Introduction
Values of δ18Osmow have been measured on seven samples of a 16 cm long quarter-piece of ice cove from 250 m depth (relative to the 1957-38. summer surface) at old “Byrd station" (10 km west of the new station), Antarctica. The core was drilled in 1957-5Ü and was shipped to the United Stales where it was stored at approximately — 20°C until the time of the analyses (June 1970). The age of the ice is based on two estimates of snow-accumulation rate. One obtains 1 400 years by using the data of Reference GowGow (1968) and 2000 years from the estimates of Reference Epstein, Epstein, Sharp and GowEpstein and others (1970).
Sample Preparation And Analysis
The core was sawn into seven approximately equal-sized samples that were put into plastic bags and then scaled to keep out room air and water vapor. The samples were allowed to melt slowly and, as they did so, the melt water was periodically decanted (five times) and discarded. In this way surface contamination was flushed away, leaving only about 15 g of pure glacier ice. The uncontaminated melt water was transferred by pipette into 200 cm3 flasks. After degassing, the samples were allowed to equilibrate at 25.3°C with tank CO2 for more than 4 d. Then, a small sample of the equilibrated CO2 was removed from each flask (in random order) and analysed with the McKinney-Nier mass spectrometer. A second analytical run was made 2 d later. This method has been described by Reference Epstein and MayedaEpstein and Mayeda (1953).
Results
The corrected values of δ18O (relative to standard mean ocean water, smow) are given in Table I together with the sample data. The δ18O values have been calculated as follows:
where R, the mass ratio, is equal to [l80]/[ l60], and the subscript 1 refers to the ice melt water. Taking into account the corrections indicated by Reference CraigCraig (1957), the equilibration correction (Reference Epstein and MayedaEpstein and Mayeda, 1953) and the machine corrections (personal communication from Mrs T. K. Mayeda), δ180 values were calculated from the measured mass ratios of the samples and the Chicago calcite working standard.

Discussion
The differences in individual values of δl80 between runs (Table I and Fig. 1) range from 0.22 to 0.01%0. while the trend of values between samples 1 and 6 in both runs changes monotonitally by about 0.74%0. Because the samples were analyzed in random order, the experimental error, while undesirably large in samples 2 and 4, was distributed randomly with respect to length along the tore and did not contribute to the trend. The experimental error was probably the result of insufficient equilibration before the first run, because the values for all samples rose in absolute value (became lighter) in run 2, and the lank CO2 was considerably heavier (-4.59 relative to SMOW) than any of the ice samples.

Fig. 1. Oxygen-isotope ratio(δl8O) versus depth in a piece of ice core from old “ Byrd station”, Antarctica. The circles and squares indicate values obtained in analytical rims 1 and 2 respectively on seven contiguous samples.
The result reported here compares very favorably with the measurements of Reference Epstein, Epstein, Sharp and GowEpstein and others (1970) on an ice core from new “Byrd station”. They obtained average values of δ18O of between -34 and 32%o at depths of between 200 and 300 m.
The orientation of the core piece (which end was up) could not be determined, therefore the Ordinate axis in the figure is labeled length rather than depth. Nevertheless, the δ18O values give additional information. If the trend in values is the result of seasonal temperature variation at the deposition site. only about half an annual layer is present, and the amount of annual accumulation for the time and place represented was in excess of 16 cm ice (14.4 cm H2O). Of course, this probably does not represent average conditions 1700 years B.P. Moreover, the approximate mean annual temperature represented by this ice is about — 30.3°C (δ 18OSMOW = -0.9T 6.4; Reference Picciotto, Picciotto, De Maere[d’Aertrycke] and FriedmanPieciotto and others, 1960). Finally, it appears that the full seasonal δ18O variation has been greatly damped, presumably by diffusion, because the apparent seasonal temperature variation is only 0.8°C
Acknowledgements
The analyses were conducted in the laboratory of R. N. Clayton at the Enrico Fermi Institute of Nuclear Studies, University of Chicago. Γ am very grateful to Dr Clayton for granting me permission to work in his laboratory and to Mrs T. K. Mayeda for her generous help with the entire project. The ice core was provided by Dr C. C. Langway, Jr of U.S. Army Gold Regions Research and Engineering Laboratory.




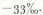 A monotonie trend in values through the 16 cm long core piece suggested that part of an annual layer was represented. Snow accumulation in excess of 14.4 g cm
A monotonie trend in values through the 16 cm long core piece suggested that part of an annual layer was represented. Snow accumulation in excess of 14.4 g cm

