Introduction
In the United States, surgical castration is commonly performed on commercial pig production farms within the first 3–5 days of life to prevent the accumulation of boar taint and agonistic behaviors (Rault et al., Reference Rault, Lay and Marchant-Forde2011), and the procedure is typically performed without administration of an analgesic or anesthetic. Current evidence demonstrates that neonates experience pain and, if left untreated, can result in permanent neuroanatomic or behavioral changes (Mellor and Gregory, Reference Mellor and Gregory2003; Sneddon et al., Reference Sneddon, Elwood, Adamo and Leach2014). Thus, pain management is essential for young animals.
The castration of piglets is recognized as a significant welfare concern, and guidelines for the use of analgesia and or anesthesia have been developed and implemented in the EU and Canada (National Farm Animal Care Council, 2014; European Commission, 2017). The European Commission reported pain intervention methods via a survey conducted from June 2016 to October 2016. The use of anesthesia and/or analgesia for piglet castration found the mixed application of pain mitigation strategies focusing on the concerns of animal welfare, economic sustainability, practical application of the method, environmental impact, and human health concerns. The application of pain mitigation for piglet castration in the EU ranges from gaseous or injectable anesthesia (CO2/O2, ketamine, azaperone, isoflurane), local anesthesia (lidocaine), non-steroidal anti-inflammatory drugs (flunixin, meloxicam, metamizol), or various combinations for anesthetic/analgesic effect (European Commission, 2017). However, there was no consensus on the best method for animal welfare with the practicality of on-farm ease of use.
Conversely, US farmers and veterinarians are currently limited in addressing this challenge due to the lack of analgesic or anesthetic drugs in the United States approved explicitly with an indication for the control of pain in swine. The lack of on-farm analgesic use may be due to a limited ability to make solid recommendations for effective pain management strategies by veterinarians, the added cost, time, and effort involved with training caretakers and implementing pain management protocols on-farm, in addition to a lack of US Food and Drug Administration (FDA)-approved analgesics labeled with an indication for the control of pain for swine (Rault et al., Reference Rault, Lay and Marchant-Forde2011; Tuyttens et al., Reference Tuyttens, Vanhonacker, Langendries, Aluwe, Millet Bekaert and Verbeke2011; O'Connor et al., Reference O'Connor, Anthony, Bergamasco, Coetzee, Gould, Johnson, Karriker, Marchant-Forde, Martineau, McKean, Millman, Niekamp, Pajor, Rutherford, Sprague, Sutherland, von Borell and Dzikamunhenga2014). The US Food and Drug Administration (FDA) has oversight of approval and safety of all products used in animals, including those animals used for human consumption. Pharmaceutical companies must use methods to assess animal responses that are well-defined and reliable to demonstrate products' efficacy and safety when seeking FDA new drug approval or label amendments. Veterinarians can prescribe FDA-approved products for extra-label purposes under the Animal Medicinal Use Clarification Act (AMDUCA). However, they must have reliable data to demonstrate the efficacy and safety of food products derived from animals treated with a drug approved for use in other species.
A literature review reveals a lack of consistent data related to the efficacy of pain mitigation products primarily due to the lack of uniform testing methodology and protocols (O'Connor et al., Reference O'Connor, Anthony, Bergamasco, Coetzee, Dzikamunhenga, Johnson, Karriker, Marchant-Forde, Martineau, Millman, Pajor, Rutherford, Sprague, Sutherland, von Borrell and Webb2016). This, in turn, makes evaluating the efficacy of pain mitigation interventions complex and has prevented consensus on best practices for pain relief (Bateson, Reference Bateson1991). Lack of consistent protocols creates difficulty for pharmaceutical companies to submit new product approvals or label claims related to pain, veterinarians to confidently prescribe products for extra-label use, researchers to reliably assess pain and potential mitigation strategies, and pig farmers to make future business decisions regarding animal welfare.
Given the need for mitigating castration pain, a consortium of researchers, veterinarians, industry, and regulatory agencies was formed to identify potential animal-based outcomes and develop a methodology based on the known scientific research, to measure pain and the efficacy of mitigation strategies. The consortium's goal is to improve pig welfare on-farm by effectively controlling pain associated with on-farm surgical procedures, such as castration, in a manner that is safe for the animal and the consumer and is compliant with US regulation. This evaluation's primary goal is to facilitate consistency and rigor by developing a research methodology utilizing validated endpoints that are well-defined and reliably measure pain in piglets. The resulting methodology, with validated outcomes, will facilitate and guide the evaluation of the effectiveness of comprehensive analgesic interventions for 3- to 5-day-old piglets following surgical castration.
Measurable outcomes were selected based on previous studies suggesting their validity, reliability, and sensitivity for the direct or indirect measurement of pain associated with surgical castration in piglets. Outcomes to be considered are observation of pain behaviors (i.e. ethogram defined behaviors and piglet grimace scale), gait parameters measured with a pressure mat, infrared thermography (IRT) of skin temperature of the cranium and periphery of the eye, and blood biomarkers. Other measures include body weight and mortality rate.
The information herein supports the inclusion of multiple endpoints to evaluate their validity and reliability for demonstrating control of pain in piglets undergoing surgical castration. For endpoint measures not included for evaluation, the consortium's decision was based on a lack of validated processes or practicality of standardizing the on-farm application to justify use within the proposed methodology [i.e. vocalization and nociceptive withdrawal response (Sheil and Polkinghorne, Reference Sheil and Polkinghorne2020)]. These endpoints may ultimately be used in studies to demonstrate substantial evidence of effectiveness, one component in the US Food and Drug Administration's approval process of a pain mitigation drug. This paper aims to describe a multidimensional methodology to directly or indirectly assess behavioral, physiological, and neuroendocrine changes in piglets associated with pain resulting from surgical castration. This methodology will use multiple outcome variables to, in summation, demonstrate analgesic efficacy in the post-surgically castrated piglet, satisfying the FDA efficacy requirement of a product.
Pain definition
Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage (IASP Subcommittee on Taxonomy, 1979). The emotional component of pain is an affective state that cannot be directly measured. The sensory component of the pain response involves nociception, including the detection, transduction, and transmission of noxious stimuli by the peripheral and central nervous systems. Collectively the sensory component of the pain response produces behavioral, physiological, and neuroendocrine responses.
Evidence of pain associated with castration
Human beings and other vertebrate mammals, such as pigs, have similar neuroanatomical structures associated with pain perception [e.g. nociceptors, a pathway connecting nociceptors to the brain, and brain structures to process pain analogous to the human cerebral cortex (Bateson, Reference Bateson1991)] and the capacity for animals to experience pain is well-described. It was long believed that neonates were incapable of experiencing pain or did so less intensely than adults because of their immature nervous system and lack of specific behavioral signs (Bateson, Reference Bateson1991). For many years, this concept was translated to veterinary medicine and livestock production practices as producers and veterinarians provided little to no analgesics or anesthetics to animals if painful procedures were conducted at a young age. Evidence now suggests neonates may have a heightened pain experience, and untreated pain could result in permanent changes to pain sensitivity and neuroanatomic or behavioral abnormalities, making pharmaceutical pain management even more critical for young animals undergoing a surgical procedure (Mellor and Gregory, Reference Mellor and Gregory2003; Sneddon et al., Reference Sneddon, Elwood, Adamo and Leach2014).
Surgical castration of piglets causes acute pain, as evidenced by behavior and physiologic changes. Piglets display several pain behaviors post-operatively in response to the surgical castration procedure, including an increase in stiffness, trembling, scratching the rump, tail wagging, awake inactive or restless behaviors, and spending more time isolated from littermates (Viscardi and Turner, Reference Viscardi and Turner2018a). Piglets also produce distinct high-frequency vocalizations associated with the castration procedure (Weary et al., Reference Weary, Braithwaite and Fraser1998; Leidig et al., Reference Leidig, Hertrampf, Failing, Schumann and Reiner2009) and spend more time in contact with the sow, which has been suggested to produce analgesic-like effects by promoting endorphin release in neonates (Field and Goldson, Reference Field and Goldson1984; Blass, Reference Blass1994). Behavioral alterations associated with castration persist beyond 24 h, with some abnormal behaviors present 4 days later (Hay et al., Reference Hay, Vulin, Génin, Sales and Prunier2003; Llamas Moya et al., Reference Llamas Moya, Boyle, Lynch and Arkins2008).
Surgical castration also causes a physiologic response. Piglets show an increase in heart and respiration rate with higher blood cortisol, lactate, and adrenocorticotropin hormone concentrations after castration (White et al., Reference White, DeShazer, Tressler, Borcher, Davey, Waninge, Parkhurst, Milanuk and Clemens1995; Prunier et al., Reference Prunier, Bonneau, von Borell, Cinotti, Gunn, Fredriksen, Giersing, Morton, Tuyttens and Velarde2006; Kluivers-Poodt et al., Reference Kluivers-Poodt, Houx, Robben, Koop, Lambooij and Hellebrekers2012). Peripheral vasoconstriction caused by activation of the sympathetic nervous system results in a decrease in cutaneous temperature, which, using an IRT camera, has been observed in piglets after castration (Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014). Surgical castration can also result in a decreased growth rate leading to production losses (McGlone et al., Reference McGlone, Nicholson, Hellman and Herzog1993; Kielly et al., Reference Kielly, Dewey and Cochrane1999; Malavasi et al., Reference Malavasi, Nyman, Augustsson, Jacobson and Jensen-Waern2006). Published studies that have examined analgesia and local anesthetic use in piglets undergoing surgical castration are summarized in Table 1.
Table 1. Results of studies that have examined analgesia and local anesthetic use to alleviate pain in surgically castrated piglets

IV = intravenous; EEG = electroencephalogram; IM = intramuscular; M = meloxicam; L = lidocaine; CRP = C-reactive protein.
a Drug efficacy is defined as successful (yes) if treatment administration minimized outcome measures or significantly minimized deviations to behavioral and physiological indicators of pain (Dzikamunhenga et al., Reference Dzikamunhenga, Anthony, Coetzee, Gould, Johnson, Karriker, McKean, Millman, Niekamp and O'Connor2014).
Development of a research protocol
The information presented herein serves as a support for developing a research protocol with the primary objective of determining the validity and reliability of endpoints for use in demonstrating efficacy for FDA product approval. The endpoints could be used to evaluate a drug's efficacy for controlling pain associated with castration of 3-to 5-day-old piglets. The 3-to-5 day-old piglet is targeted due to the standard age at which piglets are castrated in commercial production within the United States being approximately 3 days of age, and foundational research in pain mitigation has targeted the 3-day-old piglet (Sutherland et al., Reference Sutherland, Davis, Brooks and McGlone2010; Sutherland et al., Reference Sutherland, Davis, Brooks and Coetzee2012). Through a review of the literature describing pain expression measures in piglets, multiple outcome measures were identified as the best candidates for further validation and inclusion in future efficacy trials. This paper's multidimensional outcomes include observation and scoring of pain exhibition or behavior, physiological biomarkers, and automated physical measurements.
Protocol study design
In the development of a standardized protocol to determine analgesic efficacy, the following protocol was outlined to provide consistency in trial design and standardize the measurement of outcomes:
(a) Four primary treatment groups will be used in the research protocol: (1) sham castration (SHAM), (2) sham castration with the intervention (SHAM + TRT), (3) surgical castration with placebo control (CAST), (4) and surgical castration with intervention (CAST + TRT). In the absence of other confounding factors, four treatment groups will help determine the following (Weary et al., Reference Weary, Niel, Flower and Fraser2006; Ison et al., Reference Ison, Clutton, Di Giminiani and Rutherford2016):
• Differences between CAST and CAST + TRT treatments can be interpreted as the efficacy of the intervention to mitigate pain.
• Differences between SHAM and CAST can be interpreted as the effects of pain or tissue damage rather than environmental or other non-pain factors.
• Differences between SHAM and SHAM + TRT can determine if the intervention causes a difference in the tested variable in the absence of pain and tissue damage (e.g. sedative effects).
(b) To validate the measurable outcomes in this protocol, the authors would use buprenorphine (0.04 mg kg−1) (Viscardi and Turner, Reference Viscardi and Turner2018a), administered intramuscularly, before surgical castration as a ‘gold standard’ intervention. While this opioid drug is not approved for use in swine or other food animals, buprenorphine was chosen as the gold standard because of its potency as an analgesic drug and ability to bind to opioid receptors in the brain, spinal cord, and periphery to suppress pain signal transmission (Chahl, Reference Chahl1996). Furthermore, buprenorphine has proven to be effective at reducing pain and lameness in piglets (Hermansen et al., Reference Hermansen, Pedersen and Olesen1986; Meijer et al., Reference Meijer, van Nes, Back and van der Staay2015) and alleviating behavioral pain indicators and facial grimacing with no sedative effect for more than 24 h in 5-day-old piglets following castration (Viscardi and Turner, Reference Viscardi and Turner2018a).
(c) The research protocol would use a 2 × 2 factorial design with piglet (within litter) as the experimental unit. The treatment will be applied to the individual male piglet, and each treatment will be represented within a litter at least once. If more than four male piglets in the litter meet the enrollment criteria, a treatment will be assigned to each additional piglet using a treatment randomization list. Using piglet as the experimental unit and blocking/controlling allocation to treatment based on the litter (controlling for sow effect) helps control inter-litter variability (e.g. litter size, sow milk yield, and piglet sex ratio) (Festing, Reference Festing2006; Lazic and Essioux, Reference Lazic and Essioux2013). Many previous studies (Table 1) evaluating pain reduction for castrated piglets have used piglets as the experimental unit. As the experimental unit, the piglet provides more data for, and higher confidence in the power analysis calculations used to determine the research protocol's sample size.
A potential criticism for using piglet as the experimental unit rather than the litter is that having all four treatments represented within a litter could have a confounding influence on piglet behavior and activity. Emotional contagion has been observed in groups of pigs when exposed to positive or negative treatments (Reimert et al., Reference Reimert, Fong, Rodenburg and Bolhuis2017; Yun et al., Reference Yun, Ollila, Valros, Larenza-Menzies, Heinonen, Oliviero and Peltoniemi2019). For this concern to be addressed, using litter as the experimental unit in the research protocol, all uncastrated male and female littermates would need to be removed from the farrowing box so that only treated males remained, creating an unnatural environment. Having uncastrated female littermates present in the environment is important when validating outcomes and evaluating intervention efficacy, as these animals will always be present in typical commercial production.
Measurable outcomes
Validity, reliability, and sensitivity are vital characteristics that should be considered when choosing measurable outcomes for practical pain assessment (Ison et al., Reference Ison, Clutton, Di Giminiani and Rutherford2016). Each measurable outcome identified for use in the proposed protocol has been categorized as either primary or ancillary. Primary outcomes were defined as measures directly related to clinical signs of pain as recorded in the published literature and are repeatable when the proper methodology is used. Ancillary outcomes were defined as newer methodologies in the published literature directly related to clinical signs of pain or indirectly related to clinical signs of pain and support the primary outcomes. A combination of multiple outcome variables may provide a robust evaluation of a castrated piglet's pain profile and the tested intervention efficacy.
A brief justification is provided for each measurable outcome for the proposed protocol. Additionally, evidence for when and how each outcome should be measured is provided. A recommended sample size is also included for each outcome based on previous literature. With the diversity in the methodology used in the existing literature, performing a complete power analysis proved difficult.
Outcomes variables
Pain behavior and activity tracking
Diagnosis of pain in animals is a complicated process due to unique individual experiences with pain (Gaynor and Muir, Reference Gaynor, Muir, Gaynor and Muir2009) and differences in pain tolerance and reaction between breeds, sex, age, pain duration, procedure type, and stimulus severity (Matthew, Reference Matthew2000). From a scientific standpoint, a behavioral assessment may be the most practical endpoint to assess pain in livestock production systems as it is the most direct assessment of an animal's welfare. However, given that the sensory component of pain is also associated with a neuroendocrine and physiological response in the animal, measuring neuroendocrine and physiological outcomes is essential to confirm and support behavioral endpoints and outcomes. A multimodal approach is the proposed best practice because these outcomes' totality reveals an overall assessment of pain.
Piglets castrated without anesthetics or analgesics demonstrate several behavioral changes indicative of pain, as demonstrated by increased pain-specific behaviors post-procedure and deviations in maintenance behavior and piglet activity.
Piglets castrated without pain control demonstrate increased trembling, rump scratching, prostration, and tail jamming/wagging (Hay et al., Reference Hay, Vulin, Génin, Sales and Prunier2003), all of which indicate pain. Also, nursing (McGlone et al., Reference McGlone, Nicholson, Hellman and Herzog1993) and social behaviors decrease post-procedure, and greater duration and intensity of fighting with pen mates can also be observed (Hay et al., Reference Hay, Vulin, Génin, Sales and Prunier2003; Llamas Moya et al., Reference Llamas Moya, Boyle, Lynch and Arkins2008; Leidig et al., Reference Leidig, Hertrampf, Failing, Schumann and Reiner2009; Sutherland et al., Reference Sutherland, Davis, Brooks and McGlone2010; Sutherland et al., Reference Sutherland, Davis, Brooks and Coetzee2012). Lastly, a reduction in activity levels (i.e. increased inactivity) is a commonly noted behavioral response post castration (McGlone et al., Reference McGlone, Nicholson, Hellman and Herzog1993; Hay et al., Reference Hay, Vulin, Génin, Sales and Prunier2003; Llamas Moya et al., Reference Llamas Moya, Boyle, Lynch and Arkins2008).
Among studies that have recorded piglet activity and pain behavior following castration, current behavior sampling methodologies have not been validated, and there is considerable variation in the methodologies used (O'Connor et al., Reference O'Connor, Anthony, Bergamasco, Coetzee, Gould, Johnson, Karriker, Marchant-Forde, Martineau, McKean, Millman, Niekamp, Pajor, Rutherford, Sprague, Sutherland, von Borell and Dzikamunhenga2014). For example, when assessing activity and maintenance behaviors in castrated piglets, McGlone et al. (Reference McGlone, Nicholson, Hellman and Herzog1993) used continuous live observations for 6 h following castration and found reduced suckling and standing and increased lying time in castrated piglets compared to non-castrated littermates. In comparison, Hay et al. (Reference Hay, Vulin, Génin, Sales and Prunier2003) also used live observation but utilized scan sampling at 10-minute intervals and found that castrated piglets had reduced activity at the udder and spent more time inactive (i.e. lying, sitting, or standing). Similarly, Llamas Moya et al. (Reference Llamas Moya, Boyle, Lynch and Arkins2008) recorded piglet behavior for 3 h in the afternoon on the day of castration using 3-minute scan samples and found that castrated pigs spent less time walking than sham-handled piglets. These few examples highlight the wide variation in behavioral methodologies used to assess castration pain. Be that as it may, all these studies were able to quantify deviations in piglet behavior post-procedure.
To address limitations associated with behavioral methodologies used in castration studies, validating the accuracy of different behavioral sampling methodologies is needed. Also, developing a piglet behavioral pain scale using behaviors that can be reliably measured by multiple people and are sensitive to detecting pain would be valuable. Given the lack of validated behavior methodologies for pigs, the authors recommend the following methodology for all behavioral observations, regardless of how behavior data are collected (i.e. manual or automated methods). Piglets should be individually identified and filmed in their home pens continually over a 24-hour period for three days. Identification of the individual piglets would be via a unique identifier consisting of a number and a letter, with the letter randomly assigned and represents the piglet's treatment. The number identifier is placed on both back legs and the letter on the piglet's forehead and back using a black permanent marker. The markings must be refreshed twice daily during study procedures. The video should be captured digitally, and behavioral software programming utilized to capture data.
Automated technologies for measuring piglet activity at castration have not been used to date but have the potential to provide more reliable data and a significant reduction in labor (Nasirahmadi et al., Reference Nasirahmadi, Edwards, Matheson and Sturm2017). For example, accelerometers (pedometers), radio-frequency identification, and visual tracking systems have been used to assess activity in older pigs and other livestock species (Currah et al., Reference Currah, Hendrick and Stookey2009; Kashiha et al., Reference Kashiha, Bahr, Ott, Moons, Niewold, Odberg and Berckmans2013; Kulikov et al., Reference Kulikov, Khotskin, Nikitin, Lankin, Kulikov and Trapezov2014). Compared to most tracking devices, the small piglet size presents a challenge, but problems associated with these technologies are decreasing with continuing improvements in their size, accuracy, and affordability. Therefore, while automated systems may help identify effective pain control measures, they are not currently recommended for efficacy evaluation of pain mitigation strategies at castration as they require further validation for use in piglets.
Piglet behavior and activity observations will be collected using continuous behavior sampling on Day 0 (day before castration, or baseline), Day 1 (0–24 h post-treatment administration), and Day 2 (24–48 h post-treatment administration). Behaviors to be measured are defined in the ethogram in Table 2. Based on previous work outlined in Table 1, the authors recommend the sample size for behavioral observations is 10–20 piglets/treatment. Before trial initiation, observers must be trained to ensure behavioral data are recorded consistently. To ensure inter-observer reliability, a 2-h subset of continuous video is selected at random, observed, and compared until 90% accuracy is achieved (Ross et al., Reference Ross, Cressman, Cramer and Pairis-Garcia2019).
Table 2. Behavioral ethogram for piglets adapted from Hay et al. (Reference Hay, Vulin, Génin, Sales and Prunier2003)

The ethogram defined in Table 2 should be used for all observational data collected. All behaviors (Table 2) should be collected continuously by two trained observers utilizing software (e.g. Observer XT program) or any data collection, coding, and analysis tool that is easily validated with a time and date stamp. Videos are randomized and assigned to observers who are blind to treatment and time points. Inter-observer reliability is assessed before data collection and at three-time points during the behavior scoring period. Throughout the trial, all inter-observer reliability tests should produce an R-value above 0.9, indicating excellent agreement between scorers and no significant drift throughout the scoring period (Park et al., Reference Park, Cramer, Wagner, Turner, Moraes, Viscardi, Coetzee and Pairis-Garcia2020). Analysis should be completed in a repeated measures linear mixed model with the Poisson distribution.
Infrared thermography
IRT is a non-invasive method of detecting the amount of infrared energy (heat) an object radiates and can be used to measure skin temperature changes associated with activation of the sympathetic nervous system. When an animal is stressed or in pain, the sympathetic nervous system becomes activated, which causes vasoconstriction and a shift in blood flow from the skin to the organs. The blood flow change results in a loss of heat in the body's periphery and decreased skin temperature (Stewart et al., Reference Stewart, Webster, Schaefer, Cook and Scott2005; Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014).
Stewart et al. (Reference Stewart, Verkerk, Stafford, Schaefer and Webster2010) found that eye temperature measured using IRT significantly increased in response to castration. This response was reduced when calves were given an injection of local anesthetic into the testes and scrotum 7 minutes before castration. Also, SHAM procedures only caused a minor change in eye temperature compared to the painful procedures (Stewart et al., Reference Stewart, Verkerk, Stafford, Schaefer and Webster2010). These results support that IRT can be used as an objective measure of the animal's response to a painful stimulus. In pigs, IRT has been used to detect disease (Cook et al., Reference Cook, Chabot, Lui, Bench and Schaefer2015), stress (Schaefer et al., Reference Schaefer, Aalhus, Cook, Dugan, Dubeski, Fortin, Robertson and Tong2002; Magnani et al., Reference Magnani, Gatto, Cafazzo, Stelletta, Morgante and Costa2011; Sutherland et al., Reference Sutherland, Dowling, Backus and Stewart2015), and pain (Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014). Specific to pain, Bates et al. (Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014) found that pigs given analgesia before castration had greater cranial skin temperatures, as measured using IRT, than castrated pigs that did not receive pain relief.
A change in blood flow during stress can be detected using IRT on specific body regions such as the eye in cattle (Stewart et al., Reference Stewart, Verkerk, Stafford, Schaefer and Webster2010) and horses (Bartolomé et al., Reference Bartolomé, Sánchez, Molina, Schaefer, Cervantes and Valera2013), the comb in poultry (Moe et al., Reference Moe, Stubsjøen, Bohlin, Flø and Bakken2012), and head, snout, vulva, and teats in pigs (Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014; Sutherland et al., Reference Sutherland, Dowling, Backus and Stewart2015). Bates et al. (Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014) measured changes in skin surface temperature using IRT on the top of the cranium, ears, and snout in pigs castrated with and without analgesia and found that cranial skin temperature was the most reliable anatomical location for assessing pain in piglets in response to castration due to significant temperature variability in other locations. Furthermore, Sutherland et al. (Reference Sutherland, Dowling, Backus and Stewart2015) investigated the potential for IRT as a non-invasive measure of stress in pigs and compared whether the eye or the snout was a more sensitive region to measure stress. Temperature changes suggested that the eye may be a more reliable area to assess stress than the snout in pigs. The literature shows that IRT can be used to measure animal pain; however, further validation of this technique's methodology is needed, including the most reliable anatomical site for assessing pain.
Based on data from previous studies (Stewart et al., Reference Stewart, Verkerk, Stafford, Schaefer and Webster2010; Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014), assuming a 5% significance level, and 80% power, the authors recommend a sample size of 20 piglets/treatment based on an expected difference in cranial skin temperature of one degree Celsius and a standard error of the mean of 0.1 degrees Celsius at 12 h after castration, assuming data are analyzed using 2-sample t-tests.
Cortisol
Cortisol is a biomarker commonly used to measure stress and pain in animals. Several painful husbandry procedures (e.g. castration, tail docking, and dehorning) have been shown to cause an increase in cortisol concentrations in several species (e.g. sheep and cattle) (Dinniss et al., Reference Dinniss, Mellor, Stafford, Bruce and Ward1997; Kent et al., Reference Kent, Molony and Graham1998; McMeekan et al., Reference McMeekan, Mellor, Stafford, Bruce, Ward and Gregory1998; Sutherland et al., Reference Sutherland, Mellor, Stafford, Gregory, Bruce, Ward and Todd1999, Reference Sutherland, Mellor, Stafford, Gregory, Bruce and Ward2002; Stafford et al., Reference Stafford, Mellor, Todd, Bruce and Ward2002) including surgical castration and tail docking in pigs (Prunier et al., Reference Prunier, Mounier and Hay2005; Carroll et al., Reference Carroll, Berg, Strauch, Roberts and Kattesh2006; Sutherland et al., Reference Sutherland, Davis, Brooks and Coetzee2012). Numerous studies have shown that surgical castration causes a significant and marked increase in pigs' cortisol concentrations (Prunier et al., Reference Prunier, Mounier and Hay2005; Carroll et al., Reference Carroll, Berg, Strauch, Roberts and Kattesh2006; Sutherland et al., Reference Sutherland, Davis, Brooks and McGlone2010, Reference Sutherland, Davis, Brooks and Coetzee2012, Reference Sutherland, Backus, Brooks and McGlone2017). However, handling alone only causes a slight but non-significant increase in cortisol (Prunier et al., Reference Prunier, Mounier and Hay2005), suggesting that the increase in cortisol in response to castration is predominantly due to the pain of the procedure and not the stress of handling. Moreover, in pigs given analgesia (e.g. lidocaine or a non-steroidal anti-inflammatory drug), the cortisol response to surgical castration was reduced (Kluivers-Poodt et al., Reference Kluivers-Poodt, Houx, Robben, Koop, Lambooij and Hellebrekers2012; Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014).
Cortisol is commonly measured in plasma, serum, or saliva. After surgical castration in pigs, plasma cortisol concentrations peak between 15 and 60 min and return to baseline levels between 120- and 180-minutes post-procedure (Prunier et al., Reference Prunier, Mounier and Hay2005; Sutherland et al., Reference Sutherland, Davis, Brooks and Coetzee2012, Reference Sutherland, Backus, Brooks and McGlone2017). Therefore, to evaluate the efficacy of different pain mitigation strategies to reduce the pain caused by surgical castration, cortisol concentrations should be measured immediately before the procedure to assess baseline levels, between 15 and 60 min post castration to assess changes in peak cortisol levels and then again at 120 min to confirm that levels have returned to baseline. Cortisol concentrations in pigs can be measured using validated in-house radioimmunoassay (RIA) or enzyme immunoassay (EIA) techniques. However, in the recent literature, cortisol concentrations in pigs have been more commonly measured using commercially available RIA kits such as Coat-a-Count (Siemens Medical Solutions Diagnostics [formally Diagnostic Products Corp], Los Angeles, California) (Kluivers-Poodt et al., Reference Kluivers-Poodt, Houx, Robben, Koop, Lambooij and Hellebrekers2012; Bates et al., Reference Bates, Karriker, Stock, Pertzborn, Baldwin, Wulf, Lee, Wang and Coetzee2014) and EIA kits such as Assay Designs (Ann Arbor, Michigan) (Carroll et al., Reference Carroll, Berg, Strauch, Roberts and Kattesh2006; Sutherland et al., Reference Sutherland, Davis, Brooks and McGlone2010, Reference Sutherland, Davis, Brooks and Coetzee2012) and IMMULITE/IMMULITE 1000 Cortisol (Global Siemens Healthcare, Erlanger, Germany) (Sutherland et al., Reference Sutherland, Bryer, Krebs and McGlone2008).
Changes in cortisol should be included as a biomarker for pain in pigs as it has reliably been shown to increase in response to pain and be reduced or abolished in response to different pain mitigation strategies after controlling for confounding factors such as handling, restraint, and tissue trauma (Sheil and Polkinghorne, Reference Sheil and Polkinghorne2020). Besides, cortisol can be reliably measured in suckling pigs' serum or plasma using commercially available RIA or EIA kits. However, when commercial diagnostic kits developed and validated for the diagnosis of endocrine disorders such as hyperadrenocorticism are repurposed as analytical methods to measure the concentrations of biomarkers in healthy animals undergoing painful procedures, researchers must ensure that the diagnostic kit validation data provided by the manufacturer is reliable and accurate. Furthermore, it is a good scientific practice to validate the diagnostic kit's performance in the facility conducting the sample analysis.
Using data collected from previous studies (Sutherland et al., Reference Sutherland, Davis, Brooks and McGlone2010, Reference Sutherland, Davis, Brooks and Coetzee2012, Reference Sutherland, Backus, Brooks and McGlone2017; Bonastre et al., Reference Bonastre, Mitjana, Tejedor, Calavia, Yuste, Úbeda and Falceto2016), assuming a 5% significance level and 80% power, the authors recommend using a sample size of 20 piglets/treatment based on an expected difference in serum cortisol concentrations of 20 ng mL−1 and a standard error of the mean of 5 ng mL−1 at 60 min after surgical castration, assuming data are analyzed using 2-sample t-tests.
Ancillary outcomes
Stride length, contact pressure, contact area, and stance phase duration
Objective gait parameters, measured using a commercially available floor mat-based pressure/force measurement system (MatScan, Tekscan, Inc, South Boston, Massachusetts), were used in conjunction with lameness scores as primary endpoints in a pivotal study that supported FDA approval of the first analgesic drug labeled for use in cattle in the United States (Banamine Transdermal, Merck Inc, Madison, New Jersey) (US Food and Drug Administration, 2017). Pressure mat analysis is recognized within a validated foot rot model as a reliable pain assessment endpoint in cattle, thus satisfying the FDA Guidance Document 123 requirements (US Food and Drug Administration, 2006). Pressure mat technology has been used to record and analyze naturally occurring or experimentally induced changes in gait in cattle and swine due to lameness (Kotschwar et al., Reference Kotschwar, Coetzee, Anderson, Gehring, Kukanich and Apley2009; Schulz et al., Reference Schulz, Anderson, Coetzee, White and Miesner2011; Karriker et al., Reference Karriker, Abell, Pairis-Garcia, Holt, Sun, Coetzee, Johnson, Hoff and Stalder2013; Coetzee et al., Reference Coetzee, Mosher, Anderson, Robert, Kohake, Gehring, White, Kukanich and Wang2014; Pairis-Garcia et al., Reference Pairis-Garcia, Johnson, Abell, Coetzee, Karriker, Millman and Stalder2015) and surgical castration (Nasirahmadi et al., Reference Nasirahmadi, Edwards, Matheson and Sturm2017; Kleinhenz et al., Reference Kleinhenz, Van Engen, Smith, Gorden, Ji, Wang, Perkins and Coetzee2018). Taken together, these data support the assessment of stride length, contact pressure, contact area, and stance phase duration using the pressure mat in the proposed efficacy study. One of the proposed study's key outcomes would be to compare the pressure mat outcomes with behavioral and physiological outcomes.
The pressure mat will be calibrated daily using the expected body weight of the piglets, and each time the computer software is engaged using a known mass to ensure the accuracy of the measurements at each time point. The pressure mat, measuring 6–8 feet in length, must be set up on a flat surface where piglets can be directed to walk at a steady pace across the mat so that the distance between multiple footfalls, pressure, and stance can be measured. Footfalls are recorded when the foot strikes the loaded or ‘contact’ sensing elements inside a measurement box. Research grade software (HUGEMAT Research 5.83, Tekscan, Inc., South Boston, Massachusetts) is used to determine the contact pressure, contact area, stance phase duration, and stride length. The walking pig's video is captured digitally and synchronized to ensure consistent gait between and within piglets for each time point. Readings are taken before castration and at 6, 12, 24, and 48 h after castration. The per cent change from baseline for all measures will be calculated and analyzed statistically using a mixed-effects model. Before trial initiation, observers must be trained to ensure stride length data is recorded consistently. The observers will achieve 80% inter-observer reliability and be blinded to treatment to control for observer bias. The analysis's output can be converted to PDF, allowing the outcomes to be reconstructed after the study is completed.
Using data collected from an unpublished pilot study, assuming a 5% significance level and 80% power, the authors recommend using a sample size of 30 piglets/treatment based on an expected per cent change in front stride length from baseline measurements of 30%, a standard error of the mean of 10% assuming the data are analyzed by ANOVA.
Blood biomarkers
Several circulating biomarkers targeting the indirect assessment of pain and stress have been measured in piglets at the time of castration. Specifically, these include markers of the neuroendocrine response (e.g. corticotropin, β-endorphins, epinephrine, norepinephrine, and substance P), inflammatory response (e.g. haptoglobin, c-reactive protein, serum amyloid A, and prostaglandin E2), and adrenocortical response (e.g. cortisol) (Weary et al., Reference Weary, Niel, Flower and Fraser2006; Dzikamunhenga et al., Reference Dzikamunhenga, Anthony, Coetzee, Gould, Johnson, Karriker, McKean, Millman, Niekamp and O'Connor2014). Also, immune response assessments have been made using hematological endpoints such as neutrophil to lymphocyte ratio (N:L) derived from a complete blood count. These biomarkers have been correlated with stress because cortisol release is typically rapid and difficult to quantify, but the associated stress leukogram lasts longer and could be less time-sensitive. Also, the production of unconjugated pterins (neopterin and biopterin) have been associated with stressful situations such as piglet castration (Marsálek et al., Reference Marsálek, Svoboda, Smutná, Blahová and Vecerek2011; Maršálek et al., Reference Maršálek, Svoboda, Bernardy and Večerek2015).
Blood biomarkers are indirect measures of pain and inflammation. Furthermore, many of these outcomes have low specificity and can be altered by other factors such as handling stress. These outcomes are susceptible to confounding by other aspects of the experiment, specifically blood sampling and handling. Most all the analytical methods have not been validated to Good Laboratory Practice or Good Clinical Practice specifications. Attempts at validating these outcomes in piglets have been confounded by challenges with extracting the analytes from plasma and serum. Additional challenges surround the sample collection's optimal timing to ensure that the outcomes are correlated with the painful event. An initial screening of blood biomarkers relative to the painful event may help identify outcomes that warrant further investigation and validation.
Blood biomarker assessment is currently predicated using immunoassays or automated hematological methods validated for human medicine. The collection protocol (timing and amount of blood needed) will depend on the study's biomarkers. After collection, the analyte stability remains a significant challenge; therefore, samples must be stored on ice and, in some cases, liquid nitrogen. In the case of neuropeptides such as substance P, additional steps must be taken to ensure that serine proteases do not degrade after collection (Mosher et al., Reference Mosher, Coetzee, Allen, Havel, Griffith and Wang2014). One method involves the addition of benzamidine hydrochloride, a protease inhibitor, at 1 mM mL−1 of whole blood collected in EDTA. Hematological outcomes are also measured using blood collected in EDTA, while acute phase proteins, such as haptoglobin, are collected in serum tubes. Previous studies investigating blood biomarkers have generally enrolled between 10 and 20 piglets/treatment (Rault et al., Reference Rault, Lay and Marchant-Forde2011; Sutherland et al., Reference Sutherland, Davis, Brooks and Coetzee2012; Dzikamunhenga et al., Reference Dzikamunhenga, Anthony, Coetzee, Gould, Johnson, Karriker, McKean, Millman, Niekamp and O'Connor2014). Initially, all of the biomarkers mentioned above would be measured to determine usefulness as an indirect outcome variable in the multimodal protocol to measure analgesic efficacy. Upon identification of the biomarker(s) that consistently correlates with the primary outcome measures of pain, one or more biomarkers would be utilized in the multimodal protocol.
Piglet grimace scale
Facial grimace scales are a novel, non-invasive tool for pain assessment using quantifiable changes to facial features to detect pain. They have been developed for non-verbal humans as well as many animals, including mice, rats, rabbits, horses, sheep, lambs, cattle, and piglets (Langford et al., Reference Langford, Bailey, Chanda, Clarke, Drummond, Echols, Glick, Ingrao, Klassen-Ross, LaCroix-Fralish, Matsumiya, Sorge, Sotocinal, Tabaka, Wong, van den Maagdenberg, Ferrari, Craig and Mogil2010; Herr et al., Reference Herr, Coyne, McCaffery, Manworren and Merkel2011; Sotocinal et al., Reference Sotocinal, Sorge, Zaloum, Tuttle, Martin, Wieskopf, Mapplebeck, Wei, Zhan, Zhang, McDougall, King and Mogil2011; Keating et al., Reference Keating, Thomas, Flecknell and Leach2012; Costa et al., Reference Costa, Minero, Lebelt, Stucke, Canali and Leach2014; Gleerup et al., Reference Gleerup, Andersen, Munksgaard and Forkman2015; Di Giminiani et al., Reference Di Giminiani, Brierley, Scollo, Gottardo, Malcolm, Edwards and Leach2016; Guesgen et al., Reference Guesgen, Beausoleil, Leach, Minot, Stewart and Stafford2016; McLennan et al., Reference McLennan, Rebelo, Corke, Holmes, Leach and Constantino-Casas2016; Häger et al., Reference Häger, Biernot, Buettner, Glage, Keubler, Held, Bleich, Otto, Müller, Decker, Talbot and Bleich2017). A Piglet Grimace Scale (PGS) developed by Viscardi et al. (Reference Viscardi, Hunniford, Lawlis, Leach and Turner2017) described changes to three facial action units in response to piglet surgical castration and tail docking pain (Fig. 1). A facial grimace in piglets is characterized by narrowing the orbital area (eyes squeezing shut), ears pulled back against the head, and a prominent bump or bulge on the snout resulting from cheek tightening. According to the PGS, the maximum grimace score is 5, and it has corresponded well to displayed pain behaviors (e.g. an increase in pain behavior corresponded to higher facial grimacing in piglets) (Viscardi and Turner, Reference Viscardi and Turner2018a, Reference Viscardi and Turnerb).

Fig. 1. Piglet grimace scale developed by Viscardi et al. (Reference Viscardi, Hunniford, Lawlis, Leach and Turner2017).
Although an increase in piglet facial grimacing has been correlated to a decrease in activity level and corresponds to an increase in pain behavior (Viscardi et al., Reference Viscardi, Hunniford, Lawlis, Leach and Turner2017; Viscardi and Turner, Reference Viscardi and Turner2018a, Reference Viscardi and Turnerb), the PGS has not been validated as a pain assessment tool. This proposed study methodology will determine if facial grimacing can be correlated to pain behavior or other non-invasive outcome measures (e.g. IRT). The PGS has only been used to retrospectively assess pain and analgesia efficacy by scoring still-images of piglet facial expressions extracted from video recordings. Individuals used to score facial expressions were undergraduate, graduate, or veterinary students. To improve its practicality for on-farm use, the PGS should be validated for real-time detection of pain and producers and swine veterinarians' ability to use the PGS to identify pain in piglets accurately.
Previous work using grimace scales have found 1–3 facial images captured per animal per time point was sufficient to assess pain and analgesic efficacy (Sotocinal et al., Reference Sotocinal, Sorge, Zaloum, Tuttle, Martin, Wieskopf, Mapplebeck, Wei, Zhan, Zhang, McDougall, King and Mogil2011; Costa et al., Reference Costa, Minero, Lebelt, Stucke, Canali and Leach2014; Miller et al., Reference Miller, Kitson, Skalkoyannis and Leach2015; Miller and Leach, Reference Miller and Leach2015a, Reference Miller and Leachb; Miller et al., Reference Miller, Kitson, Skalkoyannis, Flecknell and Leach2016; Viscardi and Turner, Reference Viscardi and Turner2018a). These studies also used 2–5 individuals to score facial expressions. Researchers averaged the individuals' resulting scores before analysis and conducted inter-observer reliability tests to ensure scoring consistency.
Body weight
Changes in body weight can be used as an indirect measure of the pain experienced by piglets at castration. Multiple studies in a range of species demonstrate that when animals experience pain, feed consumption decreases, resulting in a reduction in body weight and average daily gain (ADG) (Malavasi et al., Reference Malavasi, Nyman, Augustsson, Jacobson and Jensen-Waern2006).
Although there is little evidence of the long-term impact of castration on body weight gain in piglets, reductions in ADG due to castration have been found in the days following the procedure. Kielly et al. (Reference Kielly, Dewey and Cochrane1999) found that pigs castrated at 3 days of age gained less weight than weight-matched controls over the 3 days following castration, while those castrated at 14 days of age showed no difference in ADG compared to controls, suggesting that delayed castration may benefit piglets. In contrast, Hay et al. (Reference Hay, Vulin, Génin, Sales and Prunier2003) compared the body weights of piglets castrated at 5 days of age and sham handled controls twice per day for 4 days after treatment and found no differences in ADG.
Most studies have measured body weights at weaning and found no effect of castration with or without pain control on body weight (Kielly et al., Reference Kielly, Dewey and Cochrane1999; Cassar et al., Reference Cassar, Amezuca, Tenbergen and Friendship2014; Burkemper et al., Reference Burkemper, Pairis-Garcia, Moraes, Park and Moeller2019). Contrary to this, McGlone et al. (Reference McGlone, Nicholson, Hellman and Herzog1993) found that piglets castrated at one day of age had lower weaning weights than those castrated at 14 days, while female pigs were intermediate. Because female pigs were used as controls rather than sham-handled males, this result is difficult to interpret. A more recent study by Morales et al. (Reference Morales, Dereu, Manso, de Frutos, Piñeiro, Manzanilla and Wuyts2017) compared ADG in over 3000 castrated versus intact males and categorized piglets based on initial body weight as low (lowest 25%), medium, or high (highest 25%). The study found that among heavier pigs, castrates had lower ADG at weaning compared to non-castrated pigs. Furthermore, low- and medium-weight piglets that were castrated had a higher likelihood of pre-weaning mortality than their non-castrated littermates (Morales et al., Reference Morales, Dereu, Manso, de Frutos, Piñeiro, Manzanilla and Wuyts2017).
While body weight changes are not a direct measure of pain, initial body weights should be considered for enrollment purposes. Control animals should be non-castrated male littermates rather than females. Piglets weighing 1.5 kg or less should be excluded.
Mortality rate
The mortality rate is not an indicator of pain, and intervention efficacy trials are not typically designed to detect statistical differences between treatments. However, when supplemented with a complete necropsy of the pigs that die, the mortality rate can be used as a non-specific indicator of negative impacts on health, toxicity when chemical interventions are applied, and secondary complications that might influence the adoption of an intervention. As some unrelated baseline, mortality is likely to occur in most populations, and occasional euthanasia of animals is warranted for unrelated reasons. Mortality is a non-specific endpoint, and a stepwise evaluation process should be implemented to use study resources effectively.
First, potential physiological impacts that can progress to death and are specific to the intervention being compared should be identified a priori at the start of the study. Any specific necropsy lesions and post-mortem diagnostic testing to confirm or refute the intervention's involvement in the mortality must be recorded. For example, if a drug intervention has the potential for harmful toxicity, the appropriate tissue, diagnostic test, and the testing laboratory should be identified and included in the study protocol as a standard component of the necropsy evaluation.
The second step is to record the pig's identity, time and date observed, and whether the death was the outcome of euthanasia or occurred naturally. In cases where euthanasia was the cause of death, the reason for euthanasia should be recorded.
The third step is to perform a complete external exam and necropsy procedure, as outlined by Torrison (Reference Torrison, Zimmerman, Karriker, Ramirez, Schwartz and Stevenson2012). The observation of gross lesions or the confirmation of absence should be recorded for all the main body systems. The use of a checklist (Fig. 2) by trained observers is necessary to maintain consistency of evaluations and data collection.
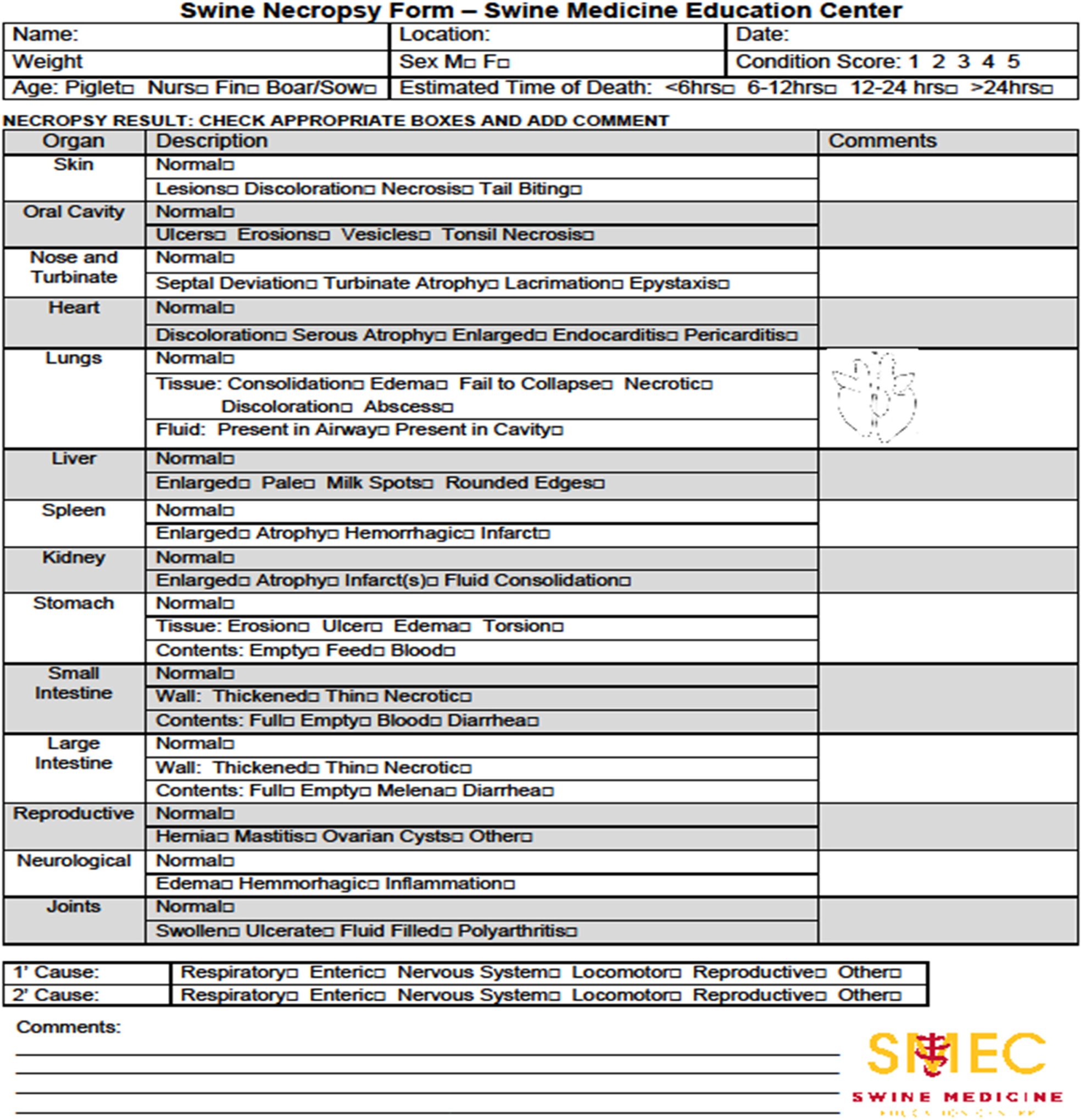
Fig. 2. Checklist of relevant mortality information, necropsy observations, including organ systems to be observed and lesions to be considered.
Suppose gross lesions indicate a specific organ system's involvement, but a cause of death cannot be determined by the necropsy procedure alone. In that case, the fourth step is to employ the appropriate tissue collection as outlined by Torrison (Reference Torrison, Zimmerman, Karriker, Ramirez, Schwartz and Stevenson2012), followed by submission to a qualified diagnostic laboratory with appropriate history and context. All correspondence with the diagnostic laboratory and test results should be copied into the study record.
Discussion
It is well documented that piglets experience pain associated with castration, and efforts to mitigate this pain should be explored. Previous studies on pain mitigation at castration have typically included similar techniques and outcomes, but wide variation in experimental design and data collection approaches give noncomparable results and hinders a comprehensive interpretation of the science (Sheil and Polkinghorne, Reference Sheil and Polkinghorne2020). This paper describes a methodology to assess behavioral, physiological, and neuroendocrine changes associated with pain in piglets resulting from surgical castration. The methodology is being further developed into a research protocol template to facilitate and guide the validity and reliability of endpoints to evaluate drugs' effectiveness to control post-operative pain in 3- to 5-day-old piglets following surgical castration.
For the experimental design and the purposes of endpoint validation, the authors recommend four primary treatments, including (1) sham castration, (2) sham castration with the ‘gold standard’ intervention, (3) surgical castration with a placebo control, and (4) surgical castration with the ‘gold standard’ intervention. Piglet (within litter) is the experimental unit with all treatments represented within each litter at least once. Outcomes being directly related to clinical signs of pain (observation of pain behaviors, activity tracking, IRT, and cortisol concentrations) and ancillary outcomes (stride length, blood biomarkers [including neuroendocrine, inflammatory, immunological, and stress response markers], piglet grimace scale, body weight, and mortality rate) being either directly related to clinical signs of pain or indirectly related to clinical signs of pain and lending support to the direct outcomes will be measured, validated and analyzed.
Conclusion
The experimental design and measurable outcomes selected from the validation study are intended to promote a consistent approach to determining more effective therapies for pain mitigation. This paper supports the inclusion of specific outcomes in the validation study and summarizes the need for further validation of emerging outcomes. The development of similar protocols for determining the validity and reliability of endpoints to evaluate the efficacy of pain mitigation therapies targeted to other painful procedures or conditions in swine, such as tail docking or lameness, should be considered.
Author contributions
Conceptualization, SRW and AD; methodology, AKB, SRW, JB, JFC, SC, AD, LAK, M.P-G., MAS, AVV; writing-original draft preparation, AKB and SRW; writing- review and editing, AKB SRW and M.P-G. All authors have read and agreed to the published version of the manuscript.
Financial support
No monetary funding was sourced for the development of this manuscript.
Conflict of interest
The authors declare no conflict of interest.









