Introduction
Congenital aural atresia and pinna malformations (microtia types I–III) occur with a prevalence of 0.83–17.4 per 10 000 births depending on the population. Caucasians and Afro-Americans have a lower prevalence compared with people of Hispanic, Asian and Andean (e.g. Ecuador or Chile) ancestry and native Americans. This might be a result of genetic or environmental factors.Reference Luquetti, Heike, Hing, Cunningham and Cox1
Rehabilitation of these patients always involves two segments: cosmetic and functional reconstruction. Therefore, two teams are needed: a plastic reconstructive team and an otological surgery crew. Close collaboration is crucial to achieve the best outcome for these patients.
Patients generally present with a conductive block of 60 dB HL and normal inner-ear function. The ear atresia plate fixes the malformed malleus–incus complex, whereas the stapes is usually intact and mobile. The lenticular incus process can be missing or may remain present as a fibrotic connection towards the stapes head. Displacement of the facial nerve canal can be expected in about 77 per cent of patients with auricular dysplasia, limiting the access towards the oval or round window niche.Reference Kiefer and Staudenmaier2 Conventional hearing aids are not an option because the ear canal is missing, and otological external and middle-ear surgery to correct the sound conduction mechanism is challenging. In the last decade, several hearing implants have been developed. They can be categorised depending on how the acoustic energy is transmitted to the cochlea.
Of the bone-conducting devices available, some are passive (vibrations are generated outside the skull) transcutaneous systems (e.g. Sophono (Medtronic, Minneapolis, USA) or BAHA Attract (Cochlear, Sydney, Australia)). Other bone-conducting devices are passive percutaneous devices (e.g. BAHA Connect (Cochlear) or Ponto Bone Anchored Hearing System (Oticon Medical, Askim, Sweden)). More recent bone-conducting devices can also be active (the vibrating part is implanted) (e.g. Vibrant Bonebridge (Med-El, Innsbruck, Austria) or Osia (Cochlear)).
Other hearing implants used to treat conductive and mixed hearing loss in congenital aural atresia are active middle-ear implants, such as the Vibrant Soundbridge (Med-El).Reference Beutner, Delb, Frenzel, Hoppe, Hüttenbrink and Mlynski3,Reference Frenzel4 This device stimulates mobile structures of the middle ear directly with a floating mass transducer. Different locations within the middle ear can be used to attach the floating mass transducer (e.g. incus, stapes and round window).Reference Tóth, Gerlinger, Bölcsföldi, Kellényi, Németh and Papp5 However, in the case of congenital aural atresia, these ossicles need to be mobilised first by otological middle-ear surgery.
A pre-operative computed tomography (CT) scan of the temporal bone is an absolute necessity for estimating the severity of the temporal bone malformation, in order to assess the dysplasia and fixation of the ossicular chain, and to identify the position of the facial nerve. This allows the surgeon to determine the chances of successful surgical reconstruction.
In 2019, the International Microtia and Atresia Workgroup provided recommendations on functional reconstruction in patients with microtia and congenital aural atresia. The Workgroup proposed general consensus recommendations on the options and timing of aesthetic reconstruction and hearing rehabilitation. Obviously, the placement of hearing implants should not interfere with the cosmetic reconstructive process, but implantation may precede, be combined with or follow the aesthetic reconstruction.Reference Zhang, Bulstrode, Chang, Cho, Frenzel and Jiang6
In June 2022, a new consensus statement on bone-conduction devices and active middle-ear implants in conductive and mixed hearing loss was released. It provided recommendations for minimal reporting standards.Reference Maier, Lenarz, Agha-Mir-Salim, Agterberg, Anagiotos and Arndt7 One major key point was to measure hearing improvement not only by calculating functional gain (unaided thresholds vs aided post-operative thresholds) but also by computing effective gain. Effective gain is the difference between bone-conduction thresholds and post-operative aided thresholds.Reference Maier, Lenarz, Agha-Mir-Salim, Agterberg, Anagiotos and Arndt7 Functional gain can sometimes be a misleading value, as it is strongly dependent on the pre-operative air–bone gap. The larger the air–bone gap, the higher the functional gain of any device that bypasses the middle ear.Reference Snik, Maier, Hodgetts, Kompis, Mertens and van de Heyning8 The following example should demonstrate this.
One patient, with a pre-operative air–bone gap of 60 dB HL, receives an imperfect middle-ear implant and reports a functional gain of 40 dB HL with this implant. Another patient, with a pre-operative air–bone gap of 30 dB HL, receives an absolutely perfect middle-ear implant and reports a functional gain of 30 dB HL with this implant.
In this scenario, the imperfect middle-ear implant will report a bigger functional gain, even though it cannot bypass the middle ear as well as the perfect middle-ear implant. In order to better evaluate and compare the capabilities of a middle-ear implant, independently of the pre-existing air–bone gap, effective gain is a more suitable measure. As effective gain is defined as bone-conduction thresholds minus aided post-operative thresholds, a perfect middle-ear implant would have an effective gain of zero. The more negative a reported effective gain, the bigger the remaining post-operative air–bone gap.Reference Snik, Maier, Hodgetts, Kompis, Mertens and van de Heyning8
The plastic and otological ear atresia team at our institution offers a wide range of cosmetic and functional rehabilitation options, individualised for each patient. This study aimed to present the results in patients with congenital aural atresia who underwent hearing rehabilitation with an active middle-ear implant (Vibrant Soundbridge) at our institution.
Materials and methods
Of our cohort of 70 microtia and atresia (congenital aural atresia) patients, 10 underwent Vibrant Soundbridge implantation between 2008 and 2021. Two of the 10 patients had binaural implantation, resulting in 12 ears for analysis (analysis was performed for each side separately).
In this retrospective study, we examined the patients’ characteristics. The CT scans for each ear were used to apply the Jahrsdoerfer grading system.Reference Shonka, Livingston and Kesser9 We determined the microtia score, the floating mass transducer coupling site and the type of Vibrant Soundbridge (vibrating ossicular replacement prosthesis) implanted.
Endpoints
The primary endpoints were the functional gain and the effective gain measurements. Secondary endpoints were complications and subjective patient satisfaction, assessed with a questionnaire. The average age at implantation was 15.5 years (standard deviation (SD) = 10.0). Seven patients were male and three patients were female. The most common coupling site was onto the mobile stapes suprastructure using a stapes coupler, in eight ears; incus coupling was performed onto the short process in four ears. Round window coupling was not performed. Patients 8 and 9 had binaural atresia (Table 1).
Table 1. Patients’ baseline characteristics

*‘Clip’ refers to a vibroplasty standard clip coupler for the stapes head; ‘SP’ refers to a short process incus coupler. Y = years; M = male; F = female; FMT = floating mass transducer; VORP = vibrating ossicular prosthesis audio processor (Vibrant Soundbridge)
Pre-operative air-conduction thresholds (unaided, masked) and post-operative soundfield thresholds (aided, masked) were obtained to calculate the mean functional gain for all frequencies. Effective gain was determined by comparing post-operative soundfield thresholds (aided, masked) with bone-conduction thresholds (masked). As secondary endpoints, patient satisfaction and further subjective measures were evaluated with a questionnaire (Appendix 1). Further data to meet the reporting standard of the consensus paper were collected (Appendix 2).
Analysis was performed for all ears and per coupling site. Statistical analysis was performed using Excel® spreadsheet software and the ENTstatistics (Innoforce, Liechtenstein) program.
Results
In the case of microtia repair, functional hearing rehabilitation was performed at the second of three rehabilitation stages, as outlined by Siegert.Reference Siegert10 Microtia repair was carried out using rib cartilage for the framework of the pinna.
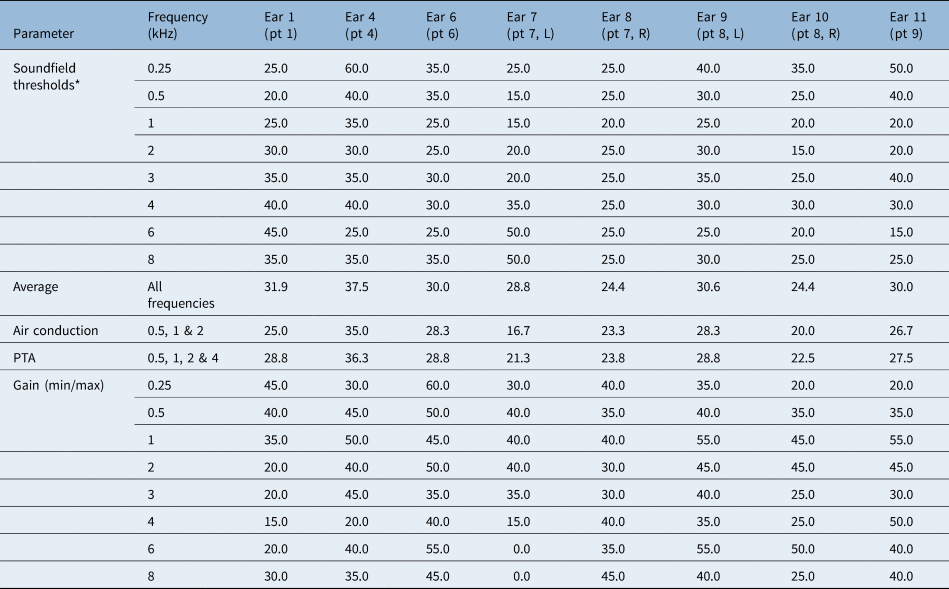
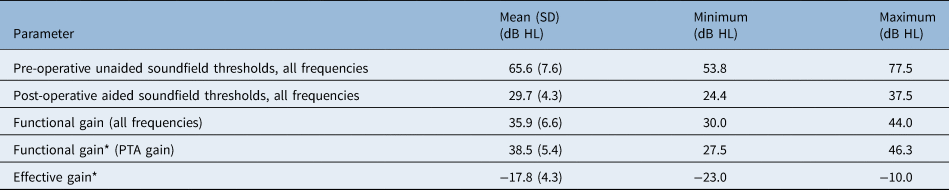
Mean air-conduction thresholds improved over all frequencies (0.25–8 kHz), decreasing from a mean of 65.6 dB HL (unaided) before surgery to a mean of 29.7 dB HL (aided) after surgery (Table 2).
Table 2. Audiometric data and functional improvement
*Frequencies 0.5, 1, 2 and 4 kHz. SD = standard deviation; PTA = pure tone average
Pure tone averages (PTA) (frequencies 0.5, 1, 2 and 4 kHz) improved from a pre-operative mean of 65.3 dB HL (SD = 8.7) to a post-operative mean of 26.8 dB HL (SD = 4.9) (Figure 1). This resulted in a mean PTA gain of 38.5 dB HL.

Figure 1. Pre-operative (pre-op) and post-operative (post-op) mean hearing level for all patients, by frequency.
Effective gain was calculated as bone-conduction thresholds minus post-operative aided soundfield thresholds (0.5, 1, 2 and 4 kHz). The mean effective gain for frequencies 0.5, 1, 2 and 4 kHz was −17.8 dB HL (SD = 4.3) (Figure 2). Optimal gain was reached at 2 kHz; the Vibrant Soundbridge was less efficient at low and high frequencies.

Figure 2. Mean effective gain over frequencies 0.5, 1, 2 and 4 kHz.
The small number of patients hinders a conclusive analysis between incus and stapes coupling. Both approaches seemed to produce similar results. The average functional gain for incus coupling between 0.5 and 4 kHz was 38.1 dB HL, while the average functional gain for stapes coupling between 0.5 and 4 kHz was 38.8 dB HL (Figure 3).

Figure 3. Mean functional gain with (a) stapes coupling and (b) incus coupling.
Secondary endpoints
No revision surgical procedures were required.
All patients wear their speech processors daily. Only 30 per cent of the participants returned the questionnaire. Those who responded reported high satisfaction with the sound quality. The patients reported that they would choose the same treatment option again.
When asked how well they were able to locate the direction of a sound before surgery (1 = never able to locate direction, 10 = perfectly able to locate direction), patients reported an average score of 2.3. The subjective scores on directional hearing after surgery improved to an average of 8.7 (Table 3).
Table 3. Subjective questionnaire

Discussion
Functional gain and effective gain
Here we discuss the functional gain and effective gain obtained using the Vibrant Soundbridge, and compare the findings with the literature. Overall, only a few studies have analysed hearing rehabilitation in congenital aural atresia patients using a Vibrant Soundbridge device. As congenital aural atresia is a rare condition, many investigations report fewer than 10 participants.Reference Célérier, Thierry, Coudert, Blanchard, Loundon and Garabédian11–Reference Wang, Zhao, Zhang, Li, Ma and Ren14 Furthermore, each study uses a slightly different approach to measure outcomes. Thus, the 2022 published consensus statement on bone-conduction devices and active middle-ear implants should be used to unify different approaches.Reference Maier, Lenarz, Agha-Mir-Salim, Agterberg, Anagiotos and Arndt7 In order to compare our findings, we focused on recent publications with more than 10 participants (Table 4).Reference Kiefer and Staudenmaier2,Reference Frenzel, Sprinzl, Streitberger, Stark, Wollenberg and Wolf-Magele15–Reference Zhao, Yang, Liu, Gao, Chen and Zhao17
Table 4. Vibrant Soundbridge and congenital aural atresia: selected studies with over 10 implanted patients

*For frequencies 0.5, 1, 2 and 4 kHz. †A graphic estimate; Kiefer et al. reported a functional gain between 20 dB HL and 36 dB HL. ‡Göttinger word recognition score gain, in children aged five to nine years, comparing after fitting and six months post operation. **Freiburger word recognition score gain, in children aged 10–17 years, comparing after fitting and 6 months post operation. §SRT50 (5–9) = sentence recognition thresholds (SRT) (Oldenburg Satztest) in quiet conditions, in children aged five to nine years, at six months post operation. #SRT50 (10–17) = Sentence recognition thresholds (Oldenburg Satztest) in quiet conditions, in children aged 10–17 years, at 6 months post operation. PTA = pure tone average; SD = standard deviation; WDT50 = word discrimination thresholds; WRS = word recognition score
Our reported mean PTA gain of 38.5 dB HL (0.5, 1, 2 and 4 kHz) compared favourably with the values of 31–38 dB HL reported in the literature. Further tests involved speech recognition and word discrimination scores (Table 4). A larger number of publications with more participant numbers exists for non-congenital aural atresia, including systematic reviews of conductive and mixed hearing lossReference Ernst, Todt and Wagner18 and of sensorineural hearing loss (SNHL).Reference Bruchhage, Leichtle, Schönweiler, Todt, Baumgartner and Frenzel19 Ernst et al. reported functional gains in conductive and mixed hearing loss patients at three months post operation ranging from 12.5 to 43.4 dB HL, averaging at 29.6 dB HL.Reference Ernst, Todt and Wagner18 In a systematic review of the Vibrant Soundbridge in patients with SNHL, Bruchhage et al. reported a functional gain ranging from 12.5 to 33 dB HL, with a higher range of 25–33 dB HL in studies with over 30 cases.Reference Bruchhage, Leichtle, Schönweiler, Todt, Baumgartner and Frenzel19
Reporting on the effective gain has only recently been discussed. Therefore, most previous publications did not present these values, and no effective gain values were reported in the congenital aural atresia literature reviewed. In our study, use of the Vibrant Soundbridge in ear atresia patients resulted in a mean effective gain for speech frequencies (0.5, 1, 2 and 4 kHz) of −17.8 dB HL, with the Vibrant Soundbridge performing best at 2 kHz.
Implantation site
There are five possible coupling sites for the floating mass transducer: incus short process, incus long process, stapes, round window and cochleostomy site.
The few existing studies that compare specific coupling sites mainly examine patients with SNHL. Edlinger et al. compared short and long incus coupling.Reference Edlinger, Hasenzagl, Schoerg, Muck, Magele and Sprinzl20 Lee et al. compared stapes and round window coupling.Reference Lee, Jung, Moon, Kim and Choi21 While all the considered studies stated there was no major difference in hearing rehabilitation depending on the coupling site, none provided large enough numbers of patients to enable a reliable and valid statistical analysis for comparison.
In our study sample, short process incus coupling and stapes coupling were used. Although our patient numbers were too low for statistical analysis, our results indicate no obvious difference between stapes and incus coupling. The average functional gain for incus coupling and stapes coupling differed by less than 3 dB HL.
In congenital aural atresia, the possible coupling site must be evaluated using careful analysis of the pre-operative CT scans. In patients with congenital aural atresia, the incus body is fused to the malformed malleus, and the long process of the incus is usually thin, short and steep towards the stapes head, and therefore not accessible for long process coupling. The round window is often hidden by a lateralised facial ridge behind the atresia plate. If short process coupling is anticipated, the floating mass transducer with the appropriate coupler has to fit onto the incus body and malleus complex once they are liberated from the atresia plate, and it also has to fit underneath the tegmen or middle cranial fossa floor. Using this access route, the facial nerve at the outer genu and in the mastoid segment is less of a concern. The proper connection between the malformed incus and the stapes must always be confirmed. In some instances, there is only a fibrous layer with limited sound transmission properties. Even if the CT scan predicts accessible positioning of the short process–floating mass transducer coupling, we suggest having a stapes clip coupler also available in the operating theatre. In cases of limited space between the incus body and the tegmen, a straightforward stapes coupling is considered. In congenital aural atresia patients, the facial nerve within the mastoid must be skeletonised carefully to allow a direct view onto the stapes suprastructure, in order to separate the incus–malleus complex safely from the stapes head, and to clip the stapes clip and floating mass transducer onto the tiny stapes suprastructure. In all our patients, the chorda was either not present or disrupted at surgery while mobilising the malleus complex from the atresia plate. No patient complained of taste disturbance.
In our study population, no patient underwent round window coupling. In our patient cohort, the Jahrsdoerfer score ranged between 8 and 10. Patients with lower scores were not considered as candidates for Vibrant Soundbridge implantation. Patients with a lower score and a distorted anatomy may, however, still benefit from bone-conducting implants. The challenging surgical procedures of canalplasty, meatoplasty and tympanoplasty in these malformations remain an option in highly selected cases. We strongly advise that patients with congenital aural atresia and microtia be sent to a centre with specialised teams working closely together, to provide full rehabilitation regarding form and function.
Strengths and limitations
All our patients were operated on by one surgeon, which makes the results more comparable. A second strength is the relatively high number of atresia patients for one centre.
• Various implants have recently become available, aimed at overcoming middle-ear hearing difficulties
• These implants provide new options for congenital atresia patients, where malformations prevent seamless acoustic transmission
• Evaluation of different implants has mainly focused on mixed or sensorineural hearing loss patients
• There are few studies on patients with mainly conductive hearing loss as it appears in many congenital middle-ear atresia cases, with none calculating effective gain
• Effective gain is favoured over functional gain in a recent consensus paper on active middle-ear implants, as it can evaluate the implant independently of pre-existing air–bone gap
One major limitation of this study is that we were not able to obtain pre-operative speech audiograms of all, or nearly all, patients. Therefore, gain in speech audiometry could not be calculated. A second limitation of our study is that, even though we provided a relatively large group of atresia patients, the population is still too small to compare subgroups statistically.
Conclusion
The placement of an active middle-ear implant of the Vibrant Soundbridge type allows hearing rehabilitation in selected cases of congenital aural atresia, with a favourable Jahrsdoerfer score, and provides long-term hearing stability in children and adults. A functional gain of approximately 38 dB HL and an effective gain of −17 dB HL can be anticipated. The strength of an implant team lies in the close co-operation between otological and plastic reconstructive surgeons.
Acknowledgements
The authors would like to thank all the participants in this study. We would also like to thank the audiometrists, and the implant fitting and programming team, at the Lucerne Kantonsspital, for providing reliable data for this study.
Competing interests
None declared
Appendix 1. Questionnaire assessing hearing rehabilitation for congenital aural atresia with implanted hearing aids



Appendix 2. Further data to meet reporting standard of consensus paper
Table 1. Demographics
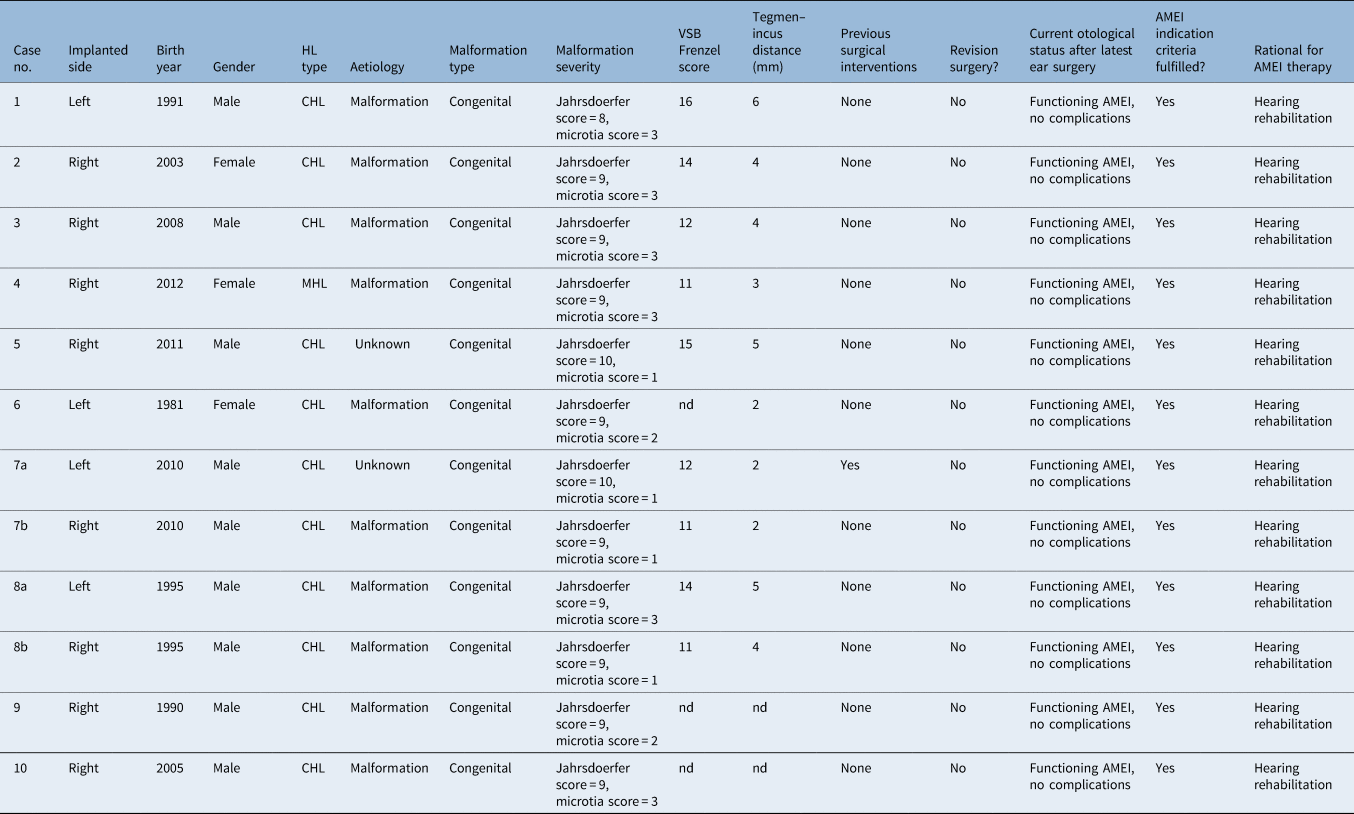
Table 2. Surgical data

Table 3. Complications

Table 4. General audiometric data
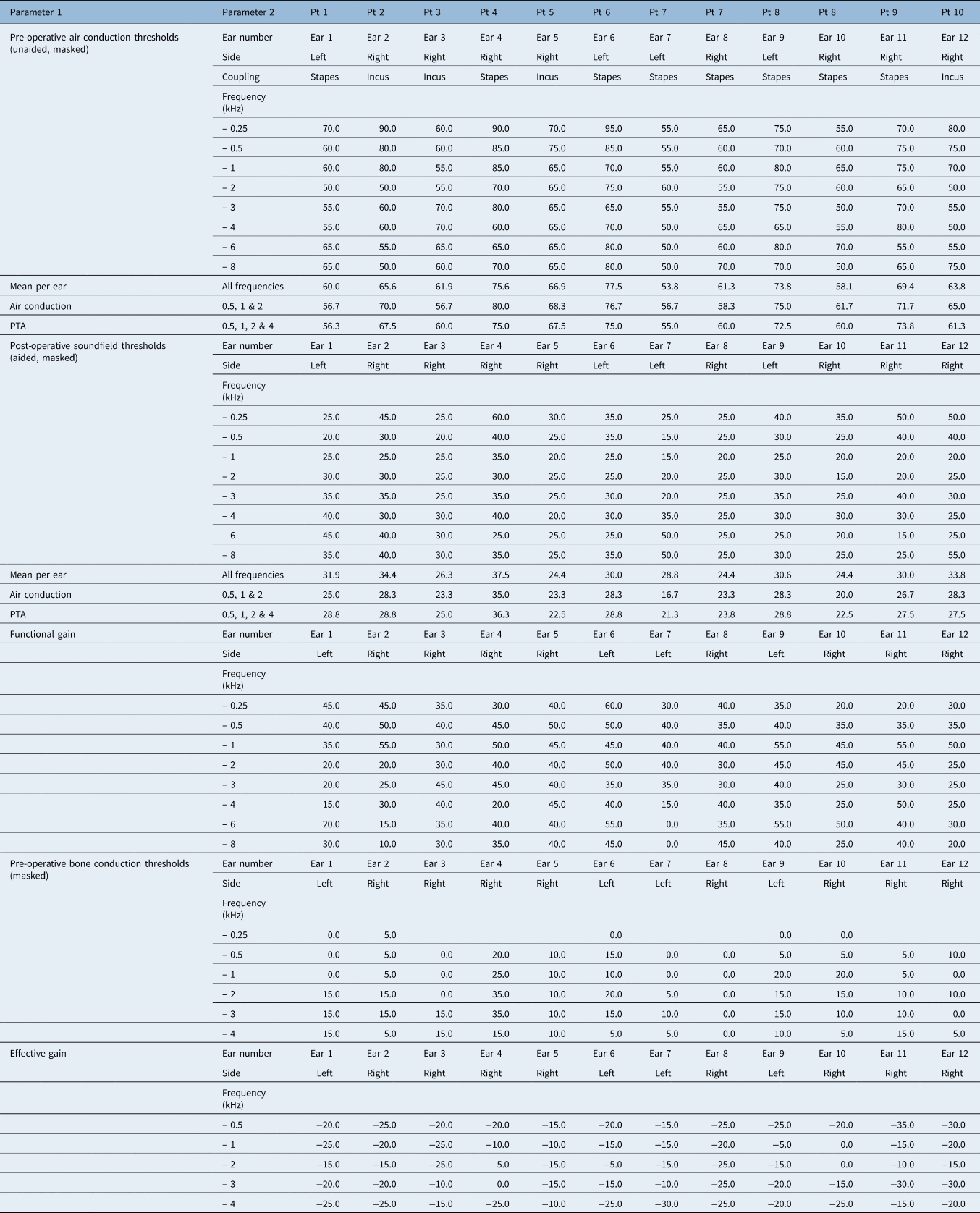
Table 5. Pre-operative air conduction threshold data for stapes

Table 6. Post-operative soundfield threshold data for stapes

Table 7. Pre-operative air conduction threshold data for incus

Table 8. Post-operative soundfield threshold data for incus