Introduction
The electron microscope study of snow nuclei was started in our laboratory in the winter of 1948–49. In the first year an expedition was made to Jōzankei, which lies about 30 km. from Sapporo in Hokkaido, the northernmost island of Japan. We succeeded in getting several samples in the field of electron photomicrographs which gave the images of something resembling the nuclei of snow crystals. The difficulty, however, was that we could not tell with certainty whether the image appearing in the photograph was that of a snow nucleus or of some dust particle of ground soil or human origin which had strayed into the sample during handling.
In the following winter, 1949–50, we went to Mt. Taisetsu, situated in the middle of this island. It is one of the highest mountains in Hokkaido. Many samples were collected at a place half-way up the mountain, at an altitude of 1050 m. In the middle of the winter season the entire district near this mountain system is covered with a snow layer more than 2 m. in depth. The nearest town is some 40 km. distant, so that there is little fear of pollution due to smoke or dust in this vicinity. The snow crystals were caught on the thin collodion film of the sample holder of the electron microscope, and kept in a desiccator at a temperature below freezing. In this condition the snow crystal does not melt, but evaporates by sublimation, and after a considerable time the entire crystal disappears, leaving what one infers to be the nucleus on the collodion film. These samples were tightly sealed and brought back to Hokkaido University, where the electron photomicrographs were taken.
The experiments were carried out by Mr. M. Kumai, of the Physics Laboratory of the Faculty of Science at Hokkaido University, and the full report was published by him in the Journal of Meteorology,Reference Kurnai 1 the advanced communication having been presented in this Journal.Reference Nakaya 2
Method of Making Specimens of the Nucleus
The difficulty of this experiment lies in making sure that the image obtained in the electron photomicrograph is the nucleus of the snow crystal. Great care must be taken in locating the centre of a snow crystal in the field of the sample holder. The effective area of the holder is circular in form and very small, the diameter being only 0.1 mm. A considerable amount of practice is necessary in locating the desired portion of the crystal in the centre of the field. All manipulations are made under an optical microscope. In Fig. 1 (p. 174) the lighter circular area in the centre is the effective field of the sample holder, and is covered with a thin collodion film. The diameter of this lighter area is 0.1 mm. A snow crystal is brought on to this holder, so that the centre of the crystal lies in this clear area, as seen in Fig. 1. The snow crystal arranged in this way is kept in a desiccator. At Mt. Taisetsu all the experiments were carried out with the desiccators kept in an igloo at a temperature of between −5° and −15° C.

Fig. 1 Locating the central portion of a snow crystal in the effective area of specimen holder
In most of the snow crystals observable in nature it is usually found that a tiny ice crystal of spherical form or of small hexagonal pattern exists at the centre of the crystal. The crystal shown in Fig. 1 is an example of a spherical ice crystal. The diameter of the sphere is usually about 0.02 mm. and the dimension of the hexagonal pattern about 0.03 mm. We called these central patterns “the central portion of the snow crystal.”
After having acquired the necessary skill, it was not so difficult to arrange the central portion of the crystal to lie in the effective area of the sample holder. Not only were specimens of the central portion prepared; other parts, for example parts neighbouring the central portion or the tip of a branch, were made ready for examination.
It is important to make sure that the presumed nucleus, destined to lie in the effective area of the sample holder, remains on the collodion film without being displaced during sublimation. Observations of the successive stages of sublimation through an optical microscope ensured that no marked displacement occurred.
The Centre Nucleus
One hundred and three successful photomicrographs were taken from the specimens obtained at Jōzankei and Mt. Taisetsu during two winters. In most of the photographs of the central portions it was found that a relatively large solid nucleus always remained in the field of the electron photomicrograph. This type of nucleus is called the “centre nucleus” in this paper. The largest extent of this centre nucleus varies between 0.5μ and 8μ. Photographs were taken of snow crystals of various shapes, and 43 photographs were successful in showing the image of the centre nucleus. The details are shown in Table I.
Table I List of centre nuclei

Three examples of the photographs of the centre nuclei are reproduced in Figs. 2–4 (p. 174). Fig. 2 shows the centre nucleus of a snow crystal consisting of an irregular combination of columns and small plates. We took electron photomicrographs of various known materials which are conceivable as nuclei in nature, and by examining and comparing their shapes we reached assumptions as to the nature of the snow crystal nuclei. The nucleus shown in Fig. 2 is very similar to the image of a kaolin particle, so this was taken to be a combination of two kaolin particles. Fig. 3 (p. 174) is a centre nucleus of a thick stellar crystal and from its shape it was taken to be a clay particle, the shape of this nucleus being very similar to that of clay particles in natural soil. Fig. 4(p. 174) is also a centre nucleus of a thick stellar crystal. This was assumed to be a lump of carbon particles. The electron photomicrograph of carbon particles obtained by burning rape-seed oil is exactly the same as this photograph, both in its general form and minutest micro-structure. Besides these three, many kinds of materials were observed. Some are believed to be micro-organisms or other organic substances. In one sample, the nuclei were considered to be certain hygroscopic chemical compounds. The number observed and the largest size of each of the supposed nuclei are tabulated in Table I.

Fig. 2 Centre nucleus of irregular combination of columns and plates, × 8400

Fig. 3 Centre nucleus of a thick stellar crystal, × 8400

Fig. 4 Centre nucleus of a thick stellar crystal, × 9000
Recently Kampe and WeickmanReference Kampe and Weickman 3 investigated the nucleus of ice crystals made artificially in a Dewar flask. Some of their photographs were surprisingly similar to our photographs of the nuclei of natural snow crystals. From these results we are inclined to consider that at least a large number, if not all, of the snow crystals observable in nature start from solid nuclei.
The Condensation Nuclei
Besides the centre nucleus described in the preceding section, innumerable very minute particles were observed in the field of the electron photomicrograph. In Fig. 3 many of these minute particles are seen scattered all over the field. They are also seen in the negatives of Fig. 2 and Fig. 4, but are not reproduced in the prints due to the weak contrast. The size of these particles is between 0.01μ and 0.4μ and the mean size is equal to that of condensation nuclei in the free atmosphere, which has been studied by many previous investigators. We shall call them “condensation nuclei” in this article.
It must be emphasized that these condensation nuclei are found not only near the central portion but in any part of the crystal. For example, when the tip of one branch is placed in the effective area of the specimen holder and allowed to evaporate by sublimation, innumerable condensation nuclei are found in the field of the electron microscope. Fig. 5 (p. 174) is an example of this, and shows the distribution of condensation nuclei of a part near the tip of one branch of a thick stellar crystal. These condensation nuclei exist all over a crystal, and we can find them in all the photographs hitherto taken. The centre nucleus is found only in the central portion, not in any other part of the crystal.

Fig. 5 Condensation nuclei of a thick stellar crystal, × 8900
A frequency curve of the size of condensation nuclei has been made from 1200 data, which were obtained from four enlarged prints of electron photomicrographs. It was found that there are two groups in size distribution, the larger ones being of the order of 0.15μ in diameter and the smaller about 0.05μ. Fig. 5 shows this clearly. The relatively larger condensation nuclei are scattered in a belt zone, and many smaller ones are seen in other parts of the field.
The concentration of condensation nuclei is larger in thick rimed crystals than in thin crystals. The relation between thickness of the snow crystal and density of condensation nuclei has been studied for many crystals, and the result is shown in Table II. It was found that the concentration of condensation nuclei was roughly 100/μ 2 in the case of thick rimed crystals, the thickness of which is about 100μ. In the case of thin crystals about 10μ in thickness, this value comes down to about 10/μ 2.
Table II Relation between thickness of snow crystals and concentration of condensation nuclei

The Growth of Snow Crystals
The elements which control the shape of a snow crystal were studied by Hanajima,Reference Hanajima 4 and the summary of the results was reproduced by the present writer in the Compendium of Meteorology.Reference Nakaya 5 The experiments were carried out for 700 crystals of various shapes. The conditions controlling the shape were found to be the temperature and the degree of supersaturation. The final result is shown in Fig. 6 above, in which each of the six regions represents the conditions of formation of a certain type of crystal. Ta is the temperature of the air where the crystal is formed, and s is the supersaturation with respect to ice. In the range of higher values of s, the air was found to contain numerous minute droplets of about 1μ, or less in diameter. s is taken as the ratio of the amount of water vapor plus droplets per unit volume to the saturation vapor density at T a with respect to ice. The former is measured by a gravimetric method using P2O5.

where w is the amount of minute droplets per unit volume. ρ′ the vapor density at water saturation at T a , and ρ 0 the vapor density at ice saturation.
The curve w = 0 in Fig. 6 shows the curve of water saturation. It is interesting to see that needles, irregular needles and cup crystals are always above water saturation, while the columns and spatial assemblage of plates are always found under water saturation. The dendritic crystals and plates are found in the range above water saturation as well as in the range of supersaturation between ice and water. The region above the water saturation curve w = 0 must be considered to be in the condition of cloud formation. The numerous minute droplets of about 1μ or less in diameter above described, are found in this region. In our experiments on the artificial snow, it was found that this minute droplet behaved in a peculiar manner in the process of condensation to the snow crystal. It did not freeze to the surface of the snow crystal in the form of droplet, but spread over the ice surface; in other words these minute droplets behaved just like vapor molecules in the process of condensation.
Rimed crystals, that is to say, snow crystals with fog particles attached, are frequently observed in nature. These particles are larger in size, and are probably about 30μ in diameter. They are frozen to snow crystals in the droplet form. In our artificial snow apparatus we also find these larger particles frozen to the crystal in the form of droplets, giving rise to a rimed crystal. In Fig. 6 the curve R shows the boundary above which the crystals are rimed.
The point A in Fig. 6 represents the most favorable condition for dendritic development of the crystal. At this point the amount of water droplets per unit volume of air is 0.06 g./m.3 The probable diameter of minute droplets in this case is about 1μ. n, the number of droplets per cm.3 is calculated from the equation

and we get

This value is in fairly good agreement with the total nucleus concentration in air. In Mason and Ludlam’s articleReference Mason and Ludlam 6 the value measured by Simpson in different localities is introduced, in which the average value in cities is given as 1.5 × 105/cm.3 At higher supersaturation, the point B in Fig. 6, the water content is about 0.2 g./m.3 In this case the mean diameter of the droplets works out at 1.5μ, assuming the same value of n. It is also confirmed by experiment that droplets of this size behave like water vapor.
Each of these minute droplets must have a condensation nucleus. Therefore it can be accepted that the number of condensation nuclei observed by the electron microscope in one crystal shows the number of minute droplets which contributed to the growth of that crystal. One example is given here in the case of a thin dendritic crystal without rime formation. The area of this crystal is approximately 1 mm.2, and the thickness is typically of the order of 0.01 mm. The volume of ice in this crystal is 106
μ
2 × 10μ = 107
μ
3. The mean concentration of the number of condensation nuclei per unit area of this crystal is about 10 as seen in Table II. The total number of condensation nuclei is 107. This is the number of droplets which contributed to the growth of this crystal. If we assume the diameter of these droplets to be 1μ, the volume of ice will be 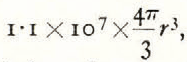 that is, 0.55 × 107
μ
3. In this case, more than one half of the ice material is supplied to the crystal in the form of minute droplets. If we assume the mean diameter of droplets to be 1.2μ, the whole of the snow crystal will be due to the condensation of these minute droplets on to the crystal. Up to the present it has been believed by all investigators of this subject that the growth of the snow crystal is the result of condensation of water molecules by sublimation. The abundant presence of condensation nuclei in a snow crystal, however, shows that the minute droplets present in The atmosphere play an important rôle in the growth of a snow crystal. In this stage of the investigations this is only a working hypothesis, but we hope that this hypothesis will be of some interest to the investigators of the physics of snow.
that is, 0.55 × 107
μ
3. In this case, more than one half of the ice material is supplied to the crystal in the form of minute droplets. If we assume the mean diameter of droplets to be 1.2μ, the whole of the snow crystal will be due to the condensation of these minute droplets on to the crystal. Up to the present it has been believed by all investigators of this subject that the growth of the snow crystal is the result of condensation of water molecules by sublimation. The abundant presence of condensation nuclei in a snow crystal, however, shows that the minute droplets present in The atmosphere play an important rôle in the growth of a snow crystal. In this stage of the investigations this is only a working hypothesis, but we hope that this hypothesis will be of some interest to the investigators of the physics of snow.

Fig. 6












