Introduction
Hydatidosis is a neglected zoonosis. The disease occurs due to infection by larval stages of the tapeworm Echinococcus granulosus. Hydatidosis is a significant public health issue and economic problem in many circumpolar, tropical, and subtropical regions (Daryani et al., Reference Daryani2007). Hydatidosis is endemic in many countries of Asia, South America, the Middle East and Australia (Pednekar et al., Reference Pednekar2009). Many species of domestic and wild animals act as intermediate hosts for metacestode stages of E. granulosus (Singh et al., Reference Singh2012). Sheep are considered the most common and successful intermediate hosts, as they harbour the most fertile cystic stages for the transmission of the infection through dog–sheep life cycles (Soulsby, Reference Soulsby1982). Hydatidosis in domestic animals is usually asymptomatic and diagnosed only during post-mortem inspection following slaughter at an abattoir.
Various studies have shown the prevalence of hydatidosis in India, particularly in food-producing animals such as cattle, pigs, buffaloes, sheep and goats (Bhattacharya et al., Reference Bhattacharya2007; Gudewar et al., Reference Gudewar2009; Pednekar et al., Reference Pednekar2009; Singh et al., Reference Singh2012). Annual economic losses attributed to hydatidosis in India are approximately USD 212.35 million (Singh et al., Reference Singh2014). Economic losses as a result of hydatidosis are associated with condemnation of infected liver and lungs (Singh et al., Reference Singh2012). The consumption of infected lungs and liver causes disease transmission to humans and definitive hosts (Singh et al., Reference Singh2012). Over 22,721 confirmed sporadic cases of human hydatidosis (17,075 and 5646 cases without and with surgical interventions, respectively) have been reported in India (Singh et al., Reference Singh2014). Ideal conditions exist for the infection biology of hydatidosis in India; in other words, the country is ideal for the establishment, propagation and dissemination of the disease in both humans and livestock (Pednekar et al., Reference Pednekar2009). However, lack of public awareness about the life cycle and transmission of E. granulosus, the absence of proper meat inspection, and improper offal disposal at illegally run abattoirs significantly contribute to the completion of domestic cycles of transmission (Singh et al., Reference Singh2012).
Until now, ten genotypes of E. granulosus have been reported by molecular genetic analysis of mitochondrial DNA sequences (Bowles et al., Reference Bowles, Blair and McManus1992; Lavikainen et al., Reference Lavikainen2003). Generally, E. granulosus genotypes are divided into four subspecies: E. granulosus sensu stricto (G1-G3 genotype), E. equinus (G4), E. ortleppi (G5) and E. canadensis (G6-G10) (Sharma et al., Reference Sharma2013). Among these strains, the G1 genotype is infamous for causing the greatest number of human cases of hydatidosis, also known as cystic echinococcosis (Moro and Schantz, Reference Moro and Schantz2009). We determined the prevalence of hydatid cysts in various organs of slaughtered hilly ‘Gaddi’ breed small ruminants of Kangra Valley, India. We also characterized E. granulosus isolates that were retrieved by targeting the mitochondrial cytochrome oxidase 1 gene (mtCO1).
Materials and methods
Screening of slaughtered animals and sample collection
A total of 427 animals (230 sheep and 197 goats) were screened for the presence of hydatid cysts. Lungs and liver of every animal were examined visually, palpated and incised for the detection of hydatid cysts. The infected organs were separated from the carcass, and incised hydatid cysts were analysed at the Department of Veterinary Parasitology, College of Veterinary and Animal Sciences, CSK HPKV Palampur (H.P.) India.
Assessment of fertility and viability of the hydatids
Hydatid fluid was aspirated with a sterile syringe after washing the cyst with normal saline. The fluid was centrifuged at 5000 rpm for 5 minutes and the pellet was observed at 10× magnification for the presence of protoscolices. The fertility of the cyst was determined by the presence of protoscolices in the hydatid fluid. The viability of the cysts was assessed by eosin exclusion method, as described earlier (Daryani et al., Reference Daryani2007).
Genomic DNA extraction and PCR amplification
The cysts were collected carefully and washed with normal saline. The genomic DNA was extracted from the hydatid fluid aspirated from the cysts. The fluid was subjected to centrifugation at 5000 rpm for 5 minutes and the pellet was screened for the presence of protoscolices. The genomic DNA was extracted from sterile and fertile hydatid cysts using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). The extracted DNA was then stored at −20°C until further use. The polymerase chain reaction (PCR) was performed for amplifying DNA sequences encoding mitochondrial cytochrome oxidase 1 (mtCO1) gene. The published primers (Bowles et al., Reference Bowles, Blair and McManus1992) employed in the present study were JB3 (forward): 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′ and JB4.5 (reverse): 5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′. The 25 μl PCR reaction mixture contained 12.5 μl Master Mix (GoTaq1 Green Mater Mix, Promega, Madison, WI, USA), 2.5 μl of each primer (forward and reverse), 5 μl genomic DNA and 2.5 μl nuclease free water. The following reaction conditions were followed in sequential order: initial denaturation (94°C for 5 minutes), denaturation (38 cycles of 94°C for 30 s), annealing (38 cycles of 50°C for 45 s), extension (38 cycles of 72°C for 35 s), and final extension (72°C for 10 minutes) (Ehsan et al., Reference Ehsan2017). The amplified PCR products/amplicons were separated by electrophoresis on 1.5% agarose gel and were visualized under UV transilluminator for detection of 446 bp amplicon size.
DNA sequencing and phylogenetic analysis
The 446 bp amplicons retrieved were custom sequenced (Eurofins Genomics India Pvt. Ltd., Bengaluru). The identification of the sequences and homology was confirmed after comparing the product sequences with the reference sequences (KX874722.1, KX874714.1, HF947595.1, KY499559.1, KT968706.1, KT446001.1, MH010310.1, KC415063.1, JX854035.1, KM100575.1, KX874713.1, HF947555.1, MH010307.1, KT382540.1, HF947553.1, KC660075.1, HM598451.1, HF947574.1, FN646371.1) available in GenBank, by using the Basic Local Alignment Search Tool (Testini et al., Reference Testini2011). To ensure an open reading frame and to exclude pseudogenes, individual mtCO1 sequences were deduced into amino acid sequences and were then analysed using MEGA X (Molecular Evolutionary Genetic Analysis) software for phylogenetic analysis (Kumar et al., Reference Kumar2018). The sequences retrieved in the present study were also used for phylogenetic tree construction along with other isolates (exhibiting similitude with the present study isolates), retrieved from GenBank using the Neighbour-Joining method in MEGA X software (Kumar et al., Reference Kumar2018). Bootstrap analyses were conducted using 1000 replicates. Analysis of the estimates of evolutionary divergence between genotype sequences (G1–G10) retrieved from GenBank (Bowles et al., Reference Bowles, Blair and McManus1992) and the present study was conducted using the maximum composite likelihood model (Tamura et al., Reference Tamura, Nei and Kumar2004).
Histopathological studies
The samples collected from infected lungs and liver were subjected to histopathological staining as per Luna (Reference Luna1968).
Statistical analysis
Mean and standard deviation values pertaining to the viability of protoscolices in the fertile cysts were assessed using Microsoft Excel.
Results
Prevalence studies
Hydatid cysts were found in 12.2% (n = 28) sheep and 10.7% (n = 21) goats. Pulmonary echinococcosis was more prevalent in slaughtered sheep and goats [sheep (56.36%) and goats (62.90%)] than hepatic infection [sheep (43.64%) and goats (37.10%)] (table 1). The cysts were recorded from both visceral and parietal surfaces of the liver. Fertility rates were higher in hepatic (81.25%) and pulmonary cysts of sheep (83.87%) compared to goats (table 1). Most viable protoscolices were recorded from fertile pulmonary cysts (68.72 ± 13.63) of sheep (table 1).
Table 1. Fertility of hydatid cysts and viability of protoscolices of fertile cysts recovered from different organs of slaughtered small ruminants.

Molecular confirmation and phylogenetic distribution analysis
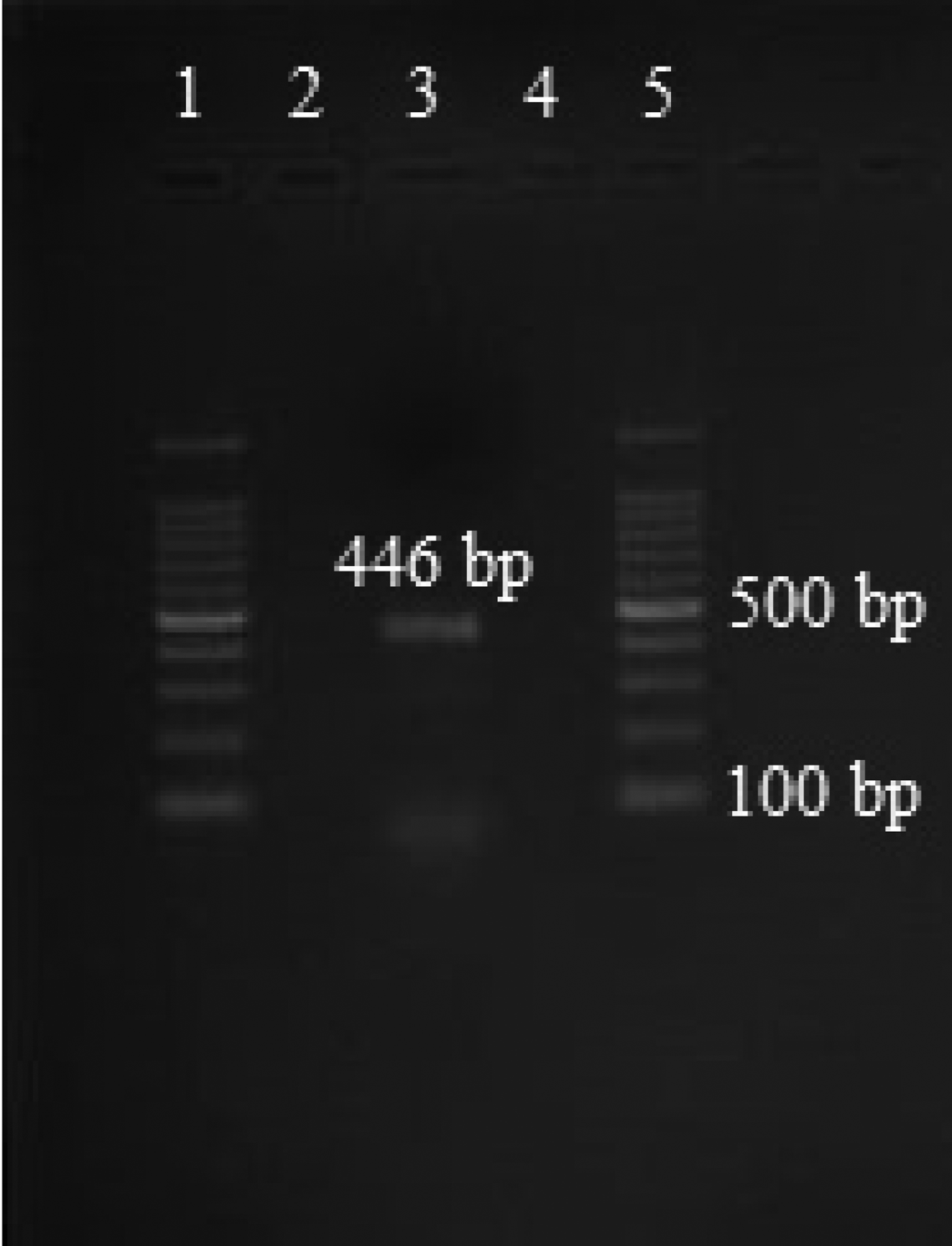
Amplicons of approximately 446 bp size were retrieved after gel electrophoresis (fig. 1). The custom sequencing analysis of partial mtCO1 gene of E. granulosus produced sequences of 410 bp for the samples analysed. Echinococcus granulosus isolates retrieved from the lung and liver hydatid cysts of humans of Turkey, accession numbers: KX874714.1, KX874722.1 and KX874721.1, exhibited 100 and 99%; G7 genotype from cattle in Portugal (HF947574.1) exhibited 99%; human hydatid cyst isolates from Iran (MH010307.1) exhibited 99% identity to the isolates retrieved from sheep/goats in the present study from the north-western Himalayas (fig. 2). The observations based on the maximum likelihood model exhibited more differences in the sequences of various E. granulosus genotypes (G1–G10) with the isolates retrieved in the present study, as the values were extremely high (> 3) in pairwise comparison (table 2).

Fig. 1. PCR amplification targeting the mtCO1 gene. 1: 100 bp plus marker; 2: PCR product of sterile hydatid cyst; 3: PCR product of fertile hydatid cyst; 4: PCR product of cysticercus; 5: 100 bp plus marker.

Fig. 2. Phylogenetic tree of Echinococcus granulosus isolate retrieved from ‘Gaddi’ breed sheep and goats in relation to different isolates of intermediate hosts submitted to GenBank based on mitochondrial cytochrome oxidase subunit gene I. The phylogenetic tree was constructed by the Neighbour-Joining method using MEGA X software. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site.
Table 2. Estimates of evolutionary divergence between sequences based on the maximum likelihood model.

Histopathological observations
Histopathological examination revealed a thick coat of granulation tissue, causing fibrosis and inflammatory reaction composed of fibroblasts and mononuclear cells around the cysts. In the liver and lungs, large cyst walls were evident, with the presence of inner germinal and outer laminated layers (figs 3 and 4). The hepato-cellular degeneration was prominent at the periphery of the cysts. The present study also supports the presence of a channel or space between the ectocyst and pericyst of the cyst wall in the liver (fig. 3). In the lungs, a germinal layer was observed separating from the laminated layer in places, and echinococcal protoscolices were also noticed in certain sections. The cyst wall was surrounded by a zone of inflammatory reaction composed mainly of lymphocytes. Pressure atrophy of adjoining parenchyma rendered alveoli to appear as slit-like structures with a narrow lumen (fig. 4).

Fig. 3. Liver section with hydatid wall and zone of inflammatory reaction. H&E × 10X.

Fig. 4. Lung section with atelectatic alveoli with narrow lumen. H&E × 10X.
Discussion
An increase in the incidence of hydatidosis in the recent past has been reported from various parts of India (Rao et al., Reference Rao, Mehra and Narang2012; Nyero et al., Reference Nyero2015). A perusal of the literature indicates a continuous decline in the prevalence of cystic echinococcosis in urban centres in the past few decades (Pednekar et al., Reference Pednekar2009). However, past studies mainly screened organized abattoirs slaughtering the livestock from intensively managed large-scale production facilities (Pednekar et al., Reference Pednekar2009). On the contrary, the present study highlights the prevalence of hydatidosis in small ruminants of poorly resourced nomadic farmers from unexplored naïve areas of the north-western Himalayas.
In rural areas, scavenger dogs contract the infection after consumption of contaminated offal from open-space non-gazetted abattoirs. These infected dogs contaminate the grazing pastures with faeces containing eggs. The slightly higher prevalence of cystic echinococcosis in sheep than goats can be attributed to their feeding habits. Being surface grazers, sheep become potential consumers of the eggs from contaminated pastures (Nyero et al., Reference Nyero2015).
Higher prevalence of pulmonary echinococcosis compared to hepatic infections in sheep and goats can be associated with the immune competence of the host. The compact tissues (such as the liver) resist the development of larger cysts (Torgerson, Reference Torgerson2003). The lung parenchyma possesses greater capillary bed and spongy consistency, which supports wider distribution of onchospheres. This provides more space for the development of larger embedded cysts (Beigh et al., Reference Beigh2017). Initial development of the cyst is generally faster (within 10–14 days), but the time required for the formation of fertile cysts with complete structure can take more than 10 months in most of the intermediate host species (Thompson and Lymbery, Reference Thompson and Lymbery1988). We detected a higher rate of fertile and viable cysts in sheep than goats, which is in agreement with the findings of Daryani et al. (Reference Daryani2007). The observation of higher rates of fertile cysts in the lungs can also be associated with the greater affinity of G7 genotype for the lungs compared to the liver (Oksanen and Lavikainen, Reference Oksanen and Laivikainen2015).
Evolution, geographical distribution of parasites and their phylogenetic relationships can significantly contribute to epidemiological studies of parasitic diseases (Lymbery and Thompson, Reference Lymbery and Thompson2012). Hence, identification and geographical distribution of parasites, their etiological roles, and phylogenetic relationships can be established by using techniques based on molecular epidemiological approaches (Archie et al., Reference Archie, Luikart and Ezenwa2008). Various studies from India have documented distribution of E. granulosus genotype strains [G1, G2, G3 and G5 (cattle, buffalo, sheep and goats) from western India (Bhattacharya et al., Reference Bhattacharya2007; Gudewar et al., Reference Gudewar2009; Pednekar et al., Reference Pednekar2009), G1 and G3 (cattle, buffalo, sheep, goats and pigs) from northern India (Singh et al., Reference Singh2012)] in meat-producing animals and in humans (Sharma et al., Reference Sharma2013). The homology of E. granulosus PCR products in the present study is in accordance with Bowles et al. (Reference Bowles, Blair and McManus1992) and Ehsan et al. (Reference Ehsan2017). The isolates of E. granulosus recovered in the present study are more closely (99%) related to the G7 genotype strain reported from hydatidosis of cattle in Portugal (Beato et al., Reference Beato2013). To the best of our knowledge, the present study is the first documented report of the G7 genotype strain of E. granulosus from any intermediate host in India. Previously, the G7 genotype strain has also been reported as a dominant genotypic strain in goats in Spain (Mwambete et al., Reference Mwambete, Ponce-Gordo and Cuesta-Bandera2004) and Greece (Varcasia et al., Reference Varcasia2007). The finding has raised concern regarding the introduction of newer infective genotypic strains in a new, unexplored area. Maximum likelihood model-based analysis of E. granulosus genotype (G1–G10) sequences compared with the present study isolates indicated diverged lineage. Interspecies hybridization can be considered as a possible explanation for this observation, as cross-fertilization occurs less frequently in hermaphrodite organisms (Gudewar et al., Reference Gudewar2009).
The findings pertaining to histopathological studies were in concordance with previous studies (Barnes et al., Reference Barnes2011; Singh et al., Reference Singh2016). The cysts had adventitial layers of varying thicknesses, protecting them from host immune responses. We also recorded the presence of the germinal membrane, laminated layer, pericyst and ectocyst in the cyst wall of the lungs, as reported by Solcan et al. (Reference Solcan2010). The space between the pericyst and ectocyst acts as a channel for the flow of tissue fluids and nutrients. Fibrosis was also evident adjoining the cyst wall. Bronchioles also appeared to be atelectatic and exhibited congestion and haemorrhages in places. Some foci of mineralization were also observed in the adventitial layer. All the observations were in line with the findings of Beigh et al. (Reference Beigh2017).
Epidemiological analyses focusing on the frequency, geographical distribution and host range of E. granulosus genetic variants are essential for the implementation of control strategies. The present study highlights the molecular confirmation and phylogenetic analysis of E. granulosus isolates with the prevalence of hydatidosis in a naïve host species and in an unexplored region. The findings are of significant medical and veterinary importance for the development of control measures to check dissemination of hydatidosis.
Author ORCIDs
A.D. Moudgil, 0000-0002-1130-2376.
Acknowledgements
We thank the Dean of Dr G.C. Negi College of Veterinary and Animal Sciences, Palampur, India, and the Head of the Department of Animal Breeding and Genetics, for providing the facilities to carry out the research work.
Financial support
The present study received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
No studies involving laboratory animals or invasive techniques were conducted. The samples were collected from slaughtered animals from local abattoirs.