Introduction
Bovine tuberculosis (bTB) caused by Mycobacterium bovis is a zoonotic disease primarily affecting animals. Although cattle are the main hosts, the disease has been reported in many other farmed and wild animals [Reference Radostits1]. Department of Agriculture, Environment and Rural Affairs (DAERA) has a European Union (EU) Commission approved bTB eradication programme which ensures compliance with the EU Trade Directive 64/432/EEC. EU approval of the bTB Northern Ireland eradication programme is vital in safeguarding the export-dependent livestock and livestock products industry (worth in excess of £1.79 billion in 2018) [2]. In 2018, the Northern Ireland bTB programme cost £39 million, an increase of £8.5 million from 2016. This increase was reflective of increased disease incidence from 2016 to 2017 (herd incidence of 9.61% in December 2017 compared to 7.45% in December 2016) requiring associated increased expenditure largely in the area of compensation payment for the purchase of cattle as part of the bTB programme [3].
In 2016, a bTB eradication strategy for Northern Ireland was published [4], providing a framework for bTB eradication from the national cattle population. Part of the implementation plan for this strategy recommended that herds chronically infected with bTB (‘chronic herds’) should be recognised as a distinct entity for action and a package of measures be targeted at them so as to minimise their impact.
In a previous publication [Reference Doyle5], data from a national database Animal and Public Health Information System (APHIS) [Reference Houston6] were used to determine definitions for chronic herds. The definitions developed for both long-duration and recurrent bTB herd breakdowns encompassed almost 40% of the total number of Single Intradermal Comparative Tuberculin Test (SICCT) reactors identified during the study period [Reference Doyle5]. This study looked at risk factors pertaining to the SICCT and cattle movement data stored on APHIS, implementing a design (same design as our present study) which compared prolonged bTB breakdowns to short-duration breakdowns [Reference Doyle5]. However, the original study [Reference Doyle5] could not investigate any of the bTB herd breakdown risk factors associated with chronic herds at a farm management level, a knowledge gap in Northern Ireland which our present study aimed to fill. Such management factors have been studied in Great Britain and the Republic of Ireland [Reference Griffin7, Reference Karolemeas8]. A previous case-control study in Northern Ireland considered risk factors for bTB relating to farm boundaries, neighbouring herds and wildlife, but it did not investigate chronic bTB herd breakdowns [Reference Denny and Wilesmith9] and in a design contrast to our study it compared herds with breakdowns to herds which did not experience bTB breakdowns.
Study objective
The objective of this study was to identify farm-level management factors associated with prolonged bTB herd breakdowns, using data collected during on-farm epidemiological investigations. A case-control study design was used where prolonged duration bTB herd breakdowns (as defined previously [Reference Doyle5]) were compared to short-duration bTB herd breakdowns.
Materials and methods
Study design and data collection
A case-control study was conducted on a study population consisting of all bTB herd breakdown investigations during the period 23 May 2016 to 21 May 2018. Data collection involved completion of an on-farm investigation form when one or more SICCT reactors or one or more confirmed lesion at routine slaughter (LRS) were disclosed in any Northern Ireland cattle herd. Confirmation of bTB in an LRS was defined as a positive histological and/or bacteriological culture result following laboratory examination. Investigations were carried out by trained Animal Health and Welfare Inspectors (AHWI) who visited each of the bTB breakdown farms. At each farm, an on-site questionnaire was completed (Supplementary Table S2) through face-to-face interview of the farmer, including identification of all herds contiguous to the bTB herd breakdown. Based on the completed questionnaire and local knowledge of the area, the Veterinary Officer (VO) responsible for the bTB herd breakdown, where possible, determined the most likely source of infection for the breakdown. Questionnaire information along with data extracted from APHIS (herd size and location) was collated into Microsoft AccessTM (Microsoft Corporation, Redmond, WA, USA). For our study, the 10 Divisional Veterinary Offices (DVOs) were aggregated into three groups according to their geographic location: south east group (Armagh, Newry, Newtownards), west group (Dungannon, Enniskillen, Strabane, Omagh) and north east group (Ballymena, Coleraine, Mallusk) (Fig. 1).
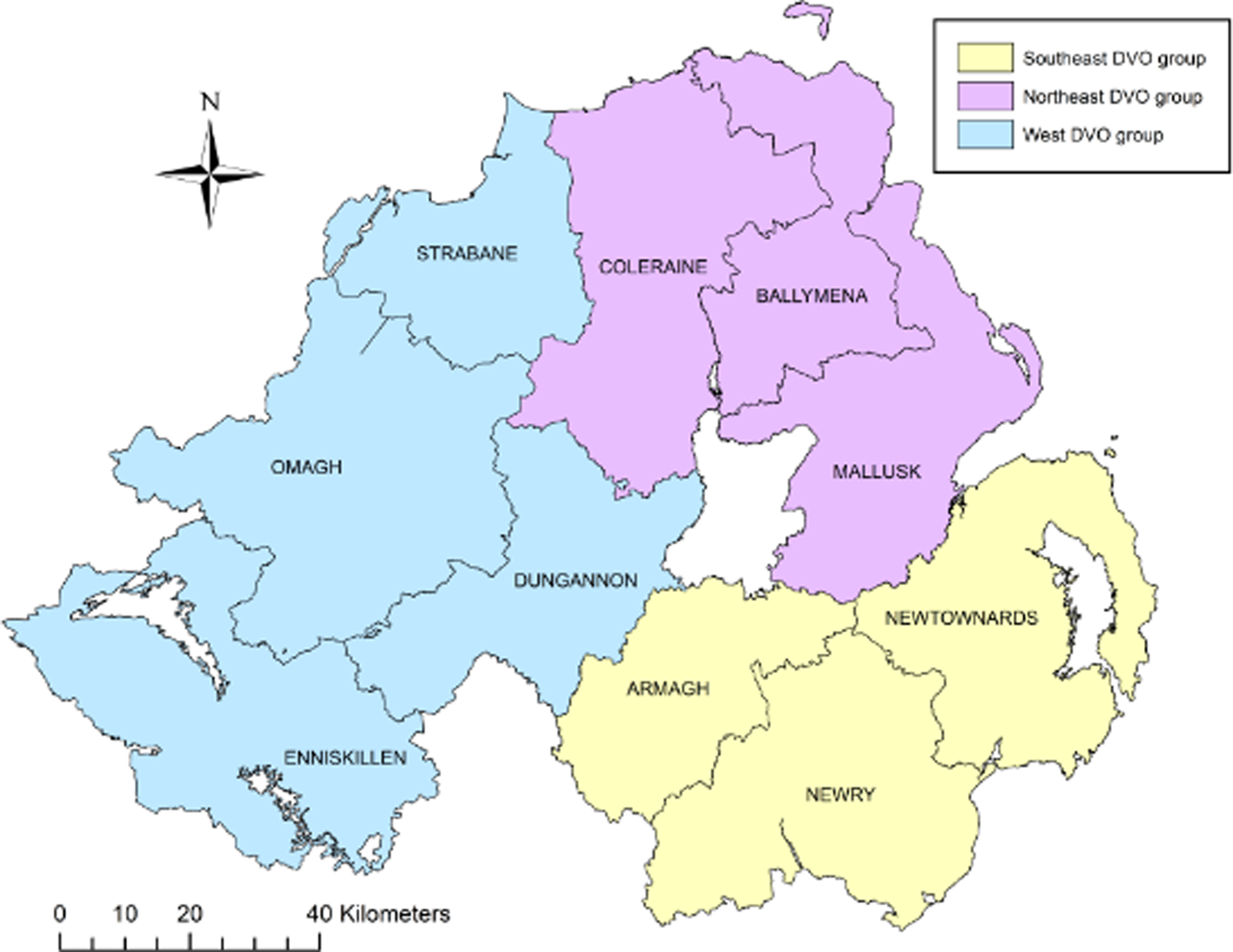
Fig. 1. Northern Ireland DVOs aggregated into three groups, southeast, northeast and west.
Case and control definitions were identical to those used in a previous study [Reference Doyle5]. Cases were bTB herd breakdowns which ended during the study period (23 May 2016 to 21 May 2018) and had a duration of ≥365 days. Controls were bTB herd breakdowns which ended during the study period (23 May 2016 to 21 May 2018) and had a duration of <365 days.
Data analysis
Microsoft AccessTM (Microsoft Corporation) and R Version 3.4.0Footnote 1 were used for data manipulations and R Version 3.4.0a and Stata/SE 15Footnote 2 were used for data analysis. The model framework used was binary logistic regression using a purposeful selection of covariates [Reference Hosmer, Lemeshow and Sturivant10] with the case definition forming the response variable. In total, 78 explanatory variables were derived from the on-farm questionnaire (see Supplementary Table S2) along with their associated factor levels. Initially, all variables were tabulated using the duration case definition against each variable's factor levels. As variables were added or removed from the model, the Akaike Information Criterion (AIC) difference was calculated between the old and new proposed model, in order to determine if the proposal reduced AIC by a value greater than two [Reference Hosmer, Lemeshow and Sturivant10]. Where the models were subsets of each other, the Likelihood Ratio test (LRT) was also calculated in order to determine if addition or removal of variables was significant at the P ≤ 0.05 level.
Initial analysis was by univariable logistic regression. Any variables containing low numbers (<10) of cases at any factor level, which could not have that factor level logically merged with another level were removed after univariable analysis. Remaining variables with P ≤ 0.25 were then analysed using a multivariable logistic regression model. The resultant model was further refined to produce a reduced multivariable logistic regression model which utilised variables with P ≤ 0.05 from the first multivariable model. Following the fit of the reduced multivariable model, its estimated coefficients were compared to those in the initial multivariable model to determine if there was a magnitude change of >20%. This magnitude change known as $\Delta \hat{\beta }$![]() % (Delta-Beta-Hat %) indicates that one or more of the excluded variables are important in the sense of providing a needed adjustment effect of the variables that remained in the model [Reference Hosmer, Lemeshow and Sturivant10]. Variables which formed the first multivariable model but not included in the initial reduced multivariable model were added back individually; being retained if they contributed to the overall model and reduced $\Delta \hat{\beta }$
% (Delta-Beta-Hat %) indicates that one or more of the excluded variables are important in the sense of providing a needed adjustment effect of the variables that remained in the model [Reference Hosmer, Lemeshow and Sturivant10]. Variables which formed the first multivariable model but not included in the initial reduced multivariable model were added back individually; being retained if they contributed to the overall model and reduced $\Delta \hat{\beta }$![]() % to below 20%.
% to below 20%.
Further to this, variables with P > 0.25 in the initial univariable analysis were also individually added back in to determine if they contributed to the multivariable model thus producing the preliminary main effects model. The only continuous variable included in the preliminary main effects model was herd size. Fractional polynomial analysis [Reference Hosmer, Lemeshow and Sturivant11] was applied to herd size in order to determine if it required scale transformation so as to satisfy the assumption of linearity in the logit outcome. Completion of this stage produced the main effects model. Using the variables present in the main effects model, all combinations of two-way interactions were statistically assessed using the LRT (P < 0.05); however, only those with probable clinical significance were accepted as potential candidates for the model. Interaction terms accepted into the final model had an odds ratio calculated as a linear combination with their associated main effects (β 0 + β 1 + β 2 + β 1.β 2) and the results placed into Table 1. The finalised model was then subjected to the Hosmer–Lemeshow goodness-of-fit-test (decile sub-grouped) to determine how well it fitted the data. Table 2 details how the methodology was applied in this study to achieve the final multivariable model.
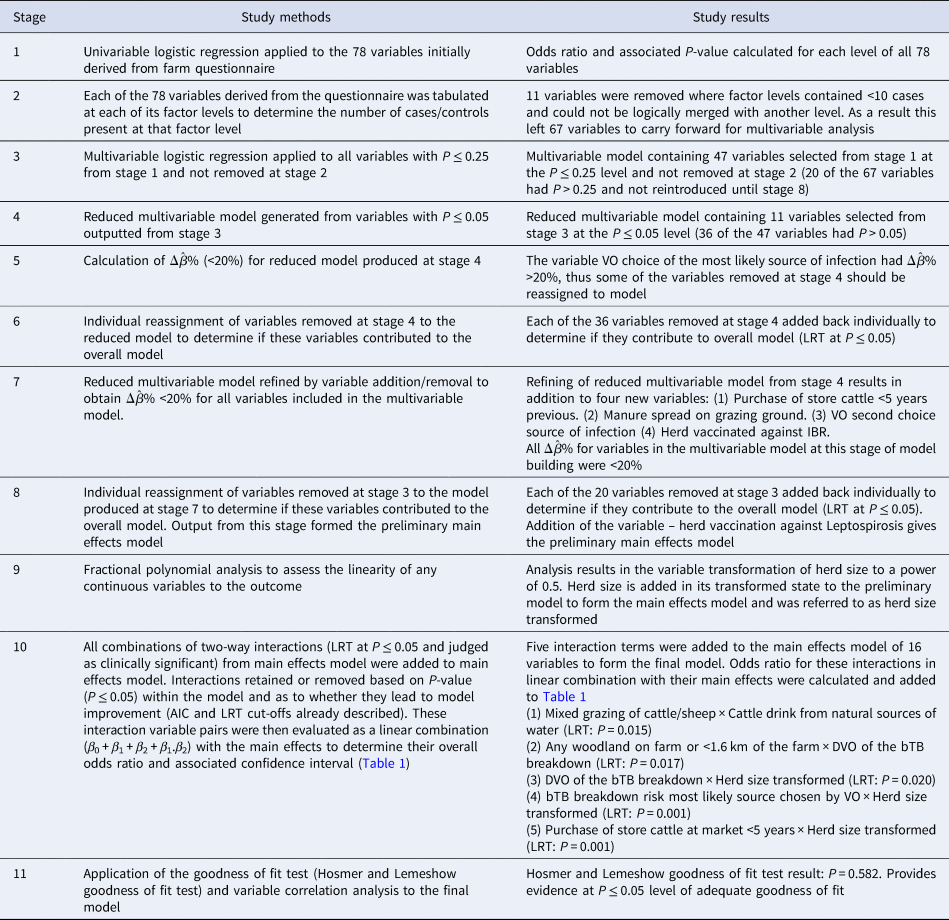
Table 1. Results of final multivariable case-control study containing calculated effects for the two-way interactions included in the model
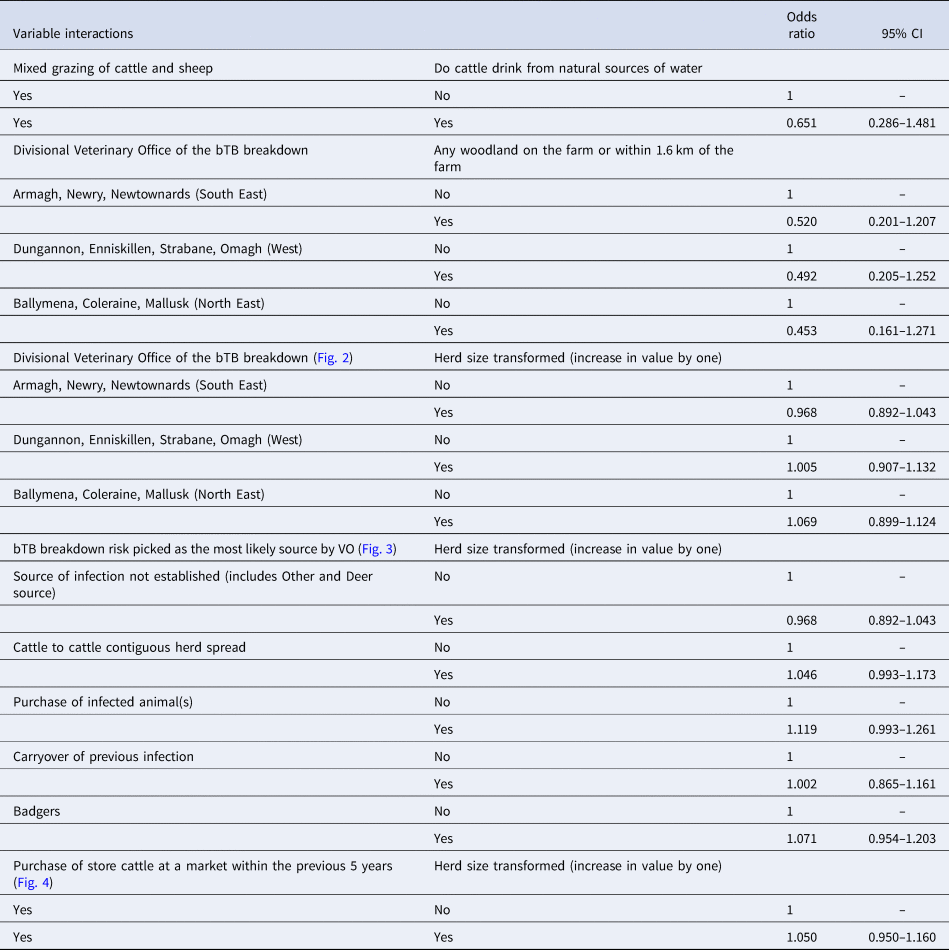
Table 2. Table showing methods applied and results observed at each stage of the study model building process
Results
A total of 2935 bTB herd breakdown investigations were completed during the study period. Supplementary Table S1 provides summary details of the 78 study variables for cases and controls. There were 126 ongoing bTB herd breakdowns at the end of the study period which were removed, leaving 2809 valid bTB herd breakdowns (191 cases and 2618 controls; Supplementary Table S1). Table 2 details the results returned at each stage of the model building process from univariable analysis through to final multivariable model. As a result of carrying out fractional polynomial analysis [Reference Hosmer, Lemeshow and Sturivant11] on herd size, it was transformed to herd size to power 0.5 and was then referred to as ‘herd size transformed’ in the subsequent analysis.
The results from the final model (Table 3) demonstrated that the odds ratio of a bTB herd breakdown persisting >365 days that contained pedigree animals was 0.594 (95% CI 0.402–0.863); where fluke treatment was carried out on the farm was 0.263 (95% CI 0.139–0.528), where cattle can access grazing ground to which slurry has been freshly applied was 0.525 (95% CI 0.283–0.915); where there was partial upgrading of boundary fences in the last 3 years was 0.383 (95% CI 0.247–0.588); where there was a full upgrade of boundary fences in the last 3 years was 0.599 (95% CI 0.406–0.886); where dead badgers were found on roads within 1.6 km of the farm in the past 3 years was 1.810 (95% CI 1.268–2.616); manure spread on grazing ground was 1.289 (95% CI 0.926–1.798); use of IBR vaccination on the farm was 1.476 (95% CI 1.005–2.158) and use of leptospirosis vaccination on the farm was 0.631 (95% CI 0.391–0.999). Dairy herds accounted for 73.2% (95% CI 59.6–86.7%) of bTB herd breakdown which carry out leptospirosis vaccination. The presence of a badger sett was recorded on 29.87% (95% CI 28.18–31.56) of investigations and of these 2.95% (95% CI 2.33–3.58%) reported fencing off badger setts and latrines.
Table 3. Results of final multivariable case-control study containing categorical and continuous variables (note interaction terms are included in Table 1)
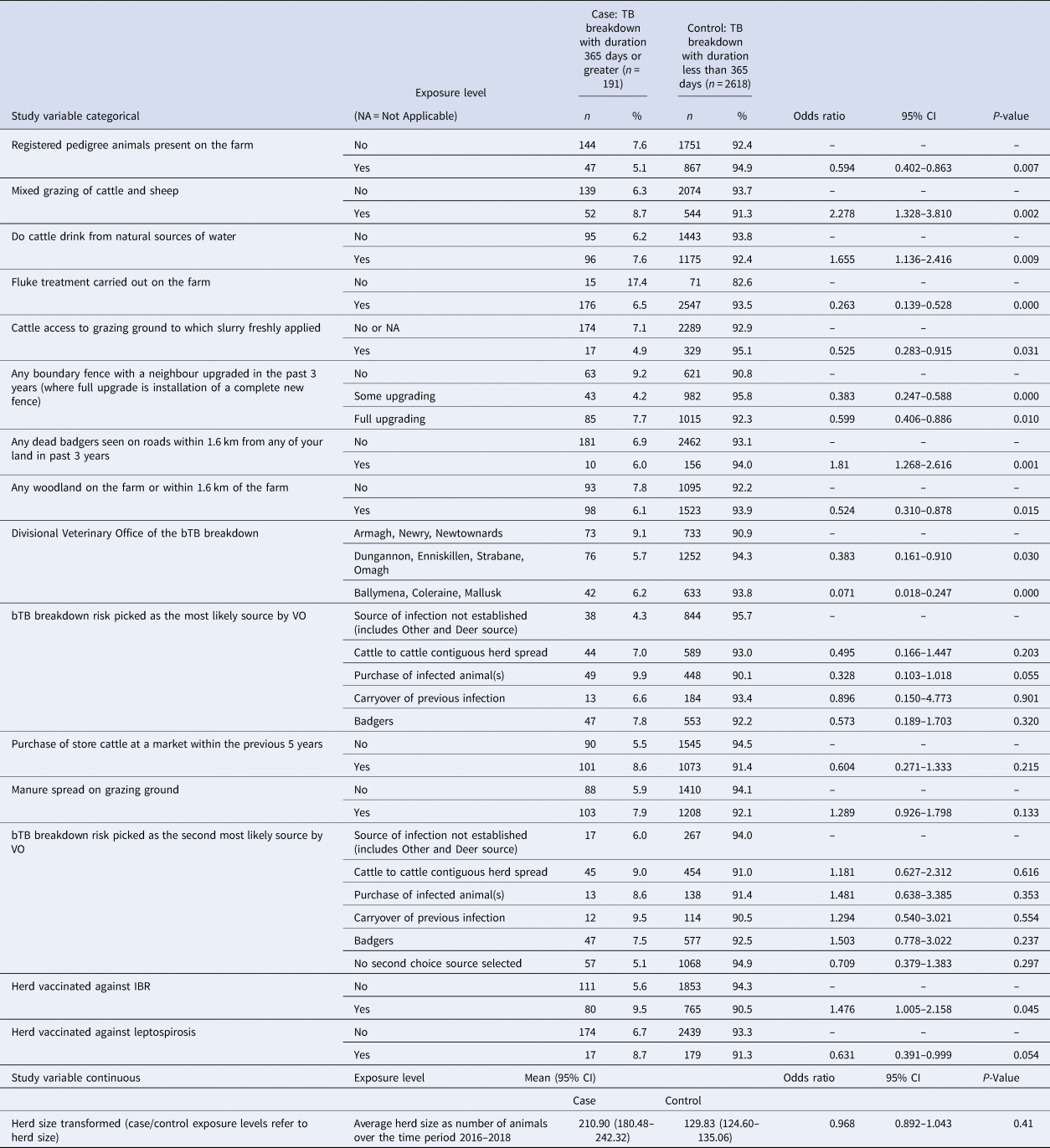
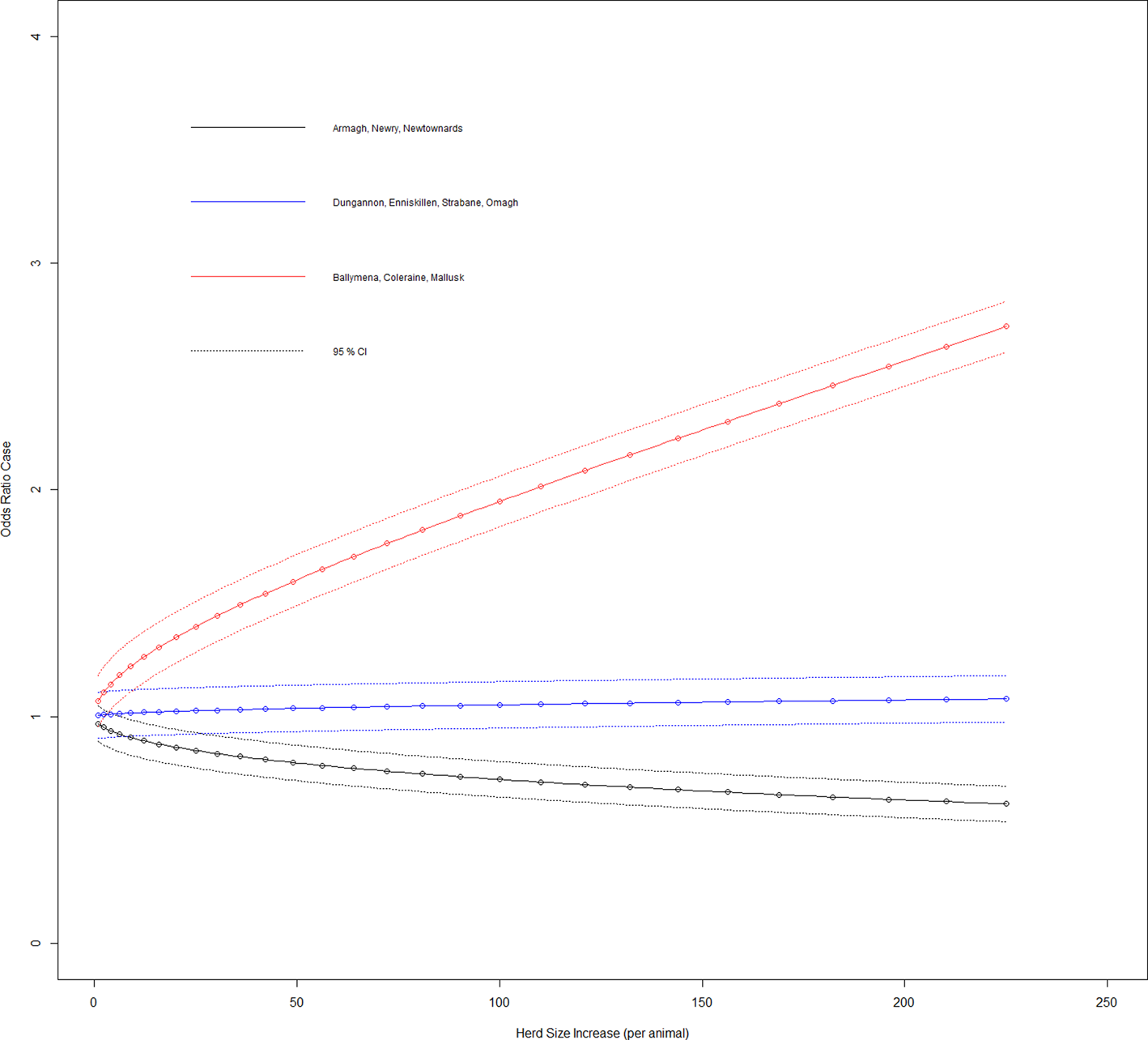
Fig. 2. Duration case odds ratio for DVO of herd given effect of increasing herd size (variable herd size graphed in untransformed state).
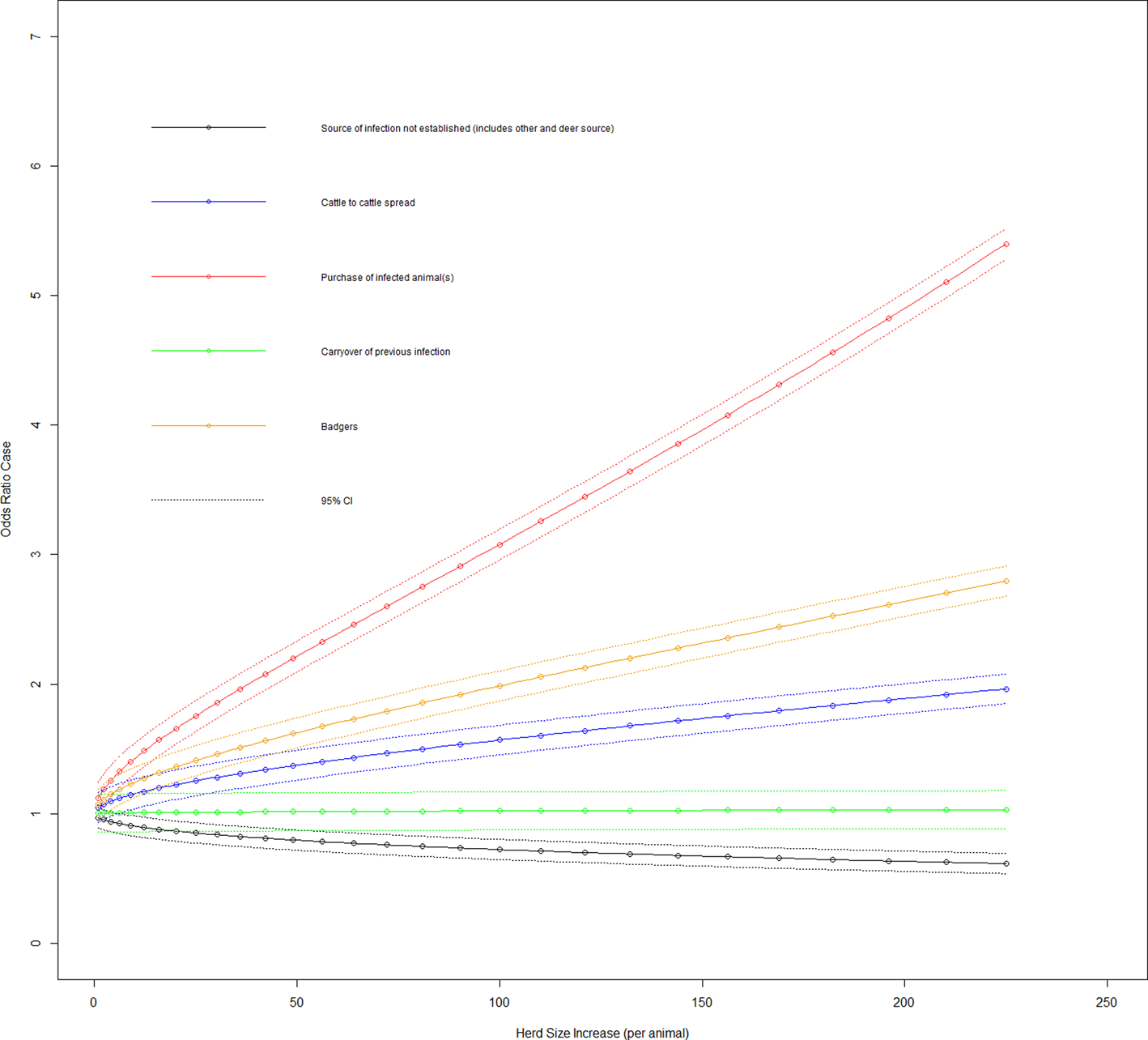
Fig. 3. Duration case odds ratio for bTB breakdown by the most likely VO source given the effect of increasing herd size (variable herd size graphed in untransformed state).
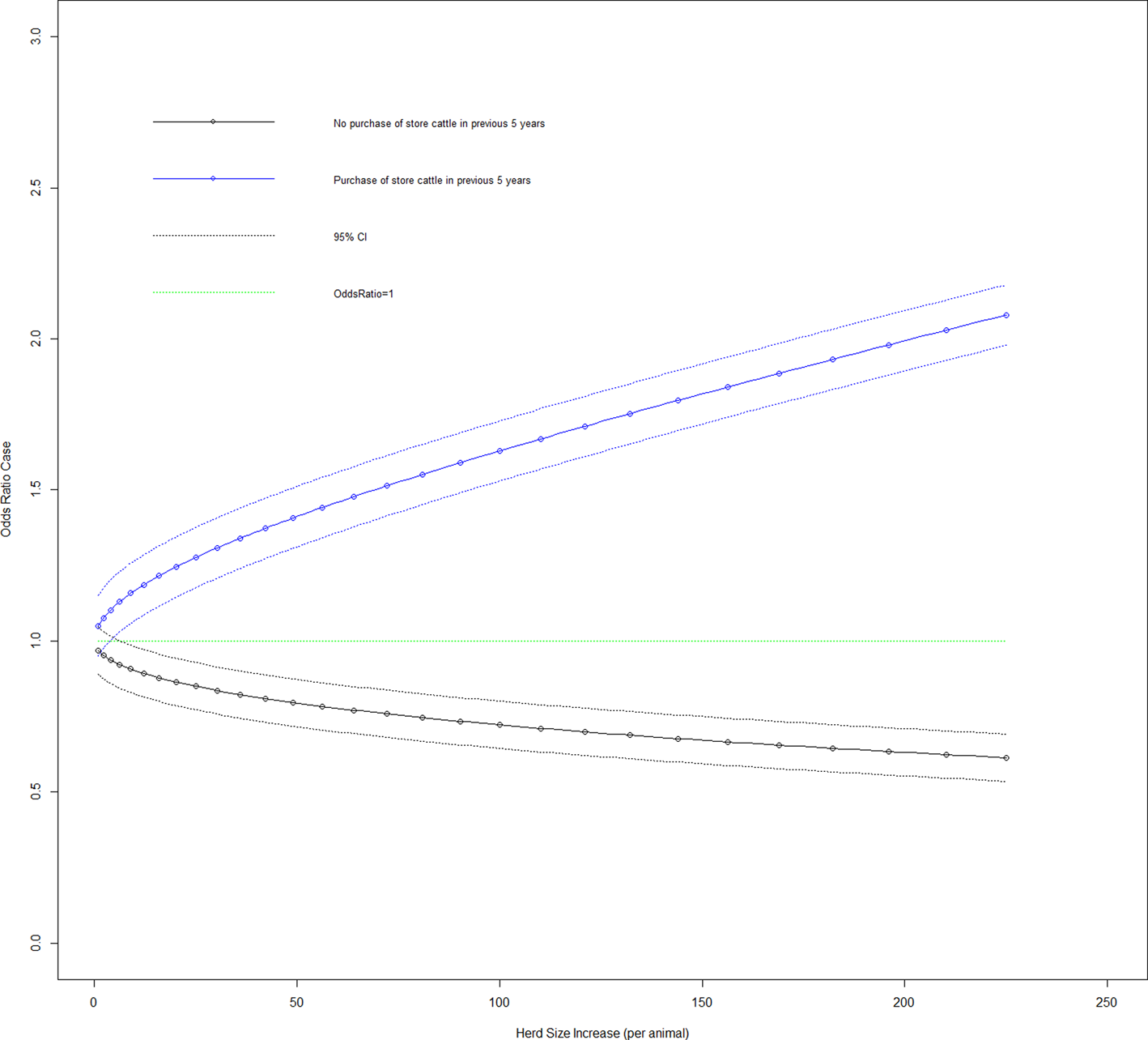
Fig. 4. Duration case odds ratio for the purchase of store cattle in the previous 5 years given the effect of increasing herd size (variable herd size graphed in untransformed state).
The results of the linear combination of the five two-way interaction terms added to the model and their associated main effects (β 0 + β 1 + β 2 + β 1.β 2) are shown in Table 1. Of the five two-way interaction terms added to the main effects model (Table 1), ‘mixed grazing of cattle and sheep’ × ‘do cattle drink from natural sources of water’ and ‘DVO of the bTB breakdown’ × ‘woodland on the farm or within 1.6 km of the farm’ returned odds ratios not significantly different from one, when interpreted as a linear combination with the main effects.
With the other three interaction terms, ‘DVO of the bTB breakdown’ × ‘herd size transformed’, ‘bTB breakdown risk picked as the most likely source by VO’ × ‘herd size transformed’ and ‘purchase of store cattle at a market within previous five years’ × ‘herd size transformed’ have their results from Table 1 shown in Figures 2–4, respectively, so that these can be interpreted in association with increasing herd size (untransformed). The odds ratio of a case where a farm is located in DVO south east group and there is an increase in herd size transformed by one was 0.968 (95% CI 0.895–1.047), with DVO west group and herd size transformed increase by one was 1.005 (95% CI 0.907–1.132) and with DVO north east group and herd size transformed increase by one was 1.069 (95% CI 0.899–1.124).
Figure 2 shows the effect on the duration case definition as herd size increases in each of the three DVO groups. The variables most likely risk source and herd size transformed had a significant interaction on addition to the main effects model (LRT: P = 0.001). The odds ratio of a case for the baseline group and where there is an increase in herd size transformed by one had was 0.968 (95% CI 0.895–1.047), with cattle to cattle contiguous herd spread of infection source and herd size transformed increase by one was 1.046 (95% CI 0.993–1.173), with purchase of infected animal(s) source and herd size transformed increase by one was 1.119 (95% CI 0.993–1.261), with a carryover of infection source and herd size transformed increase by one was 1.002 (95% CI 0.865–1.161) and with badger infection source and herd size transformed increase by one was 1.071 (95% CI 0.954–1.203).
Figure 3 shows the effect on the duration case definition of increasing herd size in each of the five infection source groups. Looking at herd types for the duration case definition based on whether they are beef fattening or other (dairy, beef cow or mixed), 11.4% (95% CI 1.9–20.7%) of beef fattening herds have source of infection as cattle to cattle spread, 73.5% (95% CI 61.1–85.9%) have purchase of infection as source, 0% for carryover of infection and 12.8% (95% CI 3.2–22.3%) have a badger source of infection. Variables purchase of store cattle at a market in the previous 5 years and herd size transformed had a significant interaction on addition to the main effects model (LRT: P < 0.001). The odds ratio of a case where a farm purchases store cattle from a cattle market, there was an increase in herd size transformed by one was 1.050 (95% CI 0.950–1.160). Figure 4 shows the effect on the duration case definition of increasing herd size for purchase of store cattle vs. non-purchase.
Discussion
One of the key perspectives of this work is to further elucidate chronic long-duration bTB herd breakdowns (>365 days) through the provision of a quantitative characterisation of their infection source thus facilitating the formulation of a more focused disease control policy. Cattle movement has been identified as a consistent herd-level risk factor for bTB [Reference Skuce, Allen and McDowell12]. In our study, two cattle movement factors were statistically associated with long-duration bTB herd breakdowns, namely, purchase of cattle generally and specifically purchase of store cattle (calves purchased for feeding over winter). Both of these factors had a significant interaction with herd size (Fig. 4), which demonstrated that given purchase of cattle risk factors, larger herds were more likely to have prolonged bTB herd breakdowns.
Cattle purchase had the strongest association as the most likely infection source (Fig. 3) followed by wildlife (badgers) and then contiguous herd spread (cattle to cattle). However, when selectively looking at prolonged bTB herd breakdowns, three-quarters (73.5%) of the herd type contributing to the purchase of infection source were beef fattening herds. This highlights two subpopulations of long-duration bTB herd breakdowns, the first being beef fattening herds which have long-duration breakdowns due to continuous purchase of infected animals and a second group of primary production herds (dairy, beef cows and mixed) with long-duration breakdowns due to infection risk from multiple sources.
After purchase of infection, badgers formed the next most likely source of a long-duration bTB herd breakdown, which was a risk factor for bTB breakdowns identified in other studies [Reference Griffin13–Reference Reilly and Courtenay16]. Skuce et al. generalise the risk from badgers to indicators of badger density/activity [Reference Skuce, Allen and McDowell12] and, in terms of chronic bTB herd breakdowns in Republic of Ireland (ROI), badger presence was reported as a risk factor for dairy herds [Reference Griffin7]. The findings from our study are consistent with these previous studies, with the association with prolonged bTB herd breakdowns being further affirmed by the finding of dead badgers on a road <1.6 kms from the home farm.
A VO attributing badgers as the most likely infection source also had significant interaction with herd size (Fig. 3) which showed this source in larger herds increased the odds of a prolonged bTB herd breakdown. This is not surprising as large herds tend to require a larger grassland area for feeding purposes, which increases the probability of exposure to a larger number of badgers, which is compounded by the larger number of cattle in such herds.
Two badger-related variables excluded from the model due to low case numbers were fencing off of badger setts and fencing off of badger latrines; both of which are considered preventive measures. An important observation in this study was that almost one-third (30%) of farms were observed as having badger setts, but only 3% of investigations reported farms where badger setts and/or latrines were fenced off, an observation also reported in other Northern Irish work [Reference O'Hagan14]. Without extensive fencing off of badger setts and latrines indirect contact between badgers and cattle cannot be curtailed [Reference Campbell17].
The third important source of infection was cattle-to-cattle contiguous herd spread. Previously, Denny and Wilesmith reported that in Northern Ireland, the two main associations with bTB breakdowns were the presence of badgers and contiguous neighbours who had confirmed bTB breakdowns (aetiological fraction for both was approximately 40% each) [Reference Denny and Wilesmith9]. They also stated that 79% of fences in Northern Ireland did not prevent nose to nose contact between herds [Reference Denny and Wilesmith9]. In a more recent Northern Ireland study, contact between neighbouring cattle was assessed as possible through 66.8% of boundaries, however no significant association was found between boundary contact and bTB breakdown [Reference O'Hagan14]. Our study, which looked specifically at prolonged bTB herd breakdowns, found that both recent upgrading or complete installation of new boundary fences showed a significant negative association with the duration of bTB breakdowns (OR 0.383; 95% CI 0.247–0.588 and OR 0.599; 95% CI 0.406–0.886, respectively). This result provides circumstantial evidence for the application of better biosecurity measures in the form of adequate boundary fences to reduce cattle-to-cattle contiguous herd contact could reduce the odds of a prolonged bTB herd breakdown.
This study also investigated associations with other common diseases found on Northern Ireland farms. Application of fluke treatment was significantly associated with a reduced odds of developing a prolonged bTB herd breakdown. Co-infection with liver fluke may mask the true bTB infection status of animals making SICCT clearance of the herd difficult [Reference Allen, Skuce and Byrne18]. Indeed, given the widespread prevalence and high level of press coverage relating to fluke infection (and its potential link to bTB) it is surprising that not more herd keepers treat their cattle against liver fluke.
Other disease-related variables investigated were use of IBR (infectious bovine rhinotracheitis) vaccination and leptospirosis vaccination. Skuce et al. stated that the influence of respiratory infections on the susceptibility to infection with M. bovis remains untested but speculated that such infections can facilitate increased aerosol spread [Reference Skuce, Allen and McDowell12]. Our study showed an association between the use of IBR vaccination (used as a proxy for IBR exposure within a herd) and increased odds of developing a prolonged breakdown (OR 1.476). With leptospirosis vaccination, there was a negative association between its use and development of a prolonged bTB herd breakdown (OR 0.631), although the significance was marginal (P = 0.054) and given that 73% of these prolonged bTB herd breakdowns were in dairy herds may suggest that other management factors confound this finding.
The presence of registered pedigree animals on a farm was significantly associated with a reduced odds of developing a prolonged bTB herd breakdown. Association with this variable indicated that herds containing pedigree animals appear to be better at removing th infection. The presence of pedigree animals on a farm is probably indicative of a herd where trade and movement are important to the business, thus providing very strong motivation for a farmer to clear the infection from the herd and employ improved biosecurity measures.
Several studies have looked at the area of risk presented by slurry and manure derived from bTB-infected premises; but definitive results on the subject are few. Given the type of cases in our study and suggestions that approximately 6 months are required for deactivation of M. bovis in contaminated slurry [Reference Scanlon and Quinn19], they must represent an extreme in terms of potential for the production of bTB-infected slurry or manure. The variable ‘manure spread on grazing ground’ had a positive association to prolonged bTB herd breakdowns (however not statistically significant in the final model, odds ratio = 1.289 (95% CI 0.926–1.798), a result consistent with previous work [Reference Scanlon and Quinn19]), but was included as it provided adjustment effects for other variables. Our study indicated no association with the use of contractors for slurry spreading (at odds with another Northern Irish study [Reference O'Hagan14]) or with applying slurry/manure to grazing ground. Even in situations where cattle can access ground to which fresh slurry has been applied, the results point to a negative association (OR 0.525) with cases. However, given the low number of cases (n = 17) in this category, it would require a guarded interpretation. The results show that herds located in the DVO north east group have the strongest statistical association to the prolonged breakdown case definition. Additionally, there is an interaction between DVO herd group and herd size (Fig. 2) where relative to the others, odds of a prolonged bTB herd breakdown increase for north east herds with increasing herd size.
Given that in this study, a statistical significance cut-off level of P < 0.05 was used for variable selection and that the final multivariable model contained 16 variables it should be realised that inclusion of at least one spurious association is a possibility. It is also possible with a study design where VOs select a breakdown source of infection there is potential for a degree of subjectivity. However, it is the trained VO with their local knowledge and standardised guidance who are best placed to make these assessments.
Conclusions
One of the central tenets of this work was investigation of disease source and its relationship to long-duration bTB herd breakdowns. The source with the strongest association to long-duration breakdowns was purchase of infection; however, as a source, it applies mainly to beef fattening herds. Beef fattening herds mostly move their stock to an abattoir with very few cattle movements to other herds. However, they do present a risk from the continuous output of infection to local wildlife and to other herds grazed contiguously. In order to reduce the input of infection to these herds, they must have the capability to risk assess their purchases [Reference Adkin20] thus reducing their overall ability to act as an infection focus in their locality. With herds other than beef fattening herds, the source of infection for long-duration breakdowns are multiple and must be addressed in a multi-faceted way.
In terms of wildlife source, more effort must be placed into breaking the transmission links between cattle and badgers. This could involve an array of methods varying from those directly applied to the badgers through to methodical and efficient fencing of badger setts and latrines. Indeed this work shows that basic segregation methods to separate badgers and cattle using fencing are not being applied. The low levels of fencing off by farmers could be as a result of confused communication, occurring where one section of government responsible for bTB control promotes it as necessary, while another section implementing subsidy payments (Basic Payment Scheme) contradicts it by officially removing these fenced off areas from field maps, potentially affecting payment. It is thus essential that governments do not create contradicting messages with policy implementation and should conceive more effective ways of promoting best practice [Reference Robinson21], such as consulting widely before introducing future subsidies, to ensure their application is biosecurity friendly and communicating this effectively to the farming public. Indeed demonstrating what is possible, some areas fenced off as part of agri-environment schemes have now been deemed eligible for BPS area-based payments, a model which should be implemented in relation to farm biosecurity.
The other source shown to be linked to long-duration breakdowns was cattle to cattle contiguous herd spread and without effective biosecure boundaries between herds, this infection route will remain present. This again is an area where it should be possible for the government to intervene, incentivising the good practice of constructing biosecure boundary fences between neighbouring farms and penalising situations where poor fencing risks contiguous disease spread. Effective boundary fencing would form a necessary part of an overall biosecurity package aimed at the structural elements of the ongoing bTB problem.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820002241.
Acknowledgements
Many thanks to the DAERA veterinary service staff who collected the information used in this study and Tim Abernethy for his diligent administrative work on this project.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Data availability statement
The data that support the findings of this study is not publically available data.












