Introduction
Exposure to trauma is a significant risk factor for psychopathology (Nemeroff, Reference Nemeroff2004). However, some individuals exposed to severe traumatic events do not develop full-blown psychiatric disorders. There are growing indications that these trauma-exposed individuals show neural alterations, even in the absence of clinical symptoms (Stark et al., Reference Stark, Parsons, Van Hartevelt, Charquero-Ballester, McManners, Ehlers, Stein and Kringelbach2015; Teicher and Samson, Reference Teicher and Samson2016). Tasks requiring the regulation of emotions and impulses seem to be particularly affected. Concurrently, in trauma-exposed psychiatric patients, significant impairments in these domains are observed, across psychopathologies. These deficiencies are believed to be responsible for hallmark trauma-associated psychiatric symptoms such as hyperarousal in post-traumatic stress disorder (PTSD) (Shalev et al., Reference Shalev, Liberzon and Marmar2017) and depression (Goldsmith et al., Reference Goldsmith, Chesney, Heath and Barlow2013) or impulsivity in borderline personality disorder (van Zutphen et al., Reference van Zutphen, Siep, Jacob, Goebel and Arntz2015).
Previously, studies have extracted distinct neural dysregulations associated with PTSD by comparing PTSD patients to trauma-exposed controls (Fani et al., Reference Fani, Jovanovic, Ely, Bradley, Gutman, Tone and Ressler2012a, Reference Fani, Tone, Phifer, Norrholm, Bradley, Ressler, Kamkwalala and Jovanovic2012b). More recently, there has been an increasing focus on trauma-exposed participants without clinical symptoms. Despite some earlier reports of decreased inhibitory performance associated with trauma (Aupperle et al., Reference Aupperle, Melrose, Stein and Paulus2012), trauma-exposed participants without current psychopathology frequently do not suffer from significant behavioural impairments (Falconer et al., Reference Falconer, Bryant, Felmingham, Kemp, Gordon, Peduto, Olivieri and Williams2008; Covey et al., Reference Covey, Shucard, Violanti, Lee and Shucard2013; Quidé et al., Reference Quidé, O'Reilly, Watkeys, Carr and Green2017; Melara et al., Reference Melara, Ruglass, Fertuck and Hien2018). However, studies of neural underpinnings paint a complex picture. For example, a recent fMRI-based study by Quidé et al. (Reference Quidé, O'Reilly, Watkeys, Carr and Green2017) examined non-affective response inhibition in patients with psychotic disorders and healthy controls. While not finding any effects of psychosis, the authors found altered left inferior frontal gyrus (IFG) functioning in a subgroup of participants that was exposed to childhood trauma. Furthermore, Melara et al. (Reference Melara, Ruglass, Fertuck and Hien2018) compared neural correlates of distractor inhibition between PTSD patients, trauma-exposed participants without PTSD and trauma-naïve healthy controls by means of EEG. Results were generally highly similar for both non-PTSD groups. However, in trauma-exposed without PTSD only, the ventromedial prefrontal cortex was associated with inhibitory control. Thus, these participants may require more active inhibition when processing emotional stimuli than trauma-naïve participants. An EEG-based study conducted by Covey et al. (Reference Covey, Shucard, Violanti, Lee and Shucard2013) compared response inhibition of trauma-exposed policemen without the current psychiatric disorder to trauma-naïve civilian controls. Here, greater P3 amplitude, suggesting greater arousal, was found in police men compared to controls. An earlier fMRI study by Falconer et al. (Reference Falconer, Bryant, Felmingham, Kemp, Gordon, Peduto, Olivieri and Williams2008), however, examining response inhibition did not find any neural differences between healthy trauma-exposed and trauma-naïve controls.
Overall, previous results are highly heterogeneous. They may point to subtle alterations that remain difficult to detect but can figure prominently. In fact, it has been suggested that differences in inhibitory functioning increase vulnerability to subsequent psychopathology (Teicher and Samson, Reference Teicher and Samson2016). Alternatively, they may mark psychiatric resilience, either as a pre-existing factor or as a product of early stress experiences. Notably, previous studies have not controlled for a lifetime diagnosis of any psychiatric disorder. Also, associations with clinical symptomatology in these trauma groups are poorly understood.
Importantly, differences may amplify in more demanding environments that induce everyday life stress. Subtle deficits in emotion regulation and inhibition may not significantly influence daily functioning until acute, ordinary and non-traumatic stress perturbates the system. Stress strengthens threat detection and rapid reactions but at the same time, it decreases the ability to inhibit automatic reactions (Starcke and Brand, Reference Starcke and Brand2012; Shields et al., Reference Shields, Sazma and Yonelinas2016). Furthermore, the sensitivity of neural emotion regulation processes to psychological everyday stress might be particularly relevant for the aetiology of trauma-related disorders (Nemeroff, Reference Nemeroff2004).
Several candidate brain areas are known to play an important role in top-down inhibition of emotions. The ventrolateral prefrontal cortex including the IFG represent the core areas facilitating response inhibition and top-down control of emotion (Kohn et al., Reference Kohn, Eickhoff, Scheller, Laird, Fox and Habel2014). Also, the bilateral middle frontal gyrus, as well as the anterior cingulate and medial prefrontal cortex, have consistently been associated with emotion inhibition (Cromheeke and Mueller, Reference Cromheeke and Mueller2014; Kohn et al., Reference Kohn, Eickhoff, Scheller, Laird, Fox and Habel2014). Moreover, the anterior insula fulfils a central role in emotion regulation processes by marking events as salient and coordinating the involvement of different, large-scale neural networks (Menon and Uddin, Reference Menon and Uddin2010; Menon, Reference Menon2011). Previous studies have further suggested that trauma-related psychopathology is associated with altered neural responses during emotion processing and inhibition in the lateral and medial prefrontal cortex as well as anterior insula (van Zutphen et al., Reference van Zutphen, Siep, Jacob, Goebel and Arntz2015; Aupperle et al., Reference Aupperle, Stillman, Simmons, Flagan, Allard, Thorp, Norman, Paulus and Stein2016). Some studies in trauma-exposed participants without current clinical symptoms have pointed to only slight anomalies in overlapping areas (Stark et al., Reference Stark, Parsons, Van Hartevelt, Charquero-Ballester, McManners, Ehlers, Stein and Kringelbach2015).
However, it is unknown how these neural systems in trauma-exposed individuals react to demanding environments and challenging circumstances. In this study, we therefore examined the effect of acute ordinary, non-traumatic stress on subsequent emotion inhibition in healthy women who had experienced multiple severe sexual or physical traumatic events before the age of 18, but never developed any psychiatric disorder (neither on DSM Axis I nor II; T+) and matched trauma-naïve healthy controls (T−).
We expected no significant differences in behavioural performance between trauma-exposed and trauma-naïve individuals, and neural differences to be subtle under baseline conditions. However, under stress, we hypothesized that trauma-exposed participants would, compared to trauma-naïve participants, show decreased top-down control, particularly in the IFG, and increased activation in areas of emotion and salience processing (Stark et al., Reference Stark, Parsons, Van Hartevelt, Charquero-Ballester, McManners, Ehlers, Stein and Kringelbach2015; Homberg et al., Reference Homberg, Kozicz and Fernández2017). In addition, we assessed the exploratory hypothesis that healthy trauma-exposed participants might show different (compensatory) mechanisms to facilitate emotion inhibition.
Methods
Participants
Twenty-five healthy women who experienced multiple severe sexual or physical traumatic events (T+) were recruited. The inclusion criterion for this group was a minimum of three traumatic events (sexual or physical) before the age of 18 with neither a history of nor current psychiatric disorders. We conducted a thorough pre-screening via phone (duration approximately 40 min) to pre-assess the authenticity of the trauma criterion. In addition, 25 matched healthy control participants without a history of trauma (T−) participated. All were recruited via public advertisements. Participants underwent a detailed diagnostic interview by a trained clinical psychologist to assess inclusion and exclusion criteria. For both groups, psychiatric disorders (lifetime and current) were assessed by the Structured Clinical Interviews for DSM-IV Axis I and II (SCID I and II; Wittchen et al., Reference Wittchen, Zaudig and Fydrich1997). The inclusion criterion of severe early life trauma (sexual or physical) was further assessed using the German version of the Early Trauma Inventory (ETI; Bremner et al., Reference Bremner, Vermetten and Mazure2000; Wingenfeld et al., Reference Wingenfeld, Driessen, Mensebach, Rullkoetter, Schaffrath, Spitzer, Schlosser, Beblo and Heim2011), a 56-item semi-structured interview for the assessment of physical, emotional and sexual abuse as well as general traumatic experience. The interview served to obtain in-depth information as well as specific (e.g. onsets, offsets, perpetrators, etc.) information about the events and to assess authenticity of the report. In order to collect an interviewer-independent measure and to increase comparability with previous studies, participants additionally completed the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003). All participants of the trauma group also fulfilled Criterion A for PTSD of the DSM-V. This criterion requires the person having been exposed to ‘death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence’ (American Psychiatric Association, 2013).
Criteria for exclusion were (1) lifetime diagnosis of a psychiatric disorder as assessed by the Structured Clinical Interviews for DSM-IV Axis I and II (SCID I and II; Wittchen et al., Reference Wittchen, Zaudig and Fydrich1997), (2) adverse health conditions affecting the central nervous or endocrine system function, (3) non-removable ferromagnetic material, (4) auto-immune and infectious diseases, (5) hypertension, (6) a transcontinental flight within the last 4 weeks, (7) excessive physical exercise of more than 10 h a week and (8) left-handedness. The study was conducted in accordance with the latest version of the Declaration of Helsinki and received approval by the local ethics committee (protocol number EA4/104/13). All participants provided written informed consent and were financially reimbursed.
Two T+ and two T− participants had to be excluded due to excessive head movement (more than 3 mm translation or 3° rotation). These participants were hence excluded from all analyses.
Study design and procedure
The study consisted of a diagnostic and an experimental session that took place on different days within a 2-week period. During the diagnostic session, inclusion and exclusion criteria were assessed and the following self-report questionnaires were obtained: Beck Depression Inventory II (BDI II; Beck et al., Reference Beck, Steer and Brown1996), the Spielberger State-Trait Anxiety Inventory (STAI-T; Spielberger et al., Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983), the Posttraumatic Diagnostic Scale (PDS; Foa et al., Reference Foa, Cashman, Jaycox and Perry1997), the Perceived Stress Scale (PSS; Cohen et al., Reference Cohen, Kamarck and Mermelstein1983) as well as the Stress Reactivity Scale (SRS; Schulz et al., Reference Schulz, Jansen and Schlotz2005).
During the experimental session, participants completed two runs of an emotional go-nogo (eGNG) paradigm during fMRI, one before and one after psychosocial stress induction by an adapted version of the Montreal Imaging Stress Task (MIST; Dedovic et al., Reference Dedovic, Renwick, Mahani, Engert, Lupien and Pruessner2005; Pruessner et al., Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner, Buss, Renwick, Dagher, Meaney and Lupien2008). The experimental procedure is depicted in Fig. 1, and the fMRI paradigm is described in more detail below.
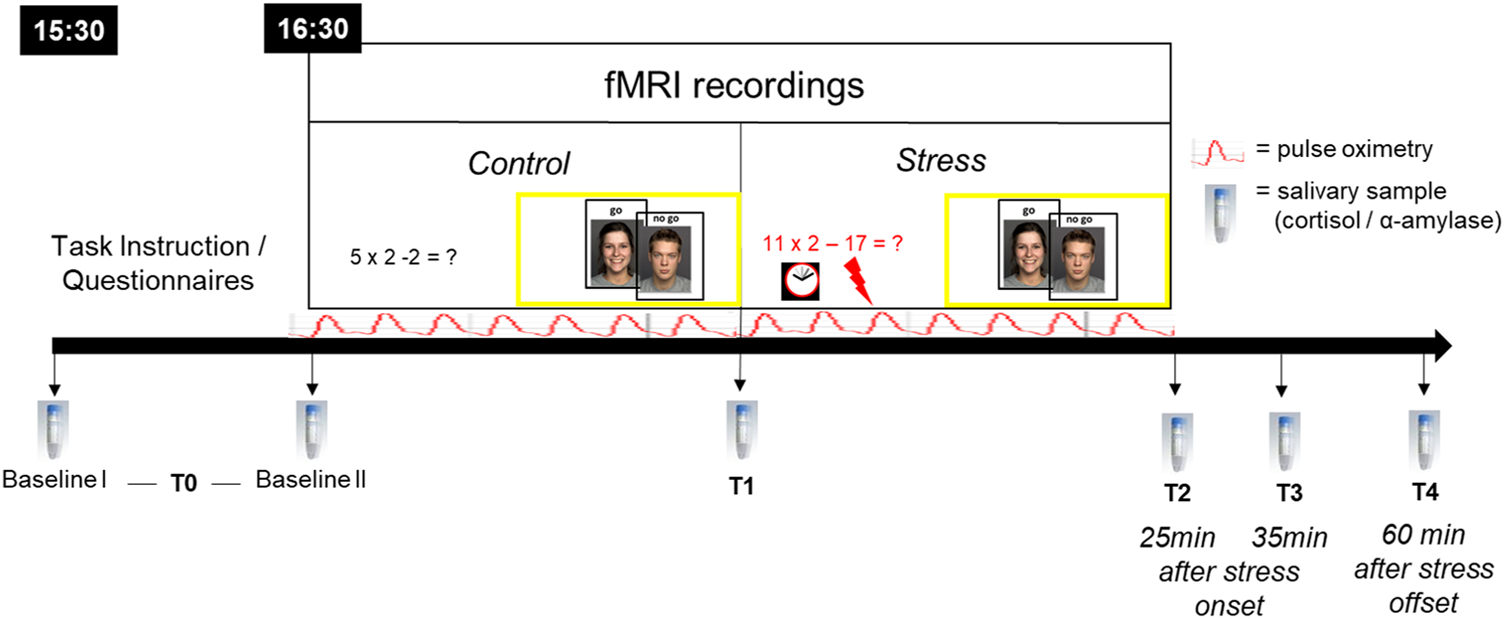
Fig. 1. Experimental procedure. All participants arrived at 15:30 h at the laboratory to control for circadian rhythmicity of cortisol release, fMRI testing began at 16:30 h. During fMRI, participants completed two runs of an emotional go-nogo (eGNG) paradigm (yellow boxes), one in the control condition and one after psychosocial stress induction. Six salivary samples were taken over the course of the experimental session. Baseline I (upon arrival) and II (before scanning, 45 min later) were averaged to a single baseline value (T0) to reduce situational influences on baseline measures. The T1 sample was taken inside the scanner, in-between control and stress condition, approximately 20–25 min after Baseline II. T2 = 25 min after stress onset, T3 = 35 min after stress onset, T4 = 60 min after stress offset. Pulse oximetry was used to measure heart rate over the course of the fMRI session.
eGNG paradigm
We used a modified version of an eGNG paradigm previously employed by Hare et al. (Reference Hare, Tottenham, Galvan, Voss, Glover and Casey2008). Subjects completed two runs (one in the control and one in the stress condition), comprised of four blocks each. Each block consisted of 30 go-trials and 10 nogo-trials. During go-trials, a target facial expression was presented, and subjects were asked to respond as fast as possible by pressing a button. During nogo-trials, non-target facial expressions were presented and subjects were asked to avoid any button press (inhibition trials). Two blocks used emotional target facial expressions (fearful or happy, respectively) in the presence of neutral non-targets, and two blocks employed neutral target faces, one in the presence of fearful non-targets and one in the presence of happy ones. Together with the participants' response, this task design provides for the analysis of four different trial types: correct go-trials (hits), correct nogo-trials (inhibition trials), incorrect nogo-trials (false alarms) and incorrect go-trials (misses).
At the beginning of each block, written instructions were displayed telling the subject to respond to the target facial expression as fast as possible by pressing with the right thumb and not to press any button for non-target facial expressions. In addition, the current task was verbally explained to the participants by the experimenter over the microphone. See Supplementary materials M1 and methods for details on stimuli selection, presentation and timing.
Stress induction and response measures
For psychosocial stress induction, we employed an adapted version of the MIST (Dedovic et al., Reference Dedovic, Renwick, Mahani, Engert, Lupien and Pruessner2005; Pruessner et al., Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner, Buss, Renwick, Dagher, Meaney and Lupien2008). See Supplementary materials and methods M2 for details. In short, during the control condition, easy arithmetic questions were presented. Stress induction then consisted of difficult arithmetic questions under time pressure in addition to negative social feedback.
To evaluate subjectively experienced stress, we asked participants to rate their stress and strain level on a 10-point scale during the control and stress condition after the experiment. Heart rate was recorded using the integrated photoplethysmograpth of the Siemens Physiological Monitoring Unit under the left index finger. As a manipulation check of physiological stress induction, six saliva samples were collected, the first two were averaged as a single baseline value (T0) to reduce situational influences on baseline measures (see Fig. 1). Details on peak detection and biochemical analysis of saliva samples are provided in the Supplementary materials and methods M3 and M4.
fMRI first-level model
Please refer to Supplementary materials and methods M5 for details on acquisition parameters and pre-processing. Effects were estimated using an event-related general linear model convolving each trial with a haemodynamic response function. A fixed-effect model was performed to create images of parameter estimates. We modelled four different emotion conditions, corresponding to four blocks of trials. These were blocks with (1) fearful nogo faces–neutral go faces, (2) happy nogo faces–neutral go faces, (3) neutral nogo faces–fearful go faces, (4) neutral nogo faces–happy go faces. For each emotion condition, we also modelled the control and stress condition separately. Within each control/stress condition and each emotion condition in turn, we modelled four different trial types: correct go trials, correct nogo trials, false alarms (incorrect go), misses (incorrect nogo). Thus there were 4 (emotion condition) × 4 (trial type) × 2 (stress v. control condition) regressors. Regressors containing correct nogo trials were the regressors of interest. Please refer to online Supplementary Table S1 for a complete list of regressors and belonging trials. Additionally, realignment parameters were included as additional regressors in the model. Individual t-contrast maps of (stress>control) for all correct inhibition (nogo) trials of all emotion conditions were computed.
Statistical group analysis
Sample characteristics and stress induction measure
All non-imaging data related analyses were carried out using IBM SPSS Statistics 22 for Windows (SPSS Inc., Chicago, IL, USA). Demographic and clinical data were analysed with two sample t tests or χ2 tests. Salivary data was winsorized (95th percentile) and log-transformed. Subjective stress, mean heart rate, cortisol and α-amylase values were analysed with a mixed design analysis of variance (ANOVA), Greenhouse-Geisser correction was used when appropriate. Post-hoc t tests were Bonferroni corrected.
eGNG paradigm
Behavioural. We explored stress-induced failures of response inhibition as indexed by false alarm rate (FAR) on inhibition (nogo) trials in the stress v. control condition (cf. Wager et al., Reference Wager, Sylvester, Lacey, Nee, Franklin and Jonides2005). First, the effect of stress on FAR in all four nogo emotion conditions: fearful, happy, neutral (fearful go), neutral (happy go) was assessed by Wilcoxon signed-rank testing. Second, the stress-induced increase ΔFAR was calculated by subtracting the control from the stress runs and Mann–Whitney U testing was employed to test group differences in ΔFAR. The Bonferroni method was used to correct for multiple testing.
Imaging (group level). On the group level, individual t-contrast images (stress>control) from first level were entered into a flexible factorial ANOVA with group (T+ v. T−), emotion condition (fearful nogo, happy nogo, neutral nogofear−go, neutral nogohappy−go) and subject as factors. We used probabilistic threshold-free cluster enhancement (pTFCE) (Spisák et al., Reference Spisák, Spisák, Zunhammer, Bingel, Smith, Nichols and Kincses2019) in addition to whole brain peak-level (voxel-wise) FDR correction with p < 0.05 and a minimum cluster size threshold of k > 30.
Results
Stress induction
Demographic and clinical data are shown in Table 1.
Table 1. Sample characteristics
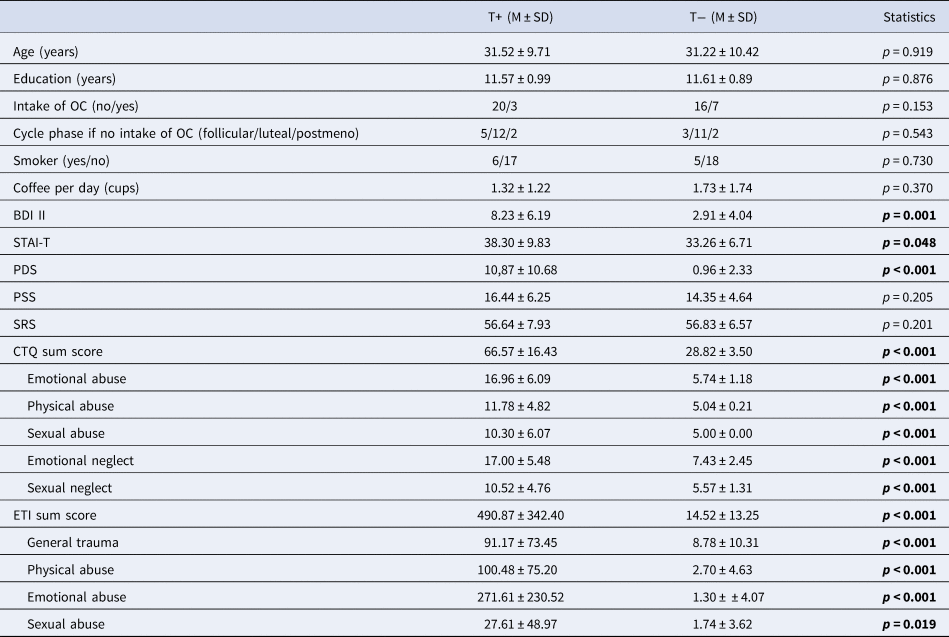
T+, trauma-exposed participants; T−, trauma-naïve control participants; OC, oral contraceptives; BDI II, Beck Depression Inventory II; STAI-T, Spielberger Trait Anxiety Inventory; PDS, Posttraumatic Stress Diagnostic Scale; PSS, Perceives Stress Scale; SRS, Stress Reactivity Scale; CTQ, Child Trauma Questionnaire; ETI, Early Trauma Inventory.
Information on cycle phase was unavailable for one T+ due to uterine agenesis.
Subjective stress and heart rate increases
An ANOVA for subjective stress showed a main effect of stress indicating higher values in the stress compared to the control condition (F 1,42 = 88.4, p < 0.001), but no main effect of group or group by stress interaction. For subjective strain, we also saw a significant main effect of strain (N = 44, F 1,42 = 92.7, p < 0.001) and a significant condition by group interaction, indicating higher strain increase in T+ (F 1,42 = 7.1, p = 0.042, see online Supplementary Fig. S1).
Heart rate was significantly higher during the stress compared to the control condition as well as during the MIST compared to eGNG in both groups (see Fig. 2a and online Supplementary Table S2).
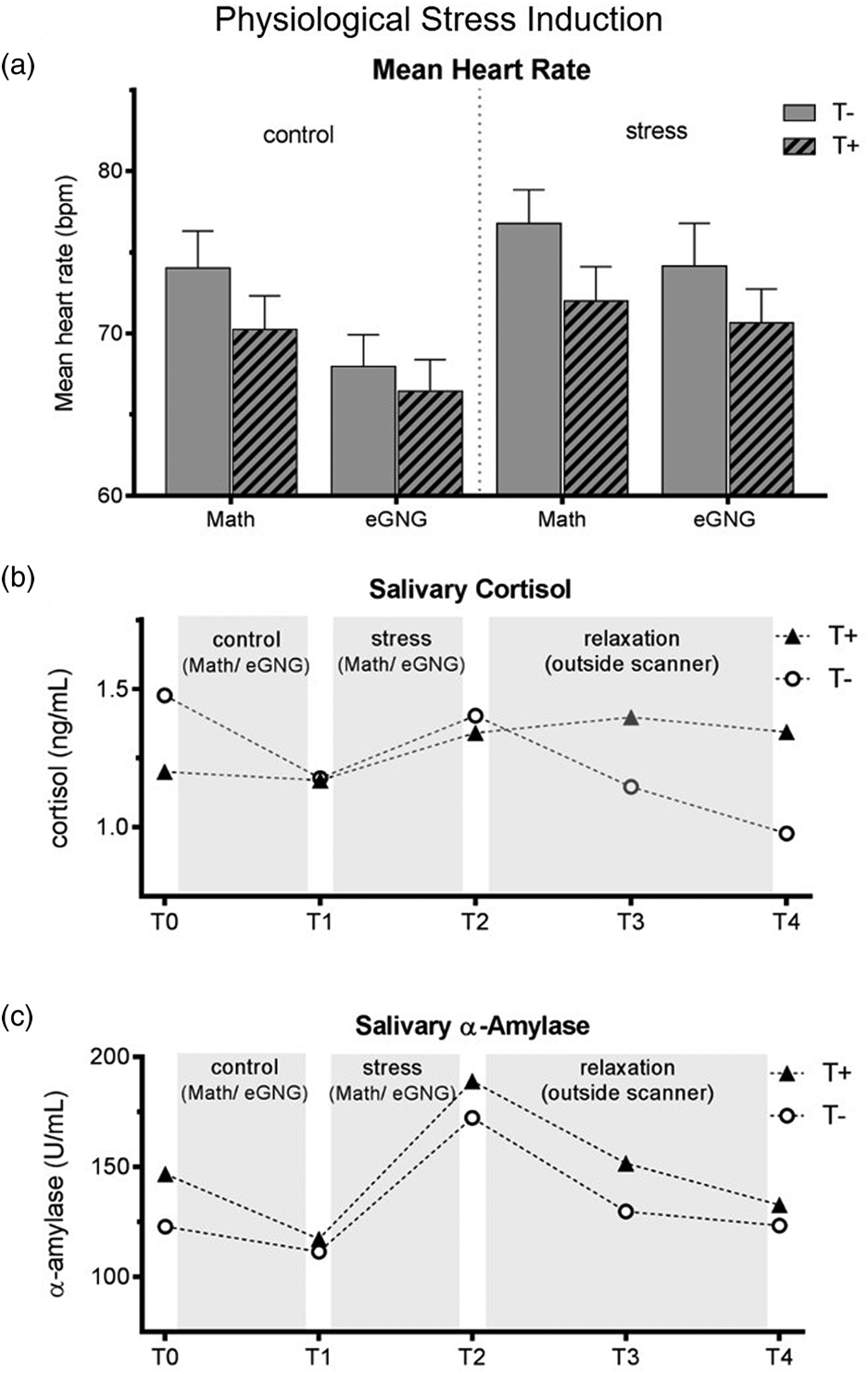
Fig. 2. (a) Mean heart rate over the experiment. Heart rate data of eight participants (3 T+ and 5 T−) had to be excluded due to scanner and movement related artefacts. We computed a mixed ANOVA including condition (control v. stress) and task (MIST v. eGNG) as within-subject factors and group as a between-subject factor. A significant main effect of condition (N = 38, F 1,37 = 13.8, p = 0.001) demonstrated elevated heart rate in the stress condition compared to control, while a main effect of task (N = 38, F 1,37 = 61.8, p < 0.001) indicated higher mean heart rate during presentation of math questions than during the eGNG paradigm. There was no main effect of group and no significant interactions. (b) Raw salivary cortisol data. Before the analysis of salivary cortisol and salivary α-amylase levels, one participant (T−) had to be discarded due to food consumption at sampling time. For statistical analysis, data were winsorized and log-transformed. Two mixed ANOVAs including a within-subject factor time (5 measurement points) and a between-subject factor group were conducted. For cortisol, we found a main effect of time (N = 45, F 4,43 = 4.8, p = 0.009), but no main effect of group or interaction effect. Post-hoc Bonferroni corrected paired samples t tests examining the increase during stress condition (T1 v. T2) and subsequent recovery (T2 v. T3) across all participants showed a significant cortisol increase during stress (T1 v. T2: N = 45, t 44 = 2.4, p = 0.044 adjusted) and recovery afterwards (T2 v. T3: N = 45, t 44 = 5.2, p = < 0.001 adjusted). (c) For α-amylase, there was a main effect of time (N = 45, F = 4172 = 17.3, p < 0.001), no main effect of group, but a time by group interaction (N = 45, F 4172 = 2.6, p = 0.037). To decode the interaction, we conducted Bonferroni corrected paired sample t tests between all consecutive time points (T1 v. T2, T2 v. T3, etc.) for both groups separately (four tests per group). This analysis revealed a marginally significant salivary α-amylase decrease from T0 to T1 in T+ participants (N = 23, t 22 = 2.7, p = 0.056 adjusted) but not in T− participants (p > 0.999 adjusted), as well as large increases from T1 to T2 in both groups (T+ group: N = 23, t 22 = 7.4, p < 0.001 adjusted, d = 1.6; T− group: N = 22, t 21 = 3.8, p = 0.004 adjusted, d = 0.9). There were no significant differences in any other paired tests. Thus, the interaction was attributable to group differences in α-amylase changes from T0 to T1, resulting from higher α-amylase baseline levels (T0) in the T+ group (two-sample t test of T0 values: N = 45, t 44 = 2. 2, p = 0.030). See online Supplementary Table S3 for all means and standard errors. T−, trauma-naïve control participants; T+, trauma-exposed participants; control, control condition; stress, stress condition; eGNG, emotional go-nogo paradigm; bpm, beats per minute.
Salivary cortisol and α-amylase
Cortisol levels significantly increased during stress induction (T2 to T3) and significantly decreased afterwards (T3 to T4), there were no group differences or time by group interactions. The α-amylase levels also significantly increased during stress induction (T2 to T3) in both groups; in addition, the T+ group had significantly higher baseline values (T1; see Fig. 2b–c).
Emotional response inhibition
Behavioural
The effect of stress on FARs on nogo stimuli in the four emotion conditions was examined across all participants. Stress significantly increased FAR on fearful faces (Wilcoxon: N = 46, Z = −3.6, p < 0.001 adjusted, r = 0.5), but had neither an effect on happy ones (N = 46, Z = −1.1, p = 0.29 unadjusted) nor on one of the neutral stimuli conditions (fearful go: N = 46, Z = −0.3, p = 0.76 unadjusted; happy go: N = 46, Z = −0.04, p = 0.98 unadjusted) (Fig. 3). The stress-associated increase in false alarms ΔFAR did not significantly differ between the groups for any emotion condition [Mann–Whitney U tests: N = 46 for all, Z happy = −0.9, p = 0.39, Z fear = −0.8, p = 0.46, Z neut(happy−go) = −0.2, p = 0.84, Z neut(fear−go) = −0.8, p = 0.46]. Thus, stress decreased inhibition of fearful nogo stimuli to a similar degree in both groups but had no effect on happy or neutral ones. Statistics on go stimuli are provided in online Supplementary Table S4.
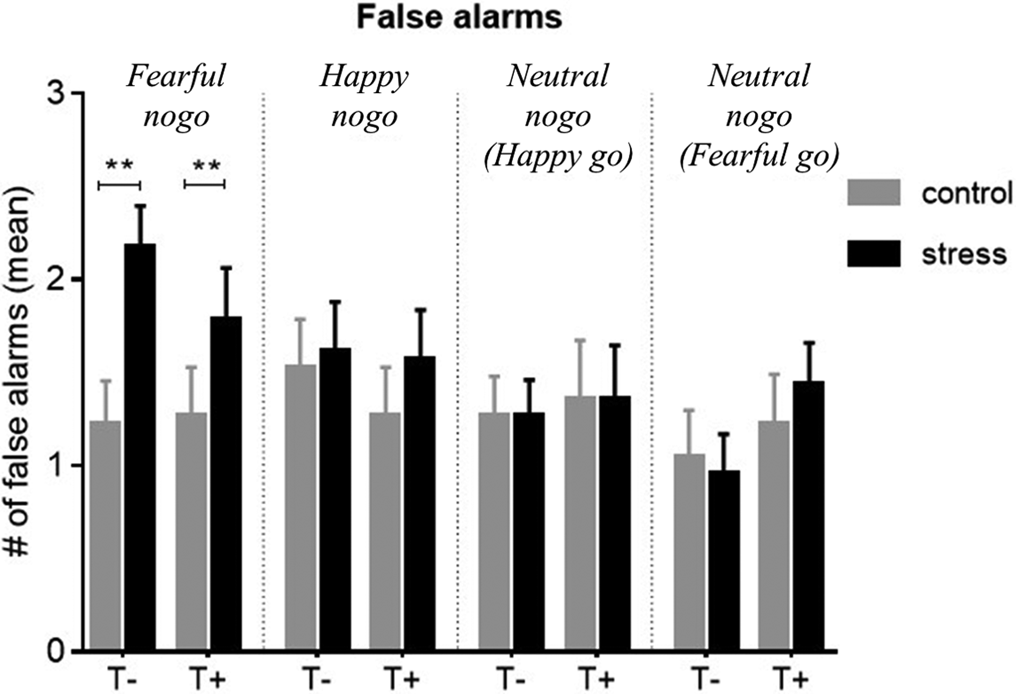
Fig. 3. Mean false alarm rate (FAR) for non-target (nogo) trials of all emotional conditions, compared between control and stress conditions. There was a significant stress-induced increase in FAR on fearful non-targets in both groups (p < 0.001), but no group differences. Stress had no significant effect on FAR in any other emotion condition. Control, control condition; stress, stress condition; T+, trauma-exposed participants; T−, trauma-naïve control participants.
Imaging
Results for neural correlates during stress induction (MIST) can be found in online Supplementary Tables S5 and S6. As stress only affected response inhibition of fearful nogo stimuli, we focused on exploring neural foundations of this stress effect on fearful stimuli in both groups. The analysis was hence on average based on 26.3 correct nogo trials for baseline and another 25.5 correct nogo trials for the stress condition. The principal contrast compared fearful nogo to neutral nogo faces and tested for an interaction effect with the group factor, i.e. [|fearful nogo–neutral nogo| × group]. This analysis revealed a significant interaction in the left IFG, indicating that T+ individuals showed blunted left IFG activation under stress compared to T− participants (MNI: −42, 38, −4, t = 5.33, p = 0.010 pTFCE and peak-level FDR corrected, 355 voxels). The reverse interaction contrast testing for increased neural activation under stress in the T+ group compared to the T− group hinted at a higher stress-induced activation in the T+ group in the right anterior insula (aIns) compared to the T− group, which however did not survive whole brain pTFCE and FDR correction (MNI: 39, 17, 5, t = 3.65, p = 0.5 pTFCE and peak-level FDR corrected, p < 0.001 uncorrected, 40 voxels) (Fig. 4a). Additionally, an analysis was run comparing the subgroup of neutral nogo trials that were part of fearful go-blocks with fearful nogo trials [|fearful nogo–neutral nogofearful−go| × group]. Here, we found that the anterior insula cluster was slightly larger (IFG: cluster: MNI: −39, 38, −1, t = 5.51 p = 0.037 TFCE and peak-level FDR corrected, 305 voxels; insula: MNI: 33, 14, 8, t = 4.8, p = 0.1 TFCE and peak-level FDR corrected, p < 0.001 uncorrected, 57 voxels). To assess whether our findings were lateralized, we lowered the threshold to p < 0.001 uncorrected, k > 5 voxels, but did not see the respective bilateral activation difference (i.e. in the right IFG or left anterior insula). Next, we extracted mean BOLD parameter estimates of the left IFG and right anterior insula (5 mm sphere around peak voxel) from individual contrast images showing stress v. control during fearful nogo trials and analysed associations with a stress-induced increase in FAR. A sphere around the peak has been shown to be a reliable and sensible single-value ROI and was therefore chosen instead of the whole cluster (Tong et al., Reference Tong, Chen, Nichols, Rasetti, Callicott, Berman, Weinberger and Mattay2016). The Spearman correlation coefficient was used to test for bivariate correlations as a stress-induced increase in FAR values deviated significantly from a normal distribution.
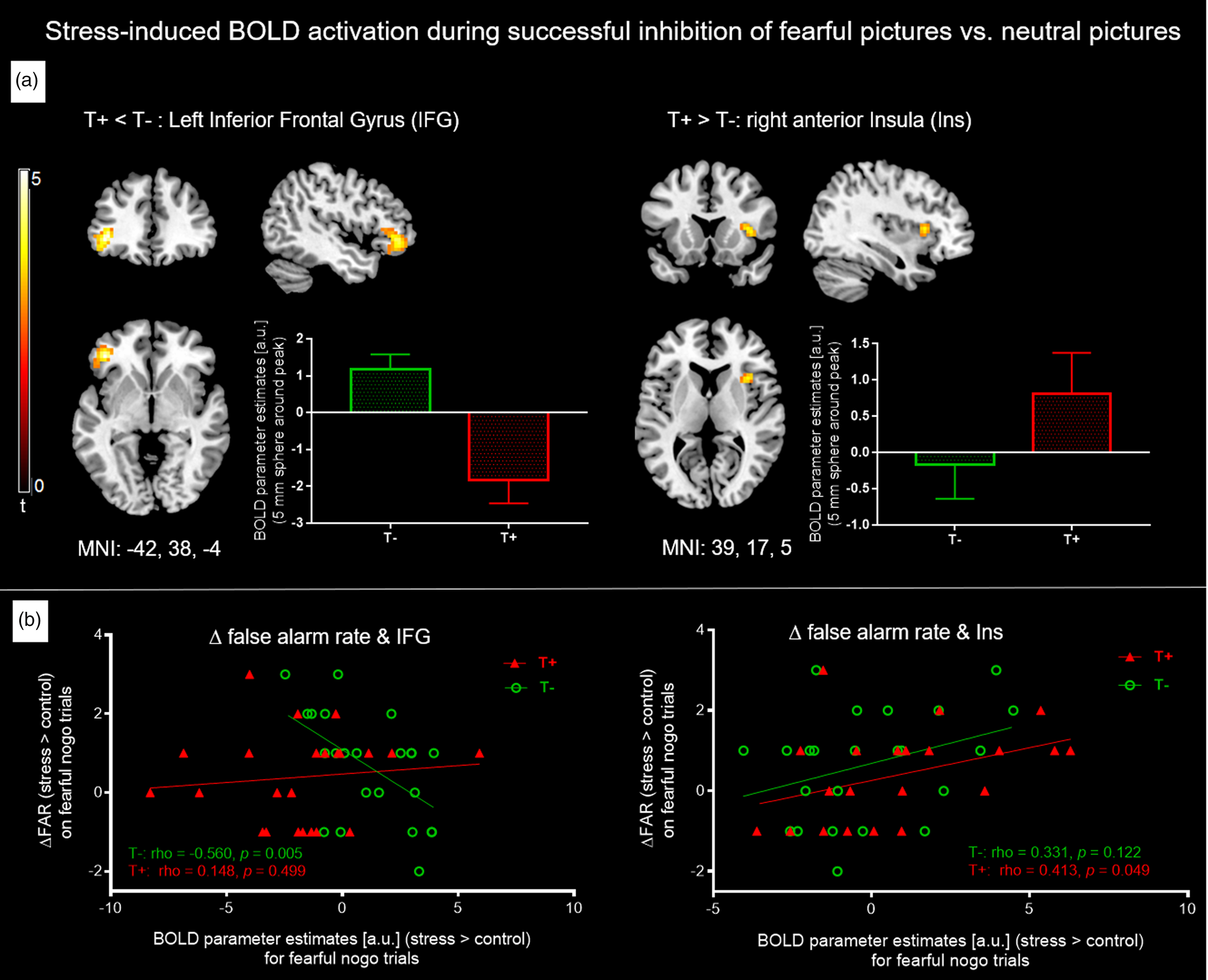
Fig. 4. (a) Individual t-maps of stress-induced BOLD increases (stress > control) for non-target (i.e. nogo) trials of all four emotion conditions were entered into a flexible factorial ANOVA and for both groups, fearful nogo-pictures were compared to neutral nogo ones [|fearful-nogo – neutral-nogo| × group]. The T− group showed higher stress-associated left IFG activation (left), whereas T+ participants showed marginally higher stress-associated right anterior insula activation. Bar graphs depict mean BOLD parameter estimates from a 5 mm sphere around the clusters' peak voxel. For IFG, results are pTFCE and FDR peak-corrected (p < 0.05) for the whole brain, minimum cluster size k > 30 voxels. For anterior insula, results are uncorrected, p < 0.001, k > 30. (b) Left: Significant negative Spearman correlation between ΔFAR (increase in FAR on fearful nogo trials from control to stress condition) and stress induced left IFG activation during fearful nogo-trials (i.e. stress > control) in T− controls but not T+ participants. Right: Significant positive Spearman correlation between ΔFAR and stress-induced right insula activation during fearful nogo-trials in the T+ but not the T− group. IFG, inferior frontal gyrus; FAR, false alarm rate; T−, trauma-naïve control participants; T+, trauma-exposed participants; control, control condition; stress, stress condition; a.u., arbitrary unit.
For the left IFG, activation increase under stress in the T− group correlated negatively with a stress-induced increase in FAR (N = 23, Spearman ρ = −0.6, p < 0.01), which was not observed in the T+ group (N = 23, Spearman ρ = 0.1, p = 0.50). This indicates that in T− participants, higher left IFG activation during stress tends to amount to better response inhibition of fearful faces under stress (Fig. 4b, left).
For the right anterior insula, we observed that stress-induced activation in the T+ group was positively related to a stress-induced increase in FAR (N = 23, Spearman ρ = 0.4, p = 0.049), but not in the T− group (N = 23, Spearman ρ = 0.3, p = 0.12). Thus, a higher activation of the right anterior insula in T+ participants is associated with a higher increase in FAR on fearful nogo faces (Fig. 4b, right).
Furthermore, we analysed associations of stress-induced neural activation with psychiatric anomalies (posttraumatic symptom severity by PDS, trait anxiety by STAI-trait, depressive symptoms by BDI-II). Left IFG activation for the whole sample correlated negatively with PDS total score (N = 46, Spearman ρ = −0.568, p < 0.001), as well as with all PDS subscales, see online Supplementary Fig. S2. There was also a significant negative correlation of left IFG activation with STAI-trait (N = 46, Spearman ρ = −0.370, p = 0.011), but none with BDI-II. There was no significant correlation between stress-induced insula activation and psychiatric symptoms.
Discussion
We investigated response inhibition in an eGNG paradigm using emotional face stimuli. We compared women who had experienced severe and multiple traumatic events before the age of 18 (T+) but have not (yet) developed any psychiatric disorder, with trauma-naïve control subjects (T−). In both groups, non-traumatic stress significantly impaired response inhibition of fearful facial expressions but did not affect inhibition of happy or neutral stimuli. When inhibiting responses to fearful faces under stress, T+ participants demonstrated a decreased brain signal in the left IFG but a marginally increased signal in the right anterior insula compared to T− controls. In T− only, IFG activation was linked to a lower rate of stress-induced false alarms, whereas in T+, insula activation was linked to a higher number of false alarms. To our knowledge, this is the first study demonstrating that trauma, in the absence of lifetime psychiatric disorder, affects neural emotion inhibition under acute stress.
While the IFG represents a central area for the top-down cognitive control of emotions (Cromheeke and Mueller, Reference Cromheeke and Mueller2014), the insula is seen as the neural locus for bottom-up salience detection and interoception (Menon and Uddin, Reference Menon and Uddin2010). It has been suggested that stress temporarily lowers top-down control in exchange for a reallocation of resources to salience detection (Homberg et al., Reference Homberg, Kozicz and Fernández2017). Our results show blunted IFG activation under stress when inhibiting responses to fearful faces in T+ participants compared to T− controls. Additionally, we found a trend towards increased response from the anterior insula in T+ participants under stress. Taken together, this pattern points to more pronounced stress-induced neural resource allocations in T+ individuals, shifting neural resources away from top-down control to salience detection, thereby likely rendering them more vulnerable to detrimental stress effects.
Alternatively, it is possible that these group differences represent markers of resilience in T+ individuals. We thoroughly excluded participants with a prior or current psychiatric disorder and did not find behavioural impairments, which indicates a relatively high level of resilience in this group. However, the current T+ group showed subtle psychiatric alterations on the behavioural level (i.e. higher depressive symptoms, higher trait anxiety, more posttraumatic stress symptoms). Importantly, a blunted IFG signal under stress was related to more posttraumatic symptom severity as well as higher anxiety values. This association further suggests that the observed alteration in the IFG signal in T+ participants is indicative of increased vulnerability rather than resilience. In line with this, both, hypoactivity of the IFG as well as hyperactivity of the right anterior insula has been implicated in the development of trauma-associated psychiatric diseases (e.g. Rauch et al., Reference Rauch, van der Kolk, Fisler, Alpert, Orr, Savage, Fischman, Jenike and Pitman1996; Stein et al., Reference Stein, Simmons, Feinstein and Paulus2007; Hayes et al., Reference Hayes, Hayes and Mikedis2012). In contrast, studies on neural markers of resilience point to increased PFC activation during top-down control (New et al., Reference New, Fan, Murrough, Liu, Liebman, Guise, Tang and Charney2009; Blair et al., Reference Blair, Vythilingam, Crowe, McCaffrey, Ng, Wu, Scaramozza, Mondillo, Pine, Charney and Blair2013), lower limbic activation during emotionally evocative events (Britton et al., Reference Britton, Phan, Taylor, Fig and Liberzon2005) and higher reactivity of the reward system (Vythilingam et al., Reference Vythilingam, Nelson, Scaramozza, Waldeck, Hazlett, Southwick, Pine, Drevets, Charney and Ernst2009). Although the period of greatest risk of developing a psychiatric disorder has passed considering the mean age of T+ participants, a subgroup may still develop a mental disorder over time, depending on the magnitude of environmental stressors. Future studies should employ a longitudinal approach to consider neural differences between those that do and those that do not.
Functional differences of the IFG only arose under stress but did not appear at baseline. In contrast, previous studies in PTSD and other trauma-related disorders have consistently found decreased IFG activity at baseline across tasks. In their meta-analysis, Hayes et al. (Reference Hayes, Hayes and Mikedis2012) highlighted the IFG as a promising target for evaluating therapeutic success in PTSD. The lack of baseline differences in our study indicates that trauma per se might not be associated with baseline IFG reactivity, but that baseline IFG activity changes only occur after the development of PTSD. Our findings therefore support the idea of a gradient in terms of durable effects of trauma upon exposed individuals.
We found an increased anterior insula response to stress in T+ participants. This finding needs to be interpreted with caution because neural activation differences did not survive our very conservative a-priori threshold (p < 0.05 whole brain pTFCE and peak-level FDR correction). However, using a more liberal threshold, we found a large cluster of activation (p < 0.001 uncorrected, k = 40 voxels). Underlining the relevance of the observed neural activation for the behavioural process in T+ individuals, anterior insula activation was related to FARs on fearful faces. Moreover, heightened insula activation is in accordance with numerous reports of trauma-induced insula sensitization and an overactive salience network in trauma-related disorders (Patel et al., Reference Patel, Spreng, Shin and Girard2012; Ruocco et al., Reference Ruocco, Amirthavasagam, Choi-Kain and McMain2013; Stark et al., Reference Stark, Parsons, Van Hartevelt, Charquero-Ballester, McManners, Ehlers, Stein and Kringelbach2015). Furthermore, our finding is plausible from a computational network perspective. This perspective suggests that the insula has three central characteristics: it represents the focal point of the salience network, it coordinates the interplay of different large-scale neural networks and it provides access to the motor system via strong coupling with the anterior cingulate cortex (Menon and Uddin, Reference Menon and Uddin2010). As executive and salience networks are believed to be competing for resources (Fox et al., Reference Fox, Zhang, Snyder and Raichle2009; Hermans et al., Reference Hermans, Henckens, Joëls and Fernández2014; Homberg et al., Reference Homberg, Kozicz and Fernández2017), heightened responsivity of the anterior insula might thus prevent adequate IFG-initiated regulation. Moreover, aberrant coordination by the anterior insula may cause impaired prefrontal network activation (Patel et al., Reference Patel, Spreng, Shin and Girard2012), potentially paving the way to typical trauma-related symptoms. Lastly, the positive correlation between stress-induced insula activation and false alarms on fearful faces in T+ participants may be due to increased coupling with the motor system.
In line with our hypothesis, we moreover observed group differences in brain–behaviour correlations. In the T− group, IFG activation under stress was related to better inhibition of fearful faces under stress, which seems to be disrupted in T+ individuals. Moreover, in T+ only, stress-associated anterior insula activation positively correlated with stress-associated false alarms on fearful stimuli. Contrary to our expectation, we did not find a compensatory mechanism that preserves neural facilitation of emotion inhibition under stress in trauma-exposed individuals. As previous studies have demonstrated alterations in neural connectivity in trauma-exposed individuals (Brown et al., Reference Brown, LaBar, Haswell, Gold, Mid-Atlantic, McCarthy and Morey2013; Cisler et al., Reference Cisler, James, Tripathi, Mletzko, Heim, Hu, Mayberg, Nemeroff and Kilts2013), future studies may examine potential compensatory mechanisms at the brain network level.
Furthermore, stress effects on emotion inhibition were limited to fearful stimuli. Previous investigations have been inconsistent with respect to the valence specificity of stress effects (e.g. Li et al., Reference Li, Weerda, Milde, Wolf and Thiel2014; van Leeuwen et al., Reference van Leeuwen, Vink, Fernández, Hermans, Joëls, Kahn and Vinkers2018). Here, relative levels of activation of sympathetic nervous system stimulation and the hypothalamic–pituitary–adrenal (HPA) axis might play a role. While exogenous cortisol (the end product of HPA axis activity) administration has mostly been found to lead to valence nonspecific effects, elevated noradrenergic signalling from the sympathetic nervous system may modulate stress-induced bias to negative cues, particularly in the presence of elevated cortisol levels (Kukolja et al., Reference Kukolja, Schläpfer, Keysers, Klingmüller, Maier, Fink and Hurlemann2008). As stress induction in the present study activated noradrenergic signal increase as marked by α-amylase levels, combined with moderate HPA axis activation marked by cortisol levels, this could explain the observed specificity of stress effects on fearful stimuli inhibition. Fearful stimuli might be particularly difficult to regulate for T+ individuals under stress.
Finally, we would like to offer some additional considerations regarding our study. It is noteworthy that we carefully selected our experimental group of individuals exposed to severe and repeated early life trauma. Lifetime psychopathology and psychotropic medication were excluded by extensive diagnostic measures including structural clinical interviewing by a trained psychologist for every participant. While trauma exposure was assessed retrospectively, and thus susceptible to recall bias, unlike most related studies, we used the ETI (Bremner et al., Reference Bremner, Vermetten and Mazure2000; Wingenfeld et al., Reference Wingenfeld, Driessen, Mensebach, Rullkoetter, Schaffrath, Spitzer, Schlosser, Beblo and Heim2011), a semi-structured interview to assess traumatic events in addition to questionnaires. Furthermore, we only recruited women to ensure homogeneity, but due to sex differences in acute HPA and autonomic reactivity (Kudielka and Kirschbaum, Reference Kudielka and Kirschbaum2005), it is unclear whether results can be extrapolated to males. Due to a technical problem at the scanner during psychosocial stress induction, we were unable to record BOLD activation during the MIST in nine trauma participants and four controls. We therefore cannot draw reliable conclusions from this neural data during the MIST. Nonetheless, subjective stress ratings, the increase in heart rate and the endocrine data clearly show a pronounced stress response to the MIST. We provide details on MIST acquisition in the Supplementary materials and methods M7 and results in online Supplementary Tables S5 and S6.
It is important to note that we did not investigate acute trauma-exposure or exposure to trauma-trigger. The present study examined emotional response inhibition after an everyday life stressor, which is distinct from stress resulting from trauma or associated triggers. Moreover, early-life trauma-exposure was not associated with differences in ordinary stress-response when considering cortisol or α-amylase levels or reported stress.
Further, for power reasons, correct inhibition trials were used for the analysis of the BOLD signal underlying emotion inhibition. While the observed brain–behaviour correlations support the link between our chosen behavioural and neural substrates, the analysis of neural correlates of erroneous trials (misses as well as false alarms) would be of significant interest. Future studies might exclusively focus on fearful/neutral pictures to provide a sufficient number of erroneous trials. Moreover, replication in a larger sample is advisable in order to confirm results and potentially give insight into subtler (potentially compensatory) mechanisms at the brain network level. Lastly, participants were subjected to the eGNG task twice. Although we used parallel versions to minimize learning effects, we cannot exclude that learning effects might have influenced the results.
In summary, our data suggest that early life trauma has a durable effect on the brain, even in the absence of lifetime full-blown psychopathology. Dysregulation of top-down control by the IFG together with heightened bottom-up salience of fear related cues may alter neural stress processing and potentially increase vulnerability to adverse effects of everyday life stress.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002198.
Acknowledgements
None.
Financial support
S. Golde was supported by a NeuroCure Ph.D. Fellowship (Deutsche Forschungsgemeinschaft, EXC257). S. Grimm, Y.F. and J. H.-R. were supported by Grants of the Deutsche Forschungsgemeinschaft (S.Grimm, Y.F.: DFG-Grant GR 4510/2-1; J. H.-R.: DFG 6939 /3-1) and A.B. was in part supported by a grant from the German Ministry for Education and Research (BMBF 01ZX1311/E and 01ZX1611/E). S.M.G. is supported by a Heisenberg Professorship from the Deutsche Forschungsgemeinschaft (GO1357/5-2) and has received honoraria (speaking fees) from Mylan GmbH and Almirall S.A. and research support from Biogen and the National MS Society. C.O. reports honoraria from Lundbeck and Neuraxpharm.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









