Pregnancy is a life stage at which significant changes in vitamin D and Ca metabolism occur to provide the required Ca to the fetus for bone mineral accretion; however, nutritional requirements for vitamin D to promote healthy pregnancy are not known. The current suggested cut-offs for serum 25-hydroxyvitamin D (25(OH)D) concentrations representing vitamin D sufficiency/deficiency are based on the evidence from non-pregnant adults; however, it remains to be established whether there is an increased requirement for vitamin D intake during pregnancy and lactation( 1 , Reference Brannon and Picciano 2 ).
A challenge of assessing vitamin D status during pregnancy is haemodilution. The expansion of maternal plasma volume begins as early as 6 weeks of gestation and continues until it reaches a net 40 % increase at 24–34 weeks of gestation( Reference Ross and Idah 3 ) and an approximately 50 % increase by 36 weeks of gestation( Reference Faupel-Badger, Hsieh and Troisi 4 ). Thus, circulating concentrations of most nutrients decrease by the end of the first 10 weeks of gestation and remain lower than non-pregnancy values until term, even though the absolute total amount of vitamins and minerals in the circulation actually increases during pregnancy( Reference King 5 ). The increased blood volume during pregnancy is evidenced by the reduction in the concentration of circulating serum albumin( Reference De Flamingh and van der Merwe 6 ). Reference values for serum 25(OH)D concentrations based on non-pregnant adults are not necessarily suitable at different stages of gestation due to the haemodilution that occurs during pregnancy as well as potentially altered vitamin D metabolism, which is not completely understood. Gestational age-specific reference intervals for serum 25(OH)D concentrations during pregnancy are required( Reference Klajnbard, Szecsi and Colov 7 , Reference Picciano 8 ).
Cord 25(OH)D concentrations are correlated with maternal levels during late pregnancy and at delivery( Reference Dror, King and Durand 9 , Reference Bodnar, Simhan and Powers 10 ), which confirms the placental transfer of 25(OH)D during pregnancy. However, it has been suggested that the concentration of free (unbound) rather than total 25(OH)D is an important determinant of the placental transfer of 25(OH)D. An earlier study( Reference Bouillon, Van Baelen and De Moor 11 ) has reported that although total 25(OH)D concentration is lower in cord than in maternal serum, the concentration of free 25(OH)D is higher in cord. Concentrations of free 25(OH)D are dependent on the capacity of vitamin D-binding protein (DBP)( Reference Bikle, Gee and Halloran 12 ), the major carrier of vitamin D and its metabolites to their target tissues in the human circulation. Concentrations of DBP increase during pregnancy from as early as 8 to 10 weeks of gestation( Reference Ritchie, Fung and Halloran 13 ), followed by an increase in serum 1,25(OH)2D levels at about 10 to 12 weeks( Reference Cross, Hillman and Allen 14 ). However, the magnitude of the increase in DBP varies among studies( Reference Ritchie, Fung and Halloran 13 , Reference Bikle, Gee and Halloran 15 , Reference Bouillon, Van Assche and Van Baelen 16 ).
If DBP concentration increases to the extent that the concentration of free serum 25(OH)D is reduced, this could have a significant impact on the availability of maternal and fetal free 25(OH)D for biological activity. There is also the possibility that DBP increases to maintain the ratio of free 25(OH)D at a consistent level throughout gestation. The aim of the present study was to assess longitudinal changes in total and free serum 25(OH)D concentrations, serum albumin and DBP concentrations in a sample of thirty women throughout pregnancy, as well as postpartum, and to attempt to describe the impact of the gestational stage by assessing serum albumin concentrations as an indicator of haemodilution.
Methods
Study design
A total of 1768 participants who attended for antenatal care at Cork University Maternity Hospital, Cork Ireland (52°N) were recruited to the Screening for Pregnancy Endpoints (SCOPE) Ireland pregnancy cohort study (http://www.scopestudy.net) before 15 weeks of gestation, between March 2008 and January 2011. The SCOPE study is an international pregnancy cohort study with the primary aim of developing screening tests to predict pre-eclampsia, infants who are small for gestational age, and spontaneous preterm birth. There were six research centres in four countries: Auckland, New Zealand; Adelaide, Australia; London, Leeds and Manchester, UK; Cork, Ireland. Data collected in Ireland were analysed in the present study. The SCOPE study was conducted according to the Declaration of Helsinki guidelines, and the Clinical Research Ethics Committee of the Cork Teaching Hospitals approved all procedures. All women provided written informed consent at first visit (14–16 weeks of gestation). The SCOPE study is registered at the Australian, New Zealand Clinical Trials Registry (http://www.anzctr.org.au, ID: ACTRN12607000551493)( Reference North, McCowan and Dekker 17 ).
The main inclusion criteria were low-risk pregnancy; singleton pregnancy before 15 weeks of gestation; nulliparous; not having had a previous pregnancy beyond 20 weeks of gestation. Information on demography, medical history, use of multivitamin supplements, alcohol consumption, and the use of cigarettes and/or recreational drugs were recorded before conception, during the first trimester, and at the time of the 15-week visit. Physical activity was assessed on work, exercise and sedentary activities by a lifestyle questionnaire. Maternal anthropometric measurements included height and weight, which were measured by a research midwife at the first visit only. Weight was measured to the nearest 100 g, without shoes or jacket, using calibrated scales. Height was measured to the nearest centimetre using a stadiometer, without shoes, according to SCOPE standard operating procedures. A non-fasting blood sample was taken from each participant and was processed within 3 h to serum, and stored at − 80°C for future analysis.
For the present nested longitudinal study, thirty Caucasian women were randomly selected at the time of recruitment to the SCOPE cohort between May and September 2009 and delivered between November and March 2010. Women participating in SCOPE Ireland had a maximum of three SCOPE visits: at 15 and 20 weeks of gestation and one visit in the third trimester. Women in the present study were sampled more frequently; non-fasting blood samples were collected at 15, 20, 24, 28, 32, 36 and 40 weeks of gestation and at approximately 9 weeks postpartum (range 8–10 weeks). Every member of the subsample had uneventful deliveries of healthy infants.
Biomarker analysis
Serum 25(OH)D concentration was measured using enzyme immunoassay (OCTEIA® 25-Hydroxy Vitamin D; Immunodiagnostic Systems Limited). The inter- and intra-assay CV were 8·3 and 4·0 %. The quality and accuracy of 25(OH)D analysis in our laboratory was assessed on an on-going basis by participation in the Vitamin D External Quality Assessment Scheme (Charing Cross Hospital, London, UK). Inter-assay variability was avoided by analysing all samples from an individual in the same run.
Serum concentrations of DBP were measured by Quantikine HS ELISA Kits (R&D Systems). The inter- and intra-assay CV were < 10 %, respectively. Serum albumin concentration was measured by colorimetric assay using Randox Albumin Reagent kits (Randox Laboratories Limited), according to the manufacturer's instructions. The inter- and intra-assay CV were 5·1 and 1·5 %.
Free concentrations of 25(OH)D were calculated (pmol/l) using the following equation (all concentrations in molar units)( Reference Bikle, Gee and Halloran 12 ):
The reported correlation coefficient between calculated free 25(OH)D using this equation and measured free 25(OH)D by centrifugal ultrafiltration is 0·925( Reference Bikle, Gee and Halloran 12 ).
Statistical analyses
Sample size was selected based on previous data from cross-sectional studies and randomised controlled trials carried out by our research group, which have consistently indicated that a sample size of approximately 30 is sufficient at 80 % power and a significance level of 5 % to detect a 10 nmol/l difference between groups, which we estimated as a clinically relevant difference over time in the present longitudinal analysis. Statistical analyses were carried out using PASW® Statistics version 20.0 (SPSS, IBM). The analysis of baseline data (at 15 weeks of gestation) revealed that serum albumin, 25(OH)D and DBP concentrations followed a parametric distribution. Free 25(OH)D concentrations were log-transformed. The overall change during pregnancy over time was analysed by repeated-measures ANOVA using the general linear model. Comparison between two time points (e.g. any pregnancy time point v. postpartum) and the magnitude of change between two adjacent time points were analysed using paired t tests. Partial correlations between serum 25(OH)D, free 25(OH)D, albumin, DBP and albumin adjusted-25(OH)D concentrations were carried out with gestational week as the covariate. Results were considered as statistically significant at P≤ 0·05.
Results
Baseline characteristics of the thirty pregnant women at 15 weeks of gestation are presented in Table 1. The age range of the participants was from late 20s to early 30s, with an average BMI of 23·5 kg/m2. Of these participants, one-third were taking a vitamin D-containing supplement, with a daily dose ranging from 2·5 to 10 μg/d, and 13 % were taking a Ca-containing supplement. The calculated free 25(OH)D concentration was 0·02 % of the total serum 25(OH)D. Age and BMI were not associated with DBP, total or free 25(OH)D. Both age (β = − 0·367, P= 0·047) and BMI (β = − 0·333, P= 0·067) were negatively associated with albumin concentrations at 15 weeks of gestation (P= 0·053) and together accounted for 20 % of the variability in albumin concentrations at baseline. Consumption of vitamin D or Ca-containing supplements was not associated with total or free 25(OH)D concentrations at 15 weeks of gestation, which may be due to the small sample size and relatively small dosage.
Table 1 Baseline characteristics of the thirty women at 15 weeks of gestation (Mean values, standard deviations and percentages)

25(OH)D, 25-hydroxyvitamin D; DBP, vitamin D-binding protein.
During gestation, an overall decrease in total 25(OH)D concentrations was observed from 15 weeks of gestation to term (P= 0·002), with a nadir at 36 weeks (see Table 2 and Fig. 1(a)). As a result, the proportion of women with serum 25(OH)D concentration < 30 and 50 nmol/l( 1 ) was 10 and 63 %, respectively, at 15 weeks of gestation and increased to 53 and 80 %, respectively, at 36 weeks of gestation. As the entire subsample was recruited between May and September, we compared longitudinal changes in total 25(OH)D concentrations of women recruited during early summer (May–July, who delivered between October and November) and late summer (August–September, who delivered between February and March) (see Fig. 2) . After an initial slight increase in the concentrations of total 25(OH)D in women recruited during May–June, the pattern of decreasing 25(OH)D concentrations during pregnancy was similar in the two subgroups, although those recruited in late summer exhibited a more rapid decline in 25(OH)D concentrations, which is typical of the seasonal variation at this northerly latitude (52°N).
Table 2 Serum total and free 25-hydroxyvitamin D (25(OH)D), percentage of free 25(OH)D, serum albumin and vitamin D-binding protein (DBP) concentrations among the thirty women at the seven time points during gestation and postpartum (Mean values and standard deviations)
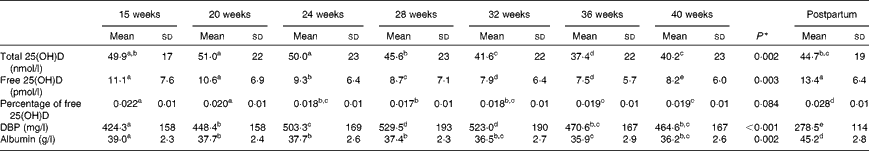
a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05; paired t test).
* Repeated-measures ANOVA.

Fig. 1 Longitudinal changes in circulating (a) total 25-hydroxyvitamin D (25(OH)D), (b) vitamin D-binding protein (DBP), (c) albumin and (d) free 25(OH)D concentrations during pregnancy and postnatal (PN) period. Values are means, with their standard errors represented by vertical bars. w, Weeks.

Fig. 2 Longitudinal changes in circulating total and free 25(OH)D concentrations in women recruited at 15 weeks of gestation in early summer (□, n 11, May–July, who delivered between October and November) and late summer (■, n 19, August–September, who delivered between February and March). Values are means, with their standard errors represented by vertical bars. w, Weeks.
Serum DBP concentrations at 15 weeks of gestation were significantly elevated compared with the postnatal level (P< 0·001). DBP concentrations continued to increase steadily from 15 to approximately 32 weeks of gestation, peaking at 28 weeks, at almost double the postnatal concentration (see Table 2 and Fig. 1(b)). Although there was a slight decrease in DBP concentrations in the last weeks of pregnancy, concentrations were still significantly higher before delivery than postpartum, and the decrease was dramatic after giving birth (P< 0·001). The increase in DBP concentrations during pregnancy was against the haemodilution gradient, which was evidenced by the decrease in albumin concentrations (Fig. 1(c)). Serum albumin concentration was 86 % of the postnatal level at 15 weeks of gestation, 83 % between 20 and 28 weeks of gestation and approximately 80 % from week 32 until term (P< 0·001), with the lowest concentration at 36 weeks of gestation (see Table 2). Albumin concentrations at all the seven time points during pregnancy were significantly lower than the postnatal level (all P< 0·001).
Similar to total 25(OH)D, free 25(OH)D concentrations also decreased from 15 weeks of gestation to delivery (P= 0·016), reaching a nadir at 36 weeks, the same time course as that observed for serum albumin (see Fig. 1(d)). Free 25(OH)D concentrations at all the seven time points during pregnancy were lower than the postnatal level (all P< 0·001). While the percentage of free over total serum 25(OH)D concentrations did not differ significantly during pregnancy, it was significantly lower than the postnatal value at all time points (Table 2). Using partial correlation and adjusting for week of gestation, associations (r) between serum total and free 25(OH)D concentrations were consistently high ranging from 0·59 at 15 weeks of gestation to 0·80 at 40 weeks of gestation, decreasing to 0·59 in the postnatal sample. Similarly, total 25(OH)D adjusted for serum albumin was consistently positively associated with free 25(OH)D throughout pregnancy, increasing from an r value of 0·56 at week 15 to 0·81 at week 40 but decreasing to 0·58 postpartum (all P< 0·05). As expected, there was a negative association between free 25(OH)D and DBP concentrations throughout pregnancy (r − 0·47 to − 0·67) and no association between DBP and total 25(OH)D concentrations, although postnatal serum 25(OH)D concentration was associated with DBP (r 0·444).
Discussion
It has been suggested for some time( Reference Bikle, Gee and Halloran 12 ) that free 25(OH)D may be a more sensitive indicator of biologically available 25(OH)D than total circulating 25(OH)D. During pregnancy, a combination of haemodilution and increasing DBP concentrations may significantly reduce the concentration of free 25(OH)D in the circulation. Therefore, in the present study, we conducted a longitudinal analysis of total and free serum 25(OH)D, DBP and albumin concentrations in a random sample of thirty women from a prospective pregnancy cohort, with sampling made at seven time points at 4-weekly intervals from 15 to 40 weeks of gestation and postpartum. While sampling was not seasonally balanced, which is a serious drawback, the data showed that as pregnancy progressed, total serum 25(OH)D concentrations decreased, even throughout summer. Free 25(OH)D was strongly correlated with 25(OH)D throughout gestation. Progressively decreasing serum albumin concentrations indicated increases in blood volume. Serum DBP concentrations increased throughout gestation against the haemodilution gradient. While absolute concentrations of free 25(OH)D decreased in line with increasing DBP, the percentage of free 25(OH)D concentrations did not vary much during pregnancy but increased substantially postpartum as the DBP level declined.
Studies investigating vitamin D and its metabolism during pregnancy have been mainly cross-sectional, and longitudinal studies have limited sampling points during pregnancy. Bouillon et al. ( Reference Bouillon, Van Assche and Van Baelen 16 ) measured DBP, 1,25-dihydroxyvitamin D (1,25(OH)2D), total and free 25(OH)D concentrations at four different time points (18, 32, 35 and 40 weeks) among forty women, and reported significantly increased levels of DBP, 1,25(OH)2D and reduced free 25(OH)D at all time points during pregnancy compared with non-pregnant controls. In contrast, total 25(OH)D concentrations during pregnancy were not significantly different from non-pregnant controls, except at 18 weeks of gestation where a significantly higher level has been reported. Seasonal variations were found in total 25(OH)D concentrations at delivery; however, the season of recruitment was not recorded in this study, and the reason why the levels of total 25(OH)D were higher at 18 weeks of gestation was not clear.
Cross et al. ( Reference Cross, Hillman and Allen 14 ) examined serum 25(OH)D and 1,25(OH)2D concentrations at each trimester among ten women, and reported increased levels of 1,25(OH)2D at the second and third trimesters. A gradual increase in 25(OH)D concentrations was observed, which were significantly higher in the third trimester compared with non-pregnant controls. However, seasonal variation in 25(OH)D concentrations was not considered. Ritchie et al. ( Reference Ritchie, Fung and Halloran 13 ) followed fourteen women from pre-pregnancy to post-menses and analysed the concentrations of 25(OH)D and 1,25(OH)2D pre- and post-pregnancy, and at each trimester. They have reported a continuous increase in the concentrations of 1,25(OH)2D during pregnancy, and concentrations at the second and third trimesters were significantly higher than pre- and post-pregnancy levels. In this study, no change in the concentrations of 25(OH)D during pregnancy has been reported, but again, seasonal variation in 25(OH)D concentrations was not considered.
More et al. ( Reference More, Bhattoa and Bettembuk 18 ) assessed 25(OH)D concentrations in a cohort of twenty women, but at only one time point during pregnancy (22–24 weeks of gestation), and reported no difference in the concentrations of 25(OH)D before, during and after pregnancy. Narchi et al. ( Reference Narchi, Kochiyil and Zayed 19 ) followed a cohort of women from early pregnancy (n 75) to shortly after delivery (n 47) and 6 months after delivery (n 24), and reported a progressive decrease in the concentrations of 25(OH)D; however, the recruitment in this study occurred during late summer (September–November). A prospective study carried out by Fernandez-Alonso et al. ( Reference Fernandez-Alonso, Dionis-Sanchez and Chedraui 20 ) in Spain reported a significant decrease in 25(OH)D concentrations from the first to the third trimester in 148 pregnant women, independent of the season of sampling. Interestingly, a comparative study carried out by Sanchez et al. ( Reference Sanchez, Idrisa and Bobzom 21 ) among Nigerian pregnant teenagers has reported a significant decrease in the concentrations of 25(OH)D in the first trimester, no difference in the second trimester, and an increase in the concentrations of 25(OH)D in the third trimester compared with non-pregnant controls. In addition, albumin concentrations were significantly lower in women during pregnancy, especially in the second and third trimesters. We conducted a prospective study( Reference O'Riordan, Kiely and Higgins 22 ) in a small pregnancy cohort (n 43) in Cork, Ireland (52°N). We reported a gradual decrease in the prevalence of vitamin D deficiency indicated by an increase in the concentrations of 25(OH)D as pregnancy progressed. We also found that the levels of serum 25(OH)D concentrations were dramatically influenced by season. A longitudinal study carried out by Holmes et al. ( Reference Holmes, Barnes and Alexander 23 ) among 120 pregnant subjects in Northern Ireland (55°N) has reported that the levels of 25(OH)D in all trimesters were lower than those found in non-pregnancy controls in the same season, although there was a slight increase in 25(OH)D concentrations as pregnancy progressed, which could be due to the season of baseline sampling (12 weeks of gestation) during winter.
A meta-analysis carried out by Papapetrou( Reference Papapetrou 24 ) assessed the relationship between the concentrations of circulating 25(OH)D and 1,25(OH)2D, during pregnancy at term, and reported that the 25(OH)D levels during pregnancy were not different from the levels found in non-pregnancy, but the 1,25(OH)2D levels were two-fold higher in pregnancy than during non-pregnancy. However, the conclusion did not consider the impact of haemodilution. Although elevated 1,25(OH)2D concentrations during pregnancy have been consistently reported, changes in the concentrations of 25(OH)D during pregnancy are contradictory, and not all studies consider the effect of season. These studies are inconclusive as they are either small, cross-sectional or with few sampling points and inadequate accounting for the seasonal effect.
DBP is the major carrier of vitamin D and its derivatives. We reported an increase in DBP concentrations during pregnancy, peaking at 28 weeks of gestation, which were almost twice the postnatal levels. Increases in the concentrations of DBP during pregnancy have been reported previously, although the magnitude of the increase in the concentrations of DBP during pregnancy varies( Reference Ritchie, Fung and Halloran 13 , Reference Bikle, Gee and Halloran 15 , Reference Bouillon, Van Assche and Van Baelen 16 ). Bouillon et al. ( Reference Bouillon, Van Assche and Van Baelen 16 ) reported a doubling in the concentrations of DBP from 18 weeks of gestation until term compared with non-pregnant subjects. Ritchie et al. ( Reference Ritchie, Fung and Halloran 13 ) reported increased DBP concentrations during pregnancy in fourteen women; the levels were highest between 23 and 26 weeks of gestation (50 % higher than pre-pregnancy) and returned to pre-pregnant levels during early lactation and post-menses. A comparative study carried out by Bikle et al. ( Reference Bikle, Gee and Halloran 15 ) has reported that pregnant subjects (n 17) had 40 % higher concentrations of DBP during the third trimester compared with non-pregnant subjects (n 24). The increase in DBP concentrations during pregnancy was accompanied by an increase in the concentrations of serum 1,25(OH)2D. In three longitudinal studies, the concentrations of 1,25(OH)2D have been reported to significantly increase at all time points during pregnancy, with the concentration being highest in the third trimester( Reference Ritchie, Fung and Halloran 13 , Reference Cross, Hillman and Allen 14 ), or near term( Reference Bouillon, Van Assche and Van Baelen 16 ), which was more than double the pre-pregnant levels.
Free 25(OH)D concentrations reported in the present study were calculated based on the total serum 25(OH)D, albumin and DBP concentrations, using the equation adopted from Bikle et al. ( Reference Bikle, Gee and Halloran 12 ). We showed a consistent decrease in free 25(OH)D concentrations from 15 to 36 weeks of gestation and a significantly lower percentage of free 25(OH)D concentrations during pregnancy compared with the postnatal value. The reduced percentage of free 25(OH)D concentrations was probably due to the increased concentrations of DBP during pregnancy. To date, only one study has reported free 25(OH)D concentrations during pregnancy. A comparative cross-section study carried out by Bouillon et al. ( Reference Bouillon, Van Baelen and De Moor 11 ) examined free 25(OH)D concentrations in maternal and cord serum at the time of delivery. Although there was no comparison of maternal free 25(OH)D levels with postnatal or non-pregnant values, significantly higher concentrations of free 25(OH)D in cord than in maternal serum have been reported alongside lower total 25(OH)D concentrations in cord. Given that the free rather than the total concentration of 25(OH)D may be more significant in the placental transfer of 25(OH)D, implications of a reduced percentage of free 25(OH)D concentrations may suggest the regulation of 25(OH)D transfer to the fetus, which warrants further investigation.
The present longitudinal study examined 25(OH)D and DBP concentrations at seven time points during gestation among thirty Caucasian women, which provided a unique opportunity to detect biological differences in vitamin D-related metabolites, taking haemodilution into consideration. The limitation of the present study was that as recruitment was between May and September, it was not seasonally balanced and the effect of season on 25(OH)D could not be fully accounted for. Nonetheless, with the exception of a seasonal slight increase in the concentrations of total 25(OH)D in women recruited in May–June at their second sample, which would have been taken during the annual peak in July, the time course of decreasing 25(OH)D concentrations observed in women who progressed through their pregnancy during summer–autumn and delivered between October and November was similar to those who progressed during autumn–winter and delivered between February and March. In addition, we were unable to measure the concentrations of 1,25(OH)2D due to the insufficient sample volume, which is an area for future investigation. Overall, the present study contributes to the current knowledge of vitamin D metabolism during pregnancy, which ultimately aims to provide a better understanding of the changes in vitamin D status as gestation progresses and the prevention of vitamin D deficiency in women and newborn infants. Dose–response randomised controlled trials with multiple sampling points and complete year-round sampling at baseline during early pregnancy would provide a definitive solution to nutritional requirements for vitamin D during gestation. Our data indicate a concomitant decrease in circulating total and free 25(OH)D concentrations as pregnancy advances, which suggests a higher requirement for vitamin D.
Acknowledgements
The present study was supported by the funding provided to M. K. from the Irish Government Department of Agriculture through the Food Institutional Research Measure, which supported J. Y. Z. and A. J. L. L. C. K. is a recipient of a Clinician Scientist Award from the Health Research Board of Ireland (grant no. CSA 2007/02) and a Principal Investigator award from Science Foundation Ireland (grant no. 08/IN.1/B2083).
The authors’ contributions are as follows: M. K. and L. C. K. designed the research; L. C. K. and R. H. were responsible for data collection and biobanking; J. Y. Z. and A. J. L. conducted the sample analysis; J. Y. Z., A. J. L. and M. K. conducted the data analysis; J. Y. Z. and M. K. wrote the paper; M. K. had responsibility for the final content. All authors read and approved the final draft. The SCOPE database is provided and maintained by MedSciNet AB (http://medscinet.com).
The authors have no conflicts of interest to declare.






