Introduction
In the past 500 years 87 vascular plant species have been officially recorded as extinct globally and 28 species are now assumed to be extinct in the wild (IUCN, 2008a). In the past 20 years 12 plant species have gone extinct globally as a result of habitat loss, invasive species and diseases (Baillie et al., Reference Baillie, Hilton-Taylor and Stuart2004). There was a marked increase in the number of plant species categorized as Critically Endangered, Endangered or Vulnerable between 2000 and 2008 (2,710 newly listed species; IUCN 2000, 2008a). This apparent accelerating rate of endangerment is to some extent a result of improved knowledge but is also an important indicator of the impact of human activities on the persistence of species in the wild (Pimm & Raven, Reference Pimm and Raven2000). Many threatened plants, such as timber trees, palms, ornamental orchids and medicinal plants, also have high commercial value and therefore their extinction will entail not only ecological impacts but also have socio-economic implications (Baillie et al., Reference Baillie, Hilton-Taylor and Stuart2004).
Given the variety of processes that threaten biodiversity a range of conservation strategies are required to slow the rate of species extinction. One task is to evaluate the processes that threaten species so as to facilitate an assessment of risks and inform the prioritization of conservation spending (Lamoreux et al., Reference Lamoreux, Akcakaya, Bennun, Collar, Boitani and Brackett2003; Mace et al., Reference Mace, Possingham, Leader-Williams, Macdonald and Service2007). Such analyses provide opportunities for the efficient use of limited resources by targeting specific strategies to abate the dominant threats to species persistence (Wilson et al., Reference Wilson, Underwood, Morrison, Klausmeyer, Murdoch and Reyers2007).
Habitat loss has been recognized as the most important threat to species globally (Wilson, Reference Wilson1992). However, country-specific causes of species endangerment have also been identified, including overexploitation in China (Yiming & Wilcove, Reference Yiming and Wilcove2005), invasive species in the USA (Wilcove et al., Reference Wilcove, Rothstein, Jason, Phillips and Losos1998) and small population sizes and restricted ranges in Australia (Burgman et al., Reference Burgman, Keith, Hopper, Widyatmoko and Drill2007). While these studies represent countries with diverse economic and biological characteristics, similar analyses have not been undertaken in any nation with a developing economy that is also classified as a biodiversity hotspot, despite such countries being a high priority for conservation efforts (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000; Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann and Lamoreux2006).
Indonesia is one of 17 mega-biodiverse countries (Mittermeier et al., Reference Mittermeier, Gil and Mittermeier1997) but is facing a rapid loss of biodiversity (Bappenas, 2003; Sodhi et al., Reference Sodhi, Koh, Brook and Ng2004). In terms of floristic richness Indonesia ranks fifth, with > 38,000 plant species, c. 18,700 of which are endemic (Bappenas, 2003). Other than the tropical Andes, Indonesia has the greatest number of endemic plant species in any region (> 6% of the total global flora; Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). The unique biodiversity of Indonesia is under pressure from high rates of habitat modification, deforestation, overexploitation, forest fires, and illegal harvesting and trade as a consequence of rapid economic development, high population growth and corrupt institutions (Bappenas, 2003; Sodhi et al., Reference Sodhi, Koh, Brook and Ng2004). In Indonesia c. 50% of forest cover has been lost in the last 50 years, with cover reduced from c. 162 million ha in 1950 to 86 million ha in 2003 (FWI/GFW, 2002; Indonesian Ministry of Forestry, 2005). Over the last decade the rate of deforestation in Indonesia is estimated to have been 1.8 million ha per year, predominately from timber extraction, transmigration, agricultural expansion, plantation establishment and mining (FWI/GFW, 2002; Bappenas, 2003; Indonesian Ministry of Forestry, 2005). A study of the processes that threaten Indonesian plants is therefore critical to assist with their conservation but research on this topic in Indonesia is rare (Sodhi & Liow, Reference Sodhi and Liow2000; Sodhi et al., Reference Sodhi, Koh, Brook and Ng2004; Meijaard & Sheil, Reference Meijaard and Sheil2007a).
Our aim here is to identify and evaluate the processes that threaten plants in Indonesia. We do this by defining, categorizing and quantifying the threatening processes to reveal the major causes of plant endangerment. We also analyse threat syndromes (sensu Burgman et al., Reference Burgman, Keith, Hopper, Widyatmoko and Drill2007), defined as a subset of processes that simultaneously threaten multiple species, to evaluate the interrelationships amongst threats to plants in Indonesia.
Methods
The primary source of data for this study is Mogea et al. (Reference Mogea, Gandawijaya, Wiriadinata, Nasution and Irawati2001), which provides an account of 240 plant species evaluated by nine Indonesian botanists. The list was an outcome of the Biodiversity Collection Project conducted between 1994 and 2000 and facilitated by the Global Environment Facility and the Research Centre for Biology of the Indonesian Institute of Sciences. Despite representing only a sample of Indonesian plant diversity, this list represents the most comprehensive list of rare and threatened plants in Indonesia. Rare plants were defined as native plants pressured by direct and indirect threats that are causing population decline or species extinction (Mogea et al., Reference Mogea, Gandawijaya, Wiriadinata, Nasution and Irawati2001). The 240 species are taxonomically diverse and cover all areas of Indonesia.
The composition of the evaluated plants differs markedly from that in the IUCN Red List for Indonesia, which comprises 687 threatened plant species of which only 21 are on both lists (IUCN, 2008b). Palms and orchids dominate Mogea et al. (Reference Mogea, Gandawijaya, Wiriadinata, Nasution and Irawati2001) whereas the IUCN Red List contains only six palm species and no species of orchid. The differences between the nationally-derived list and the globally maintained database are probably because of the differences in spatial scale, experts, taxonomy and the frequency of information update of each database (Burgman, Reference Burgman2002; Possingham et al., Reference Possingham, Andelman, Burgman, Medelin, Master and Keith2002). For 671 species on the IUCN Red List the conservation status was last updated in 2000 or before, with only 16 species updated after 2001 (IUCN, 2008b). We consider the Mogea et al. (Reference Mogea, Gandawijaya, Wiriadinata, Nasution and Irawati2001) list of threatened species a more comprehensive and accurate source of information for a country-level analysis.
We categorized the proximate (i.e. direct) threatening processes to each of the 240 species using the IUCN–CMP Unified Classification of Direct Threats (Hilton-Taylor et al., Reference Hilton-Taylor, Stattersfield, Salafsky, Salzer and Neugarten2006). Four general threat classes were identified: habitat loss, overexploitation, intrinsic biological factors and natural factors (Table 1). Within the four general threat classes we identified 16 specific threats that are potentially important in the context of Indonesia.
Table 1 Definitions of general and specific threats.
We allocated each plant to one of five groups: palms (Arecaceae, 60 species), orchids (Orchidaceae, 52 species), trees (57 species), shrubs (41 species) and others (30 species; Appendix 1). Of the Arecaceae and Orchidaceae 22 and 8 genera, respectively, were evaluated (Uhl & Dransfield, Reference Uhl and Dransfield1987; IUCN/SSC Orchid Specialist Group, 1996). The tree group evaluated comprises 21 families and 35 genera. The shrub (defined as a ligneous plant with multiple stems and a height < 7 m; Baumer, Reference Baumer1983) group includes woody climbers and comprises 11 families and 20 genera. The other group is used for the remaining species, in 10 families and 14 genera, predominantly herbaceous and parasitic plants. The categorization of plants by these major groups was undertaken to facilitate the development of conservation strategies specific to each group.
The processes that threaten a species were first determined by expert judgement. Two botanists from the Indonesia Botanic Garden and six botanists from Herbarium Bogoriense undertook the threat assessment for the species with which they are familiar. A score of 1 was assigned to those species considered to be negatively affected by a particular threatening process and 0 if the threat was considered to have no impact. The expert evaluation is potentially subject to bias and, although focusing on species with which they are familiar reduces this, it is likely to produce a conservative evaluation of the effect of key threatening processes. We therefore cross-checked the expert evaluation with secondary data and available literature, including herbarium specimen databases, journal articles, scientific reports, taxonomic books and official websites (Appendix 2). To maximize the level of repeatability and transparency the final evaluation was determined according to the following process: (1) the threats were allocated by the most experienced expert for each species; (2) the evaluation was adjusted if evidence from the secondary information sources contradicted an expert evaluation of no impact of a particular threat on a species; (3) if evidence from the secondary information sources suggested no impact and this contradicted the expert assessment of there being an impact, then the expert assessment took precedence.
Unweighted Pair Group Method with Arithmetic Mean Analysis (UPGMA; Legendre & Legendre, Reference Legendre and Legendre1998) was used to identify sets of specific threats (threat syndromes) that simultaneously affect a large number of species (sensu Burgman et al., Reference Burgman, Keith, Hopper, Widyatmoko and Drill2007). Before being clustered the similarities between threats were calculated using the Bray-Curtis dissimilarity method and the distance of the resultant matrix was ordinated using Nonmetric Multi-Dimensional Scaling (Legendre & Legendre, Reference Legendre and Legendre1998). To provide a more detailed assessment of threat syndromes we also undertook this analysis for each major plant group. All statistical analyses were conducted using R v. 2.7.2 (R Development Core Team, 2008).
Results
General and specific threats
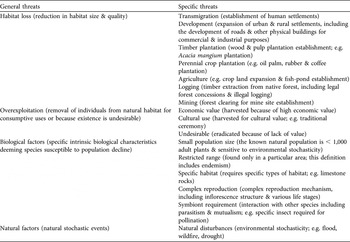
Intrinsic biological factors and habitat loss are the most significant general threats to the 240 species of plants evaluated, affecting 83 and 82% of species respectively. Overexploitation has also contributed considerably to species endangerment, affecting 64% of species, whereas natural factors are endangering a relatively small proportion of the studied species (6%; Fig. 1).
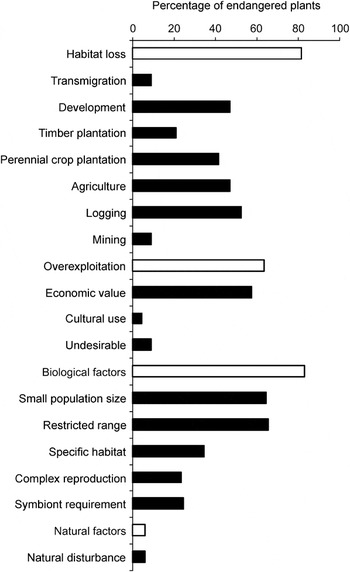
Fig. 1 The percentage of a sample of Indonesian threatened plant species (n = 240; Appendix 1) affected by general and specific threats. The white bars represent the four general threats; black bars represent the 16 specific threats. Categories of specific threats are not exclusive and therefore do not total 100%.
Of the specific threats, restricted ranges and small population sizes are the most prevalent threats, both associated with 65% of all plants studied (Fig. 1). Other significant specific threats are overexploitation because of high economic value, logging activities, development of human settlements, agriculture and perennial crop plantations. The two threatening processes that were thought to be potentially important in the context of Indonesia (transmigration and cultural use) were found to endanger plants to a lesser extent (9 and 5% respectively).
Threats by major plant group
The type of general threat affecting species varies significantly between groups (Fig. 2). Habitat loss is the most important threat to palms (95%) and trees (98%), whereas biological factors are the major threat to shrubs (88%) and the other species investigated (97%). However, orchids are most adversely affected by overexploitation and biological factors (98 and 100%, respectively).
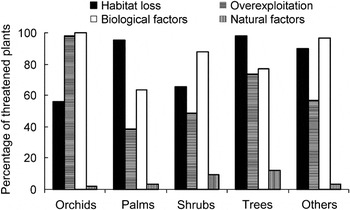
Fig. 2 The percentage of 240 plant species (Appendix 1) affected by the four general threats (habitat loss, overexploitation, biological factors, natural factors) is significantly different among major plant groups (χ2 = 32.07, df = 12, P < 0.001). Five plant groups are represented: orchids (n = 52), palms (n = 60), shrubs (n = 41), trees (n = 57) and others (n = 30).
Of the specific threats, overexploitation because of economic value and symbiont requirements are the most important threats to orchids (both 98%), and logging is the major threat to trees (77%). Palms are threatened mostly by development of human settlements (78%), and small population size is the most important threat to shrubs (68%) and the remaining species investigated (97%).
Threat syndromes
Using UPGMA three sets of interrelated threats that concurrently affect a large number of species (threat syndromes) were identified (Fig. 3): (1) species with small populations that are endemic to particular areas; these are also overexploited because of their economic value and are located in areas that are threatened by logging activities (e.g. Calamus ciliaris, Arecaceae; Phalaenopsis gigantea, Orchidaceae; Upuna borneensis, Dipterocarpaceae); (2) species in natural habitats simultaneously disturbed by agriculture, development for human settlements and perennial crop plantations (e.g. Arcangelisia flava, Menispermaceae; Alyxia reinwardtii, Apocynaceae; Schizostachyum castaneum, Poaceae); (3) species with complex reproduction mechanisms that also have specific habitat requirements and symbiotic relationships with other organisms (e.g. some flagship species such as Amorphophallus spp., Araceae; Rafflesia spp., Rafflesiaceae; Paphiopedilum spp., Orchidaceae).
Fig. 3 Dendrogram of threat syndromes to Indonesian plant species. The three threat syndromes are (1) economic value, logging, restricted ranges and small population sizes; (2) agriculture, development and perennial crop plantation; (3) symbiotic requirement, complex reproduction and specific habitat.
Threat syndromes by major plant group
Two sets of inter-related threats were identified for species of orchid (Fig. 4a): (1) species that are overexploited because of their economic value and also have restricted ranges, small population sizes, symbiont requirements, complex reproduction mechanisms and specific habitat requirements (e.g. Paphiopedilum kolopakingii, P. mastersianum) or are threatened by logging (e.g. Paraphalaenopsis serpentilingua and Paraphalaenopsis laycockii); (2) species for which their natural habitats are disturbed concurrently by agriculture and development of human settlements (e.g. Phalaenopsis amabilis and Phalaenopsis javanica).
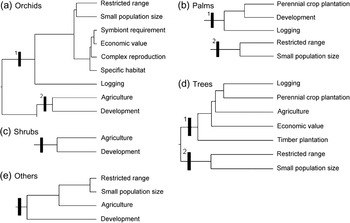
Fig. 4 Dendrogram of the three threat syndromes (Fig. 3) to five groups of plants: (a) orchids; (b) palms; (c) shrubs; (d) trees; (e) others.
Two threat syndromes were identified for palm species (Fig. 4b): (1) species for which their natural habitats occur in areas important for perennial crop plantations, development of human settlements and logging (e.g. Nenga gajah and Borassodendron borneensis); (2) restricted range species with small population sizes (e.g. Ceratolobus glaucescens and Johannesteijsmannia altifrons).
Only one threat syndrome was identified for shrub species (Fig. 4c): species for which their natural habitats intersect with areas of agriculture and areas of human settlement development (e.g. some species used for traditional medicine such as Anaxagorea javanica, Annonaceae, Rauvolfia serpentina, Apocynaceae).
There are two sets of inter-related threats affecting tree species (Fig. 4d): (1) species that are overexploited and are also threatened by habitat loss because of logging activities, perennial crop plantations, agriculture and timber plantations (e.g. major commercial timber trees such as Shorea spp., Dipterocarpaceae; Diospyros spp., Ebenaceae; Eusideroxylon zwageri, Lauraceae, and also some agarwood species such as Aquilaria spp., Thymelaceae); (2) restricted range species with small population sizes (e.g. Mangifera casturi, Anacardiaceae; Vatica bantamensis, Dipterocarpaceae).
Two threat syndromes were identified for the other species: (1) species with complex reproduction systems that typically have symbiont requirements (e.g. Amorphophallus spp., Rafflesia spp.; this threat syndrome was not represented in the cluster analysis because the coefficient of Bray-Curtis dissimilarity was zero, which reveals a perfect relationship between the threats); (2) restricted range species with small population sizes occurring in areas where habitat has been destroyed for development of agriculture and human settlements (Fig. 4e; e.g. Gigantochloa manggong, Poaceae; Musa acuminata var. nakaii, Musaceae).
Discussion
Indonesia has high levels of plant endemism but significant socio-economic constraints to the conservation of biodiversity. In addition to habitat loss, the greatest threat to biodiversity at a global scale (Wilson, Reference Wilson1992; Baillie et al., Reference Baillie, Hilton-Taylor and Stuart2004), overexploitation and intrinsic biological factors (such as restricted ranges and small population sizes), are important threats to plant species in Indonesia. We identified natural factors (such as floods and drought) as a minor cause of endangerment to the plant species evaluated.
Species extinction in tropical biodiversity hotspots is often linked with habitat loss caused by the destruction of primary forests (Brooks et al., Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands and Konstant2002, Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands and Konstant2003). Our study reveals that logging activities are a major threat to plants in Indonesia. These results concur with the causes of deforestation in Indonesia between 1950 and 2000 being timber extraction (69 million ha), clearing for shifting cultivation (4 million ha), establishment of perennial crop plantations (7 million ha), creation of timber plantations (9 million ha), and forest conversion in transmigration areas (4 million ha; FWI/GFW, 2002). The establishment of oil palm Elaeis guineensis plantations is expected to increase species endangerment in the future (Fitzherbert et al., Reference Fitzherbert, Struebig, Morel, Danielsen, Bruhl, Donald and Phalan2008).
In our analysis of threat syndromes habitat loss caused by logging often occurs simultaneously with development of human settlements, agriculture, and perennial crop and timber plantations. This syndrome is dominant for the plants that occur in Sumatra and Kalimantan, the two regions in Indonesia with the most extensive areas of forest concessions, illegal logging, wood plantations for pulp production and oil palm plantations (FWI/GFW, 2002). A combination of development of human settlements and agriculture is the major threat to the plant species evaluated that occur in the centres of human population (i.e. Java and Bali) where threats from other causes of habitat destruction are less prevalent. Further spatial analysis of the distribution of species and threats is needed to verify these patterns.
Conventional conservation strategies of creating new protected areas are likely to be important for abating threats caused by habitat loss. However, commitment of the Indonesian government to strengthening the role of existing protected areas by more stringent law enforcement and good governance of these areas will also be essential for conserving such species. The Indonesian government has officially preserved 24 million ha (13% of the total land area) as protected areas (WRI, 2003) yet the pressures on biodiversity are still high because the reserved areas are threatened by forest fires, illegal logging, mining and establishment of oil palm plantations. Such activities are thought to reduce the effective size of these protected areas by > 50% (Curran et al., Reference Curran, Trigg, McDonald, Astiani, Hardiono and Siregar2004; Gaveau et al., Reference Gaveau, Handono and Setiabudi2007). Prudent decision making is also needed in allocating land-uses, especially if increasing demands for timber and oil palm plantations are to be satisfied. The location of such land uses must be carefully considered in the context of habitat for threatened species (Wilson et al., Reference Wilson, Meijaard, Drummond, Grantham, Boitani and Catullo2010). Another strategy is to mandate timber and plantation companies to undertake more conservation-friendly management practices and set aside areas within timber concessions such as high conservation value forests (Dennis et al., Reference Dennis, Meijaard, Nasi and Gustafsson2008). Such set-asides are increasingly necessary to certify production forests and oil palm plantations (Meijaard & Sheil, Reference Meijaard and Sheil2007b; Fitzherbert et al., Reference Fitzherbert, Struebig, Morel, Danielsen, Bruhl, Donald and Phalan2008; Venter et al., Reference Venter, Meijaard and Wilson2008).
We found that overexploitation is a substantial threat to the plant species investigated. Conservation strategies such as strict regulation of management practices (e.g. harvesting methods) and trade (e.g. chain of custody and certification) are needed to control the exploitation and utilization of species of economic value (Soehartono & Newton, Reference Soehartono and Newton2001).
Restricted ranges and small population sizes were associated with the endangerment of Indonesian plants but the consequences of such traits are expected to be emphasized in Indonesia because of habitat loss and overexploitation. The susceptibility of species with restricted ranges to human-induced disturbance can be mitigated by strictly protecting the remaining habitat of these species, combined with ex situ propagation, reintroduction and population enrichment in natural habitats.
We also found that each group of plants has a different dominant threat syndrome. For orchid species the combination of threats caused by biological factors and overexploitation for economic purposes is common. A combination of financial reward and difficulties associated with propagating these species motivate poachers to harvest orchids in their natural habitats. Epiphytic orchids of the genera Phalaenopsis and Paraphalaenopis are threatened by logging activities because they require host trees. In contrast, terrestrial orchids of the genus Paphiopedilum are relatively protected from threats caused by habitat loss because they are commonly found in specific habitat niches (e.g. limestone rocks, river banks and steep slopes).
The palm and tree species evaluated are threatened simultaneously by overexploitation for economic purposes and habitat destruction caused by logging activities, perennial crop plantations, development of human settlements and agriculture. Compared to orchids, both palms and trees are habitat generalists and are consequently more susceptible to several types of habitat loss. Strong threat syndromes for shrubs are not apparent, except the co-occurrence of threats caused by agricultural activities and development of human settlements. This is presumably attributable to their diverse uses, simple biological characteristics and widespread geographical distribution.
For the remaining herbaceous and parasitic species the threat syndrome is a combination of threatening processes caused by biological factors and habitat loss. Compared to orchids threatened species such as Amorphophallus spp. and Rafflesia spp. are not economically valuable. However, because of their complex reproduction mechanisms and specific habitat requirements such species are sensitive to habitat disturbance, so that even minor habitat destruction will severely affect these species.
Our findings indicate that invasive alien species, pollution, disease and climate change are not important threats to Indonesian plants. In the IUCN Red List no Indonesian plants are listed as threatened by these processes (IUCN, 2008b). Wilson (Reference Wilson1992), however, noted that introduced species are the second most important threat to biodiversity after habitat loss. Further research is therefore required on the impacts of invasive alien plant species on the flora of Indonesia (Lawler et al., Reference Lawler, Aukema, Grant, Halpern, Kareiva and Nelson2006; Meijaard & Sheil, Reference Meijaard and Sheil2007a).
In Indonesia there is a lack of information on the processes that threaten native species. This inevitably leads to difficulties in developing conservation policy and setting conservation priorities, and precludes the development of integrated conservation strategies across a wide range of taxonomic groups. This study is the first comprehensive evaluation of a small but representative sample of the plant diversity of Indonesia. We therefore recommend that information on the processes that threaten Indonesian species be expanded and periodically updated, with this research serving as a benchmark and a baseline to evaluate trends through time. In addition, an evaluation of threat syndromes at a local scale (for provinces or protected areas) will help guide the development and implementation of conservation programmes.
Acknowledgements
This research was supported by a postgraduate Australian Development Scholarship to SB from AusAID and funding to KAW from the Australian Research Council.
Appendices
The appendices for this article are available online at http://journals.cambridge.org
Biographical sketches
Sugeng Budiharta’s research interest is the conservation of threatened plants and degraded ecosystems, especially in the tropics. Didik Widyatmoko’s research is focused on the conservation of threatened palms. Irawati’s research interests are in orchid species and their conservation. Harry Wiriadinata is a taxonomist specializing in the Fabaceae and Thymelaceae. Rugayaha is a taxonomist specializing in the Cucurbitaceae. Tukirin Partomihardjo is an ecologist with interests in ecosystem dynamics, especially in degraded forests and volcanic ecosystems. Ismail is a field botanist with a special interest in trees. Tahan Uji is interested in species of the Rutaceae and Bombacaceae. Ary Prihardhyanto Keim has a particular interest in the taxonomy of Pandanaceae. Kerrie A. Wilson’s research interests are in conservation decision making.









