I. Introduction
With the great progress of the applications of GaN on optoelectronic devices and high temperature / high power electronics, GaN is more attractive than ever[Reference Nakamura, senoh, Nagahara, Iwasa, Yamada, Matsuahita, Kiyoku and Sugimoto1,Reference Nakamura, senoh, Nagahara, Iwasa, Yamada, Matsuahita, Kiyoku, Sugimoto, Kozaki, Umemeoto, Sano and Chocho2]. The metal-oxide-semiconductor field effect transistor (MOSFET) based on β-Ga2O3/GaN has been reported[Reference Ren, Hong, Chu, Marcus, Schurman, Baca, Pearton and Abernathy3], and it shows a better performance at 400°C than that at room temperature. In the many polymorphs of Ga2O3, β-Ga2O3 is believed to be the equilibrium phase. It is very important to understand the oxidation mechanism of GaN at high temperature for the realization of these electronic devices. The kinetics of the oxidation of GaN powder in dry air has been reported by Wolter et al.[Reference Wolter, Mohney, Venugopalan, Wickenden and Koleske4]. An oxide forms at 900°C for 1 h, and is identified as the monoclinic β-Ga2O3. The initial stage of the oxidation is found to be limited by the rate of an interfacial reaction. The same mechanism was also found in the oxidation of GaN epilayers at 900°C in dry air in their report. However, there is little known about the oxidation of GaN epilayers in dry oxygen.
In this letter, we report a scanning electron microscope (SEM) observation and the oxidation behavior of GaN epilayers at temperatures from 800°C to 1100°C in dry oxygen. The results reveal the different oxidation mechanism at different temperatures.
II. Experiment
The GaN epilayers (about 1-µm thick) used in the experiments are grown on a c-plane of sapphire substrates by rapid thermal processing low-pressure metalorganic chemical vapor deposition (RTP/LP-MOCVD). The detailed growth process has been published previously[Reference Shen, Zhou, Chen, Chen, Zhang, Shi, Zheng, Tong and Park5]. The full width at half maximum (FWHM) of 9.8 min of X-ray rocking curve (XRC) indicates the high quality of the GaN film. A 1.5-inch diameter sample is divided into several pieces for oxidizing under various conditions. After cleaned in the solvents and DI water, the samples are etched in HCl:DI water (1:1) to remove surface contamination and the native oxide. Then the samples are placed in a quartz boat, which will be transferred into a horizontal quartz tube, and the tube is placed in a electric furnace to oxidize the samples at temperatures (from 800°C to 1100°C) for a certain time (from 10 min to 6 h). During the oxidation, dry oxygen flow maintains 1 SL. After completing each experiment, the quartz boat is brought out to room temperature within 5 min.
The samples are studied by the θ−2θ scan X-ray diffraction (XRD) with Cu Kα radiation and SEM.
III. Results and Discussion
The oxide is characterized by a θ−2θ scan XRD. The XRD data of GaN epilayers which are oxidized at temperatures 800°C, 900°C, 1000°C, 1050°C and 1100°C for 1 h are shown in fig.1. The data for various oxidation times are also collected. After oxidized at 800°C for 6 h, the crystalline oxide is observed and identified as the monoclinic β-Ga2O3 phase by comparing the data list in Powder diffraction file, 11-370.

Fig.1. The XRD data of GaN epilayers which were oxidized at temperatures of 800°C, 900°C, 1000°C, 1050°C and 1100°C for 1 h. The GaN peaks disappeared at 1100°C for 1 h.
The GaN films oxidized at lower than 1050°C show a relative smooth surface to eyes, but show a serious rough surface at 1050°C for 1 h or 1100°C for 35 min. The intensity of the oxide peaks increases quickly with the increasing oxidation time, and GaN peaks eventually disappear at 1100°C for 1h, which indicates a very high oxidation rate in this case. The recent studies of thermal stability of GaN film have also shown that the GaN film is unstable over 1000°C[Reference Koleske, Wickenden, Henry, DeSisto and Gorman6]. From fig.1, it can be seen that the strongest peak of β-Ga2O3 is (11-3), and the second strongest is (30-6). Either (11-3) or (30-6) is not the peak which has the highest intensity listed in Powder diffraction file, 11-370 and reported in the oxidation of GaN powder by Wolter[Reference Wolter, Mohney, Venugopalan, Wickenden and Koleske4]. This fact indicates that there may be some selective relationship between the oxide orientation and (0001) GaN. The little change of the XRD intensity ratios (1.2∼1.6) between (11-3) and (30-6) at various oxidation conditions indicates that the structure of the oxide film on (0001) GaN is almost unchangeable in this case.

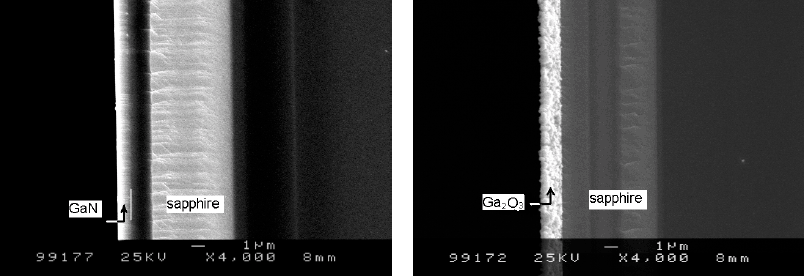
Fig.2. SEM images of a GaN film and an oxide layer. (a), the 1 µm-thick GaN film before oxidation; (b), completely oxidized at 1050° C for 2h and the thickness of the oxide layer is about 1.4 µm.
Fig.2 shows the cross-sectional SEM images of a GaN epilayer and an oxide layer formed at 1050°C for 2h. The GaN layer is almost completely oxidized at 1050°C for 2 h. Contrast to a very smooth surface of the GaN film before oxidation (fig.2a), the oxide layer shows a very rough surface (fig.2b), and has a number of oxide grains. An MOCVD-grown GaN epilayer on sapphire always has high density of dislocation, which may prevent the GaN film from the oxidation on the way of layer-by-layer. From the images, a significant expansion of the volume is clearly observed. The thickness of the oxide layer formed by a 1 µm GaN is about 1.4 µm, which indicated an expansion of the volume of 40%.
Since the intensity of XRD diffraction peak is related to the volume of the sample[Reference Zevin, Kimmel and Mureinik7] and the structure of the oxide film on (0001) GaN is almost unchangeable. One can chose the intensity of β-Ga2O3 (11-3), I, to monitor the oxide thickness. It should be noticed that the premises of the approach are the fixed X-ray power. In addition, the very small X-ray absorption by β-Ga2O3 film, less than 3% in a ∼1 µm-thick gallium oxide film [Reference Wolter, Mohney, Venugopalan, Wickenden and Koleske4], indicates a thickness which is much greater than the estimated oxide thickness in this work.
Fig.3 shows the intensity of the β-Ga2O3 (11-3) diffraction peak plotted vs. oxidation time at different temperatures. The two different oxidation processes can be clearly observed over 1000°C. When the oxidation temperature is higher than 1000°C, there is a rapid oxidation process in the initial stage of oxidation, followed by a relatively slow process. The oxidation rate in the initial stage is dependent on the oxidation temperature, which can not be observed at lower temperatures. Obviously, the top oxide film formed in the rapid process could be affected by the decomposition of GaN film.

Fig.3 The intensities of β-Ga2O3 (11-3) peak plotted vs oxidation time for different temperatures, 800°C (diamond); 900°C (square); 1000° C (circle); 1050° C (triangle). Two different oxidation process were observed over 1000°C.
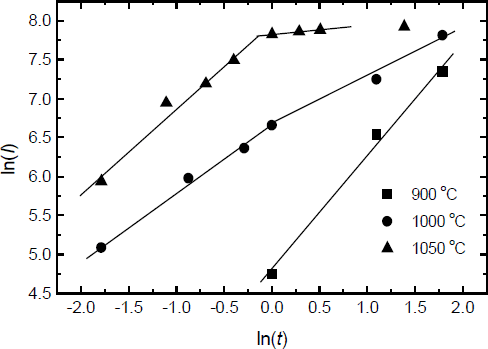
The equations for the oxide growth have been described by Wolter[Reference Wolter, Mohney, Venugopalan, Wickenden and Koleske4]
where n is the reaction order, k is the reaction rate constant, t is the oxidation time. If n is equal to1, the oxidation is under interfacial reaction-controlled; if n is equal to 2, the oxidation is under diffusion-controlled. The reaction rate k is the function of the oxidation temperature T and the activation energy. Fig.4 displays a plot of ln(I) vs. ln(t) from fig.3, which is used to determine the reaction order during the slow oxidation process and the rapid oxidation process. From fig.4, a linear relationship exists for all temperatures, but the slops which are equal to 1/n from eq.2 are different each other. During the slow oxidation process, the values of n increase from 0.71 at 900°C to 1.6 at 1000°C, which indicates that the dominating oxidation mechanism changes from interfacial reaction-controlled to diffusion-controlled with increasing the oxidation temperature. It is beyond our expectation that the n is 7.8 at 1050°C. At this temperature, the film is almost completely oxidized, and the top oxide layer may affect the later oxidation of the GaN film. Therefore, the oxidation at 1050 °C after 1 hour is different from the oxidation under other conditions. It is very different from the oxidation of GaN powder in dry air. However, during the rapid oxidation process, the values of n are 1.1 at 1000°C and 0.89 at 1050°C, which indicates that the dominating oxidation mechanism is under interfacial reaction controlled.
IV. Conclusions
In conclusion, the oxidation of GaN epilayers in dry oxygen has been investigated. The θ−2θ scan XRD data identify that the monoclinic β-Ga2O3 begin to form on GaN epilayers at 800°C for 6h. The GaN epilayers are completely oxidized at 1050°C for 4 h or at 1100°C for 1 h. Two different oxidation processes are found when the temperature is over 1000°C. There is a rapid oxidation process in the initial stage of oxidation, and a relative slow process follows. The oxidation is limited by the rate of an interfacial reaction mechanism in the rapid oxidation process and changes from the interfacial reaction-controlled mechanism to the diffusion-controlled mechanism in the slow process. When the temperature reaches 1100°C, the oxidation rate is very fast, which is considered as the results of the GaN decomposition at high temperature. SEM images show the rough surface of oxide layer and a number of oxide grains in it. An expansion of the volume of 40% is also observed.
Acknowledgements
This work was supported by National Natural Science Foundation of China with contracts #69976014, #69636010, #69806006, #69987001, the National High Technology Research & Development Project of China, and MOTOROLA(China Inc.) Semiconductor Scholarship.