behavioural mechanisms showed a non-significant effect [bedtime variability (F = 1.99, p = .086) and rise-time variability (F = 0.64, p = .642)].
Table 3. Estimated marginal means, standard deviations and effect sizes for the putative mechanisms – from pre-treatment to the post-treatment assessment

APSQ-2, Anxiety and Preoccupation about Sleep Questionnaire, 2-item version; d, Cohen’s d; DBAS-16, Dysfunctional Beliefs and Attitudes about Sleep scale, 16-item version; Pre, pre-treatment; Post, post-treatment; SAMI-8, Sleep Associated Monitoring Index, 8-item version; SRBQ, Sleep-Related Behaviours Questionnaire.
Discussion
The purpose of the current study was to explore the efficacy of CBT-I among patients with insomnia disorder co-morbid with GAD. The main findings were that CBT-I resulted in significant, large improvements in insomnia symptoms as well as significant improvements on the secondary clinical outcomes with moderate to large effect sizes, including GAD symptoms. Several of the putative mechanisms were reversed in the expected direction during CBT-I.
The efficacy of CBT-I in this trial was primarily directed at exploring changes in insomnia symptoms. Regarding insomnia severity, CBT-I resulted in large improvements (d = 2.5–2.8). Based on ISI cut-offs, approximately 61% of the participants responded to treatment and 48% remitted at post-treatment. Compared with a previous study using the same study protocol (but not specifically recruiting patients with co-morbid GAD), the response and remission rates were slightly lower (61 vs 67% and 48 vs 57%) (Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014). It is also important to underscore that only 26.1% of the patients reached insomnia remission at follow-up; this is markedly lower than the previous study mentioned above (56%; Harvey et al., Reference Harvey, Bélanger, Talbot, Eidelman, Beaulieu-Bonneau, Fortier-Brochu and Soehner2014). The reasons for a blunted response in rates of insomnia response and remission is not possible to tease out in the current study but might be due to GAD characteristics, such as excessive worry. Concerning night-time symptoms, CBT-I improved all four sleep diary parameters with large effect sizes. For the majority of insomnia symptoms, the significant changes occurred from pre-treatment to the week 6 assessment, which was followed by maintained improvements. Although CBT-I was delivered based on case conceptualization, resulting in slight variations across patients regarding which treatment components were used and in which order, all patients had been administered sleep restriction, stimulus control, Socratic questioning, and at least one behavioural experiment up to the sixth session. Based on a relatively solid evidence base for sleep restriction (Miller et al., Reference Miller, Espie, Epstein, Friedman, Morin, Pigeon and Kyle2014), it is likely that restricting time in bed was one important component for reducing insomnia symptoms in this trial.
Beyond reductions on insomnia symptoms, CBT-I resulted in significant improvements in anxiety, worry, depression, functional impairment and quality of life with medium to large effect sizes. The significant changes emerged between pre- and post-treatment for four of the domains with maintained results at follow-up. For worry, the significant change occurred across the whole study period, meaning that additional improvements were noted between post-treatment and follow-up; this continued reduction after CBT-I completion could not be observed on any other outcome at follow-up. Albeit not significant, the effect size for GAD-7 also suggested a continued improvement after CBT-I. In relation to the findings on anxiety and worry, it should be emphasized that the responses were limited using cut-offs; 33.3–61.9% of the patients scored below on the GAD-7 and 13.0–26.1% on the PSWQ. Compared with the previous trial on insomnia disorder and GAD (Belleville et al., Reference Belleville, Ivers, Belanger, Blais and Morin2016), the larger effects on GAD symptoms might hypothetically be due to a strengthened cognitive therapy format of CBT-I in the current study. In particular, an apparent difference across studies is that the current study’s version of CBT-I relied heavily on the main component in cognitive therapy, namely behaviuoral experiments. Such experiments build on a Socratic approach that relies on the patient drawing own conclusions with marked benefits, such as being rated as more helpful, supportive and engaging (Heiniger et al., Reference Heiniger, Clark and Egan2018). Behavioural experiments also have the potential to be generalizable to other clinical presentations and problems. Although identical measures were not used across studies (Belleville et al., Reference Belleville, Ivers, Belanger, Blais and Morin2016), insomnia and GAD symptoms appear very similar (e.g. for ISI and PSWQ); pre-treatment symptom severity is thus not a likely explanation.
While the improvements on insomnia symptoms were expected, the large and continued reductions on worry and anxiety symptoms were not anticipated. Hypothetically, we present two explanations for the large improvements in worry and anxiety. First, the CBT-I components in this trial might be beneficial in decreasing GAD symptoms. For example, sleep restriction is likely to result in high sleep pressure and associated fatigue/exhaustion and reduced energy at the start of CBT-I (Kyle et al., Reference Kyle, Morgan, Spiegelhalder and Espie2011), which might reduce opportunities for worrying. Also, skills training on the cognitive components in CBT-I might generalize from insomnia to worry, through testing of worries concerning poor sleep and daytime symptoms. Concerning treatment components, it should be noted that no evidence-based elements for GAD, such as relaxation training (Borkovec and Costello, Reference Borkovec and Costello1993), were used in the current study’s CBT-I. Second, improved sleep during CBT-I might be viewed as an establishing operation (Michael, Reference Michael2000). If so, it is conceivable that enhanced sleep quality and quantity is a stimulus condition that influences alternative behavioural patterns, such as activation, distraction, and problem-solving. In turn, such new behaviours might undercut the tendency to worry.
Approximately one-third of the patients experienced an adverse event at post-treatment. Based on previous findings showing that sleep restriction therapy results in increased fatigue/exhaustion and extreme sleepiness as well as reduced motivation/energy (Kyle et al., Reference Kyle, Morgan, Spiegelhalder and Espie2011), the adverse events reported in this study are likely to be due, at least partly, to sleep restriction. All seven putative mechanisms were changed in the expected direction following CBT-I. However, significant changes were noted on five of the seven mechanisms with large effect sizes. Similar improvements have been observed in previous trials (Harvey et al., Reference Harvey, Sharpley, Ree, Stinson and Clark2007; Schwartz and Carney, Reference Schwartz and Carney2012). It should be noted that the design of the study precludes conclusions regarding the putative mechanisms as mechanisms of change (Kazdin, Reference Kazdin2007); this would, for example, require statistical analyses investigating temporal relations between changes in mechanisms and subsequent improvements in symptoms.
The current study has several methodological limitations that should be kept in mind when interpreting the findings. First, the efficacy of CBT-I was evaluated without a comparison group; future research should include a control group (e.g. waitlist or CBT for GAD conditions) to control for threats to internal validity. Second, based on previous research showing that GAD patients display objective sleep disturbance (Monti and Monti, Reference Monti and Monti2000), future treatment studies might include objective sleep assessment. Third, CBT-I was administered by two therapists in the present study, thereby precluding conclusions regarding the efficacy of CBT-I to other clinicians. Other methodological alterations in future research could include recruiting patients from health care settings, having multiple interviewers, and assessing GAD symptoms (i.e. anxiety and worry) at more time-points during CBT-I.
The current study provides preliminary evidence for the efficacy of CBT-I for patients with insomnia disorder co-morbid with GAD. Before firm conclusions can be made on the efficacy of CBT-I for GAD, there is a need for replication of the present study and research using more robust designs.
Data availability
The data that support the findings of this study are available on request from the corresponding author (M.J.F.). The data are not publicly available due to containing information that could compromise the privacy of research participants.
Acknowledgements
We would like to express our appreciation to the Centre for Psychotherapy Research and Education, Centre for Psychiatry Research, Karolinska Institute for supporting the execution of this study.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
Markus Jansson-Fröjmark and Kalle Jacobson have no conflicts of interest with respect to this publication.
Ethical statements
We as authors have abided by the Ethical Principles of Psychologists and Code of Conduct as set out by the APA. The study was reviewed and approved by the Regional Ethical Board in Stockholm, Sweden (reference number 2016/856–31).










Comments
No Comments have been published for this article.