Introduction
Cholelithiasis, one of the most common causes of hospitalization for gastrointestinal disorders, affects 10–20% of the global population. Nutritional and lifestyle changes in modern life lead to an increase in the diagnosis of cholelithiasis (Lammert et al., Reference Lammert, Gurusamy, Ko, Miquel, Méndez-Sánchez, Portincasa, van Erpecum, van Laarhoven and Wang2016; Littlefield & Lenahan, Reference Littlefield and Lenahan2019; Stokes & Lammert, Reference Stokes and Lammert2021), which places a significant burden on healthcare systems worldwide (Everhart & Ruhl, Reference Everhart and Ruhl2009; Peery et al., Reference Peery, Crockett and Barritt2015; Fairfield et al., Reference Fairfield, Wigmore and Harrison2019).
Clonorchiasis is an important food-borne zoonosis caused by the intake of raw or undercooked freshwater fish or shrimp contaminated with living Clonorchis sinensis metacercariae (Qian et al., Reference Qian, Utzinger, Keiser and Zhou2016; Tang et al., Reference Tang, Huang and Yu2016). When ingested, the metacercariae excyst in the duodenum and ascend to the liver biliary tract through the ampulla of Vater. It takes several days for adult flukes to become mature when they enter the gallbladder. The life span of adult flukes can reach nearly 30 years in humans (Hong & Fang, Reference Hong and Fang2012). It is estimated that one worm produces approximately 2500–3000 eggs per day by sexual reproduction (Hong & Fang, Reference Hong and Fang2012). The eggs flow down to the intestine and gallbladder with bile, which may cause gastrointestinal symptoms due to mechanical stimulation and secretions and metabolites related to inflammation and intermittent obstruction of the bile ducts. In mild cases, symptoms include abdominal pain, nausea, anorexia, and diarrhoea. Severe infections can cause pyogenic cholangitis, which may progress to atrophy of the liver parenchyma and portal fibrosis (Petney et al., Reference Petney, Andrews, Saijuntha, Wenz-Mücke and Sithithaworn2013; Qian et al., Reference Qian, Chen and Yan2013).
Cholecystolithiasis is a common disease associated with C. sinensis infection. A few studies have suggested that the formation of intrahepatic bile duct stones is highly correlated with C. sinensis infection (Jang et al., Reference Jang, Kim, Yu and Lee2007; Liu et al., Reference Liu, Zhu, Cai, Lv and Li2019). Moreover, our previous study demonstrated that C. sinensis infection was a significant cause of gallbladder stones, especially calcium carbonate (CaCO3) gallbladder stones (Qiao et al., Reference Qiao, Ma, Luo, Luo and Zheng2012b, Reference Qiao, Ma, Luo, Yang, Luo and Zheng2014; Ma et al., Reference Ma, Luo, Wang, Qiao, Huang and Zhong2017). This study was consistent with another group indicating that calcified parasitic ova of C. sinensis might contribute to the formation of gallbladder stones (Huang et al., Reference Huang, Chen, Eng and Chen1994). However, these studies were based on C. sinensis eggs, and there is no definite straightforward evidence from C. sinensis adult worms to date. To investigate whether C. sinensis adults participate in the early stage of gallbladder stone formation, we recruited patients with cholecystolithiasis and collected stones similar to adult worms. We systematically analysed these stones and confirmed that adult C. sinensis tissues were present in some of the stones resembling adult worms characterized by calcium salts wrapped around C. sinensis eggs, indicating direct calcification and packaging after C. sinensis adults entered the gallbladder, which led to the early formation of CaCO3 or bilirubinate–CaCO3 mixed gallbladder stones.
Materials and methods
Subjects and specimens
Gallbladder stones resembling adult C. sinensis worms were obtained from 33 cholecystolithiasis patients who received gallbladder-preserving cholelithotomies (Qiao et al., Reference Qiao, Huang, Luo and Zhang2012a) in the Department of General Surgery of The Sixth People's Hospital of Nansha, Guangzhou, China, between July 2017 and June 2019. They consisted of 24 men, ranging in age from 38 to 72 years, with a mean age of 52.8 ± 11.2 (mean ±standard deviation (SD) ), and nine women, ranging in age from 36 to 69 years, with a mean age of 53.1 ± 11.1 (mean ± SD). There was no significant difference when comparing the age of male vs. female groups by an independent sample t-test (P > 0.05).
In some patients, live C. sinensis adult worms can be seen under endoscopy, with internal organs that can be seen vaguely. All the stones were washed twice with distilled water and then dried.
Composition analysis of gallbladder stones by Fourier transform infrared spectroscopy (FTIR)
The main components were analysed using a Bruker (TENSOR27, Germany) FTIR spectrometer. A 2 mg sample of each layer was weighed if the layered structures were distinct. In amorphous stones, 2 mg samples were weighed directly. The samples were mixed with potassium bromide at a ratio of 1:150, ground thoroughly and used to make discs. The main components were analysed using a Bruker (TENSOR27, Germany) FTIR spectrometer in the frequency range of 400–4000 cm−1 at 4 cm−1 resolution. Control substances (99% pure standard) were obtained from Sigma Chemical Company (St. Louis, MO). Gallstone composition was determined by comparison of the gallbladder stones with standard control spectra.
Microscopic analysis with scanning electron microscopy and X-ray energy spectrometry
In the present study, all gallbladder stones resembling adult C. sinensis worms were analysed by scanning electron microscopy (SEM). The stones were split, and 1–2 pieces (3–5 mm in size) were sampled from each layer if the layered structures were distinct. In amorphous stones, 1–2 pieces, 3–5 mm in size, were sampled and fixed on the sample table using an electroconductive adhesive. One piece was secured to the surface while the other was placed facing the opposite direction. The surface to be analysed was polished, thus making the surface and the bottom surface parallel. The samples were then dried at 40°C overnight. The dried samples were subsequently sputter-coated with gold (ETD-2000, Beijing Elaborate Technology Development, China), observed using a ZEISS (EVO LS10, Cambridge, Germany) SEM and photographed. The extra high tension was 20 kV. The microstructure was then analysed through amplification of 400, 1000, 3000, 6000, 10,000 and 20,000.
The region of interest was amplified to 100 and 3000 to analyse element composition and distribution by X-ray energy spectrometry (X-Max, Oxford, United Kingdom). The elements from B5 to U92 can be detected only if the weight percentage was more than 0.01%.
Microscopic examination of bile sediments
Two-millilitre bile samples were centrifuged at 1450×g for 10 min. The supernatant of each sample was transferred to another clean tube for analysis of the chemical composition of the bile (data not shown), and approximately 0.5 ml of sediment was kept. The sediment was then smeared onto labelled slides and observed with a BX51 system microscope (Olympus, Japan).
Histopathological examination of gallstones
All gallbladder stones resembling adult C. sinensis worms were analysed by histopathological examination. Gallbladder stone samples fixed in formalin were taken to the pathology laboratory, decalcified in 10% nitric acid solution, dehydrated in gradient alcohol, embedded in paraffin and sectioned into 3- to 5-μm-thick slices (HM315, Thermo, USA). Microscopic examination was performed on all slides of the sample after haematoxylin and eosin staining (Bessot, China). We used an Olympus microscope (model BX51) equipped with a digital camera to examine the slides as well as to take pictures. To reduce bias, the microscopic examination of pathology slides was performed in single blinded conditions.
Statistical analysis
The experimental data analysis was executed by SPSS v.16.0 software. Ages are presented as the means ± SDs. The detection rate was analysed using a Chi-square test. P < 0.05 was regarded as statistically significant.
Results
Thirty-three gallbladder stones resembling adult C. sinensis worms were obtained
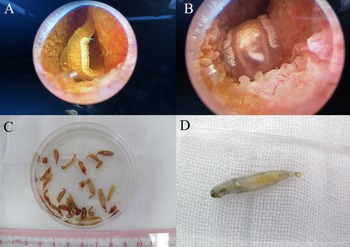
Clonorchis sinensis adults were obtained from patients, and the adult worms and internal organs could be clearly seen under endoscopy (fig. 1A, B). The size of the fresh worm is approximately 10–25 mm × 3–5 mm, and organs such as mouth suckers, abdominal suckers, ovaries, testes, seminal vesicles, etc., were visible to the naked eye (fig. 1C, D). All 33 gallbladder stones were black melon seed-like in appearance, with a similar shape and size to C. sinensis adult worms (fig. 2). Some of the stones had a hard texture, and others had a brittle texture. There was no obvious layered structure in the section of the stones.
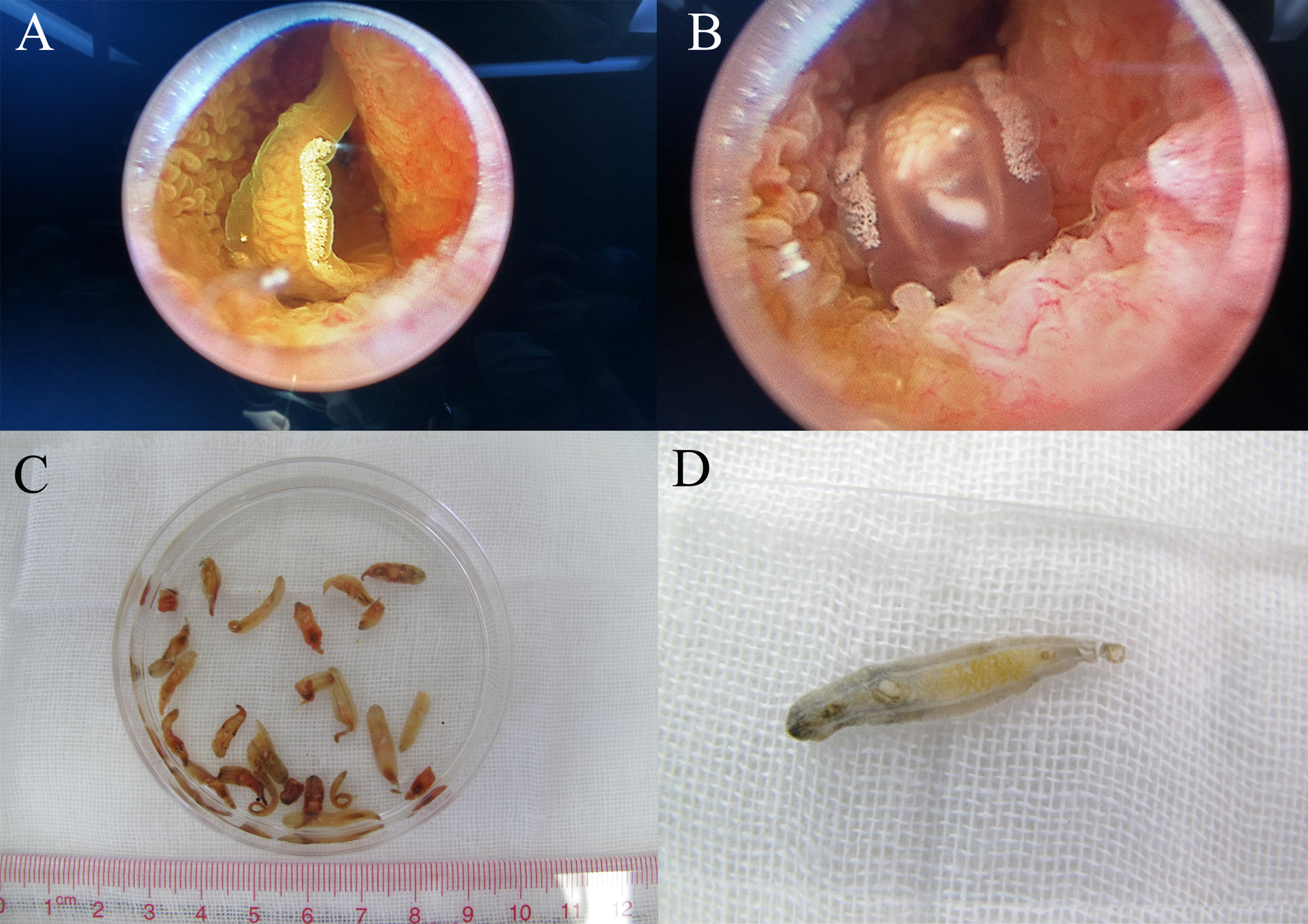
Fig. 1. Adult worms of Clonorchis sinensis: (A, B) adult worms under endoscopy; and (C, D) adult worms collected from infected patients.

Fig. 2. Clonorchis sinensis adult worms and gallbladder stones resembling adult worms: red arrowhead indicates the stones resembling adult C. sinensis worms, and blue arrowhead indicates the adult worms collected from patients.
C. sinensis eggs were found in the corresponding bile sediment
Mostly, the bile was dark yellow and clear with low viscosity. Microscopic examination revealed that 28 of the 33 cases of bile sediments were positive for C. sinensis eggs, which were adhered and packaged with CaCO3 crystals and bilirubinate particles. Most of the eggs had a complete structure with a size of (26–33) μm × (15–17) μm, plus a classic sesame-like shape and opercular shoulders, a small operculum on the front end, a visible abopercular knob on the posterior end and a miracidium inside, as shown in fig. 3.
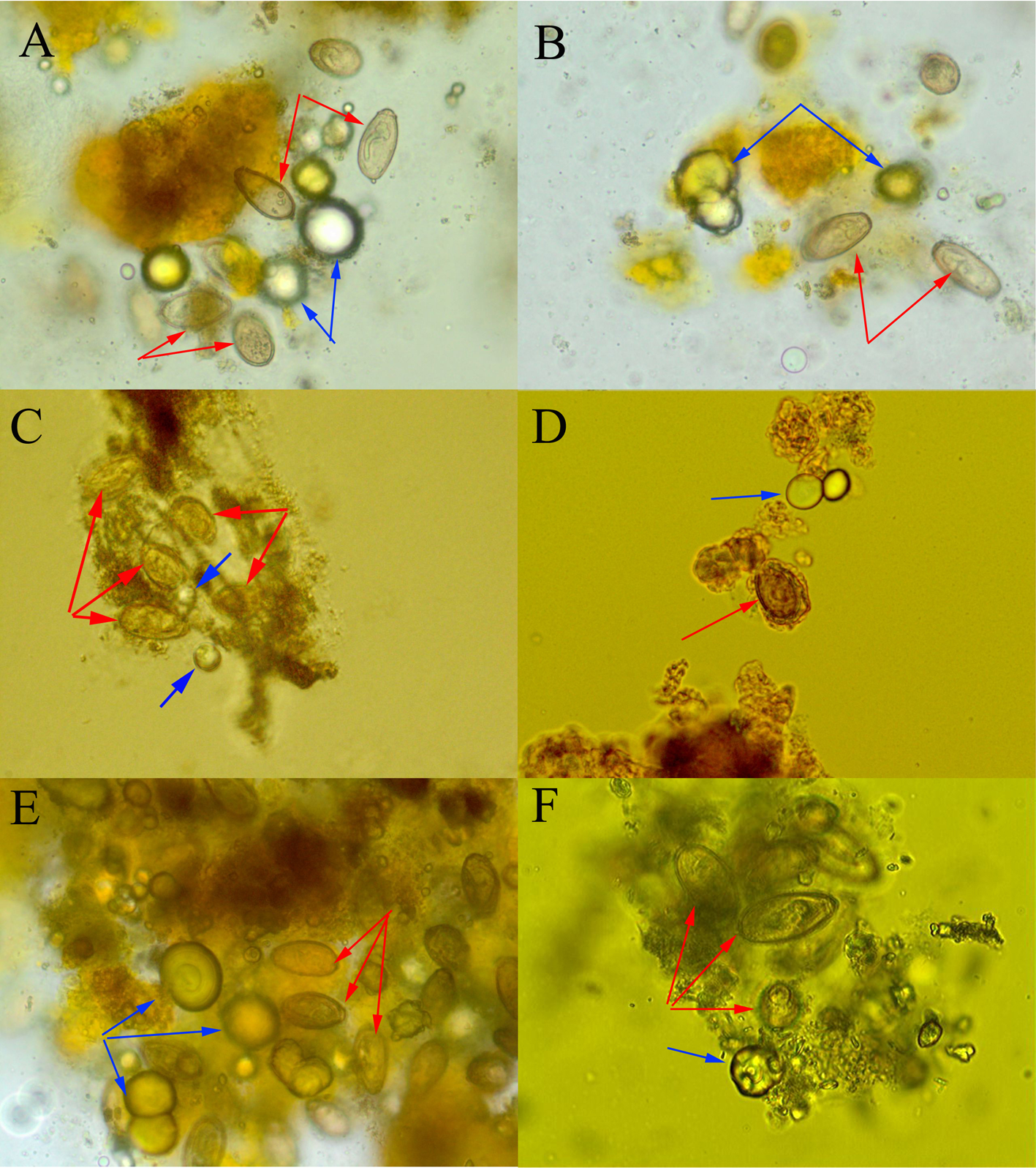
Fig. 3. Clonorchis sinensis eggs in the bile sediment: blue arrowhead indicates the calcium carbonate (CaCO3) crystals; and red arrowhead indicates the C. sinensis eggs. Most of the eggs have a complete structure with a size of (26–33) μm × (15–17) μm, with a classic sesame-like shape and opercular shoulders, a small operculum on the front end, a visible abopercular knob on the posterior end and a miracidium inside. Clonorchis sinensis eggs were adhered and packaged with CaCO3 crystals and bilirubinate particles.
Gallbladder stones resembling adult C. sinensis worms were mostly CaCO3, bilirubinate and bilirubinate–CaCO3 mixed stones
Of the 33 gallbladder stones resembling adult C. sinensis worms, nine cases were CaCO3 stones and mainly calcite, 12 cases were bilirubinate stones and 12 cases were mixed stones, including nine cases of bilirubinate–CaCO3 mixed stones, two cases of bilirubinate–calcium phosphate mixed stones and one case of bilirubinate–CaCO3–calcium phosphate mixed stones. The infrared spectrograms of the CaCO3 and bilirubin standard control as well as CaCO3 and bilirubinate–CaCO3 mixed gallbladder stones are shown in fig. 4. Cholesterol and calcium stearate stones were not found in the present study. Under SEM, C. sinensis eggs were found in 30 of the 33 gallbladder stones resembling adult C. sinensis worms, including all the CaCO3 and mixed stones.

Fig. 4. Infrared spectrogram of the calcium carbonate (CaCO3) and bilirubin standard control as well as CaCO3 and bilirubinate–CaCO3 mixed gallbladder stones: (A) infrared spectrogram of the CaCO3 standard control; (B) infrared spectrogram of the bilirubin standard control; (C) infrared spectrogram of CaCO3 gallbladder stones; and (D) infrared spectrogram of bilirubinate–CaCO3 mixed gallbladder stones.
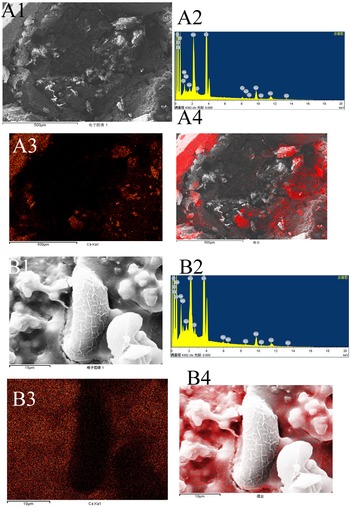
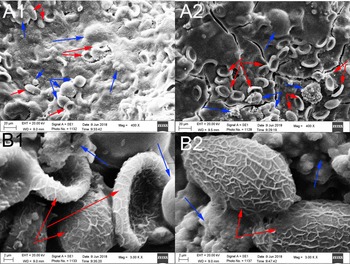
Mapping analysis of element distribution in general stones using an X-ray energy spectrometer with a magnification of ×100 showed that in gallbladder stones resembling adult C. sinensis worms, calcium ions, with an amount of 12.88% (table 1), were mainly distributed in the outer layer (fig. 5A). When the stones were magnified to × 400, a large number of C. sinensis eggs in clusters and surrounding calcified particles could be seen in some stones (fig. 6A); when magnified to × 3000, we found that C. sinensis eggs were packaged by mucoid matter and adhered to each other with CaCO3 crystals and bilirubinate granules (fig. 6B). Mapping analysis of element distribution in the micro field showed that a large amount of calcium ions with an amount of 18.87% can be detected on the surface and surroundings of C. sinensis eggs, suggesting that C. sinensis eggs adsorbed a large amount of calcium salts, and the eggs as well as their surroundings were wrapped by calcium salt particles (fig. 5B). The elemental composition in the general stone and in the micro field is shown in table 1.
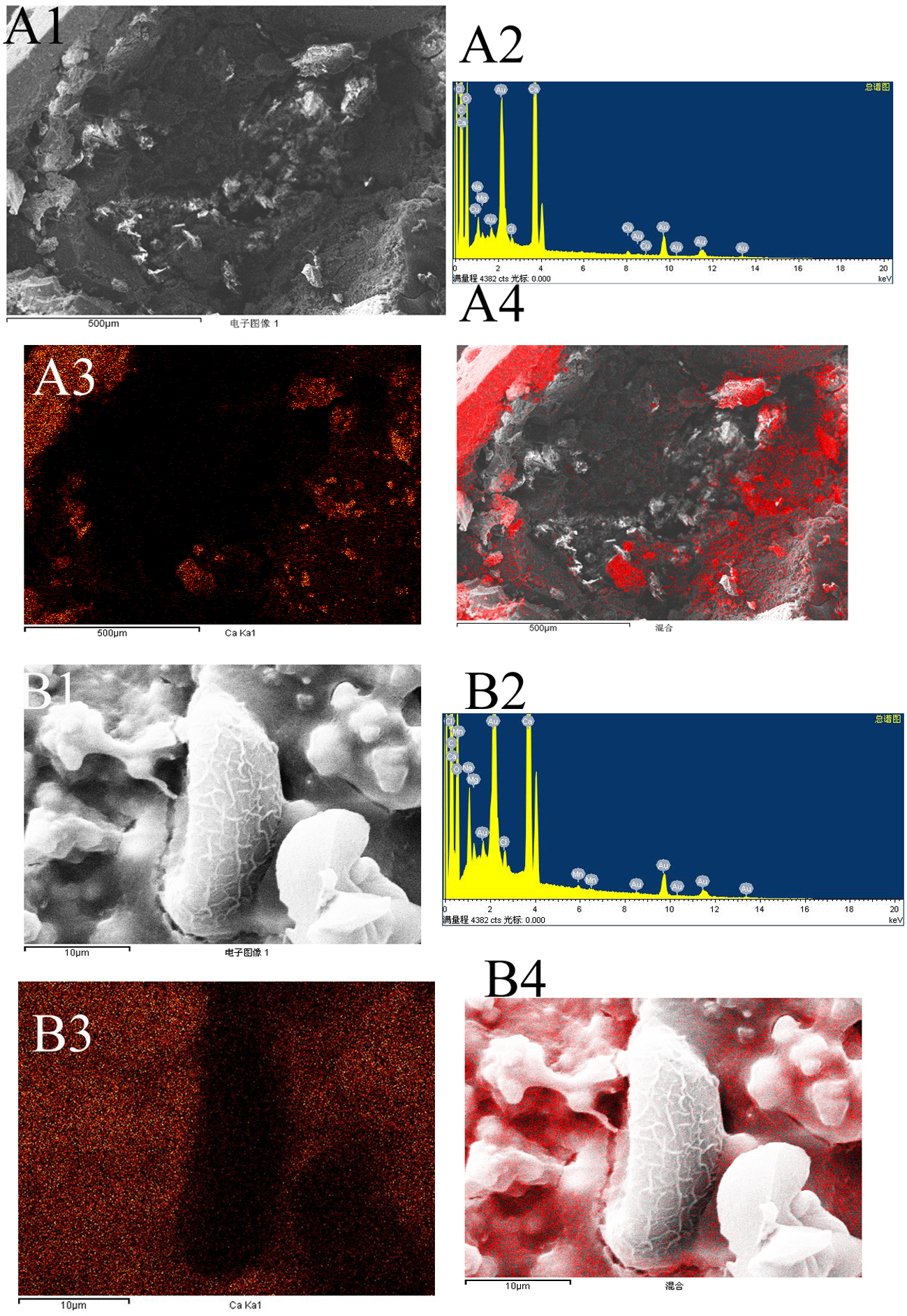
Fig. 5. Mapping analysis of element distribution in general stones and in the micro field (red dot represents calcium distribution): (A1) general stones with a magnification of × 100; (A2) energy spectrum of general stone; (A3, A4) calcium distribution in general stones, mainly in the outer layer; (B1) micro field with a magnification of × 3000; (B2) energy spectrum of the micro field; and (B3, B4) calcium distribution in the micro field, mainly in the surroundings of Clonorchis sinensis eggs.
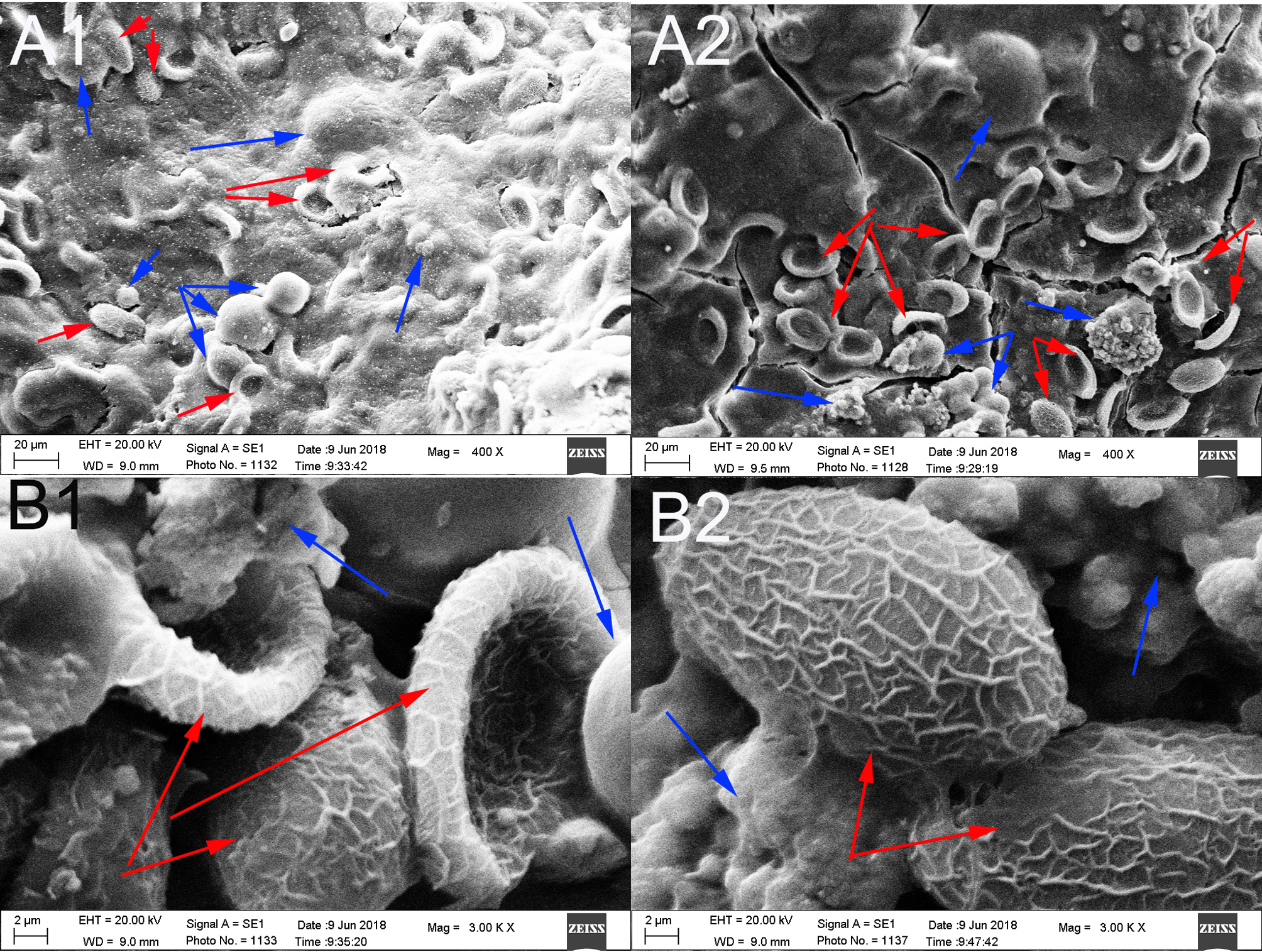
Fig. 6. Clonorchis sinensis eggs and calcified particles or calcium carbonate (CaCO3) crystals were seen in the stones: blue arrowhead indicates the CaCO3 crystals; and red arrowhead indicates the C. sinensis eggs. (A) Clonorchis sinensis eggs in clusters and surrounding calcified particles were seen (original magnification, × 400); and (B) C. sinensis eggs were packaged by mucoid matter or adhered to CaCO3 crystals and bilirubinate granules (original magnification, ×3000).
Table 1. Elemental composition in general stone and in the micro field.

*Gold was from the coating layer.
Parasite tissues were found in 12 gallbladder stones resembling adult C. sinensis worms
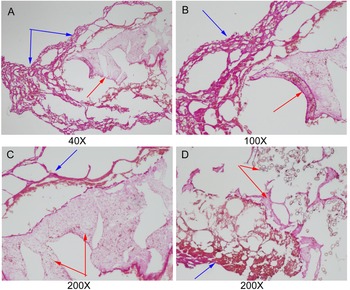
According to the histopathological examination, parasite tissues were found in 12 gallbladder stones resembling adult C. sinensis worms, including seven CaCO3 stones, three bilirubinate–CaCO3 mixed stones and two bilirubinate stones. Under the optical microscope, we found that parasite tissues were surrounded with stone particles or crystals when the slide was magnified × 40 or × 100, and a large number of C. sinensis eggs in clusters could be seen in the parasite tissues when the slide was magnified × 200 (fig. 7).
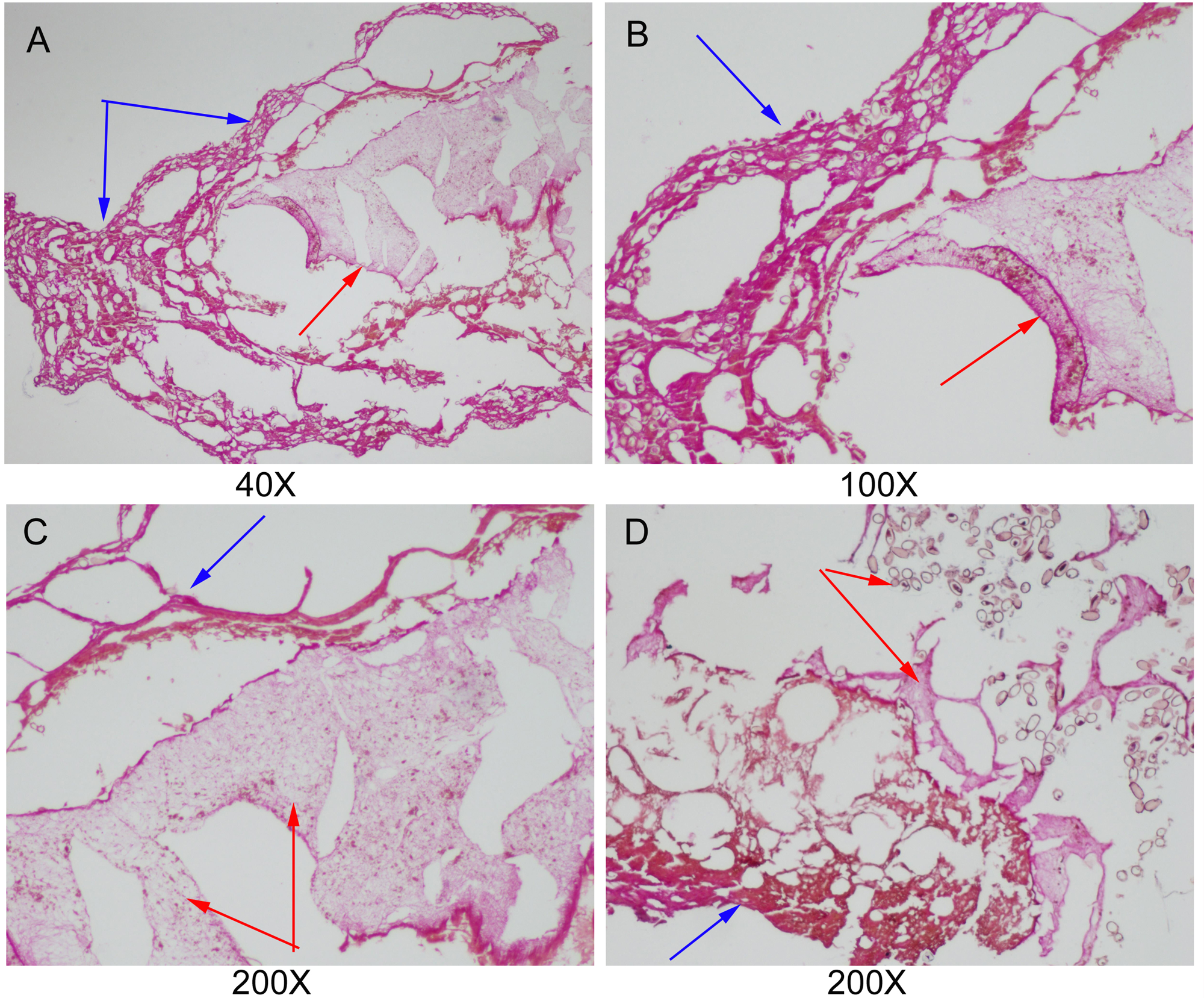
Fig. 7. Parasite tissues were found in gallbladder stones resembling adult Clonorchis sinensis worms: blue arrowhead indicates the stone particles or crystals; and red arrowhead indicates parasite tissues. (A–C) parasite tissues were surrounded with stone particles or crystals (original magnification, ×40×100×200); and (D) a large number of C. sinensis eggs in clusters could be seen in the parasite tissues (original magnification, ×200).
C. sinensis adult worms may be more likely to calcify and form CaCO3 stones or bilirubinate–CaCO3 mixed stones
Of the nine CaCO3 gallbladder stones resembling adult C. sinensis worms, seven cases contained parasite tissue and three of the nine bilirubinate–CaCO3 mixed gallbladder stones resembling adult C. sinensis worms contained parasite tissue, while only two of the 12 bilirubinate stones embraced it. The detection rate of parasite tissues was highest in CaCO3 stones, followed by bilirubinate–CaCO3 mixed stones and bilirubinate stones, with a statistically significant difference (P < 0.05, table 2). A large number of C. sinensis eggs in clusters were observed under optical microscopy and SEM. Parasite tissues were surrounded by CaCO3 crystals and other particles, suggesting that C. sinensis adults may more easily absorb calcium salts or calcify to form CaCO3 stones or bilirubin–CaCO3 mixed stones.
Table 2. The detection of parasite tissues in different types of gallbladder stones resembling adult Clonorchis sinensis worms.

Discussion
Gallbladder stones, as a highly prevalent disease in general populations with substantial hospital admission (Peery et al., Reference Peery, Crockett and Barritt2015; Pape et al., Reference Pape, Swart and Duvenage2019; Joshi et al., Reference Joshi, Singh, Shrestha, Shrestha and Karmacharya2020), were deemed to be formed in a complex process involving multiple factors (Bonfrate et al., Reference Bonfrate, Wang, Garruti and Portincasa2014; Lin et al., Reference Lin, Yang, Wu, Yeh, Liou, Lin and Chiang2014; Shabanzadeh et al., Reference Shabanzadeh, Sørensen and Jørgensen2016; Shabanzadeh, Reference Shabanzadeh2018; Di Ciaula et al., Reference Di Ciaula, Garruti, Frühbeck, De Angelis, de Bari, Wang, Lammert and Portincasa2019; Wang et al., Reference Wang, Hao and Yao2020). Analysis of the chemical components of gallstones contributes extremely great value to analyzing disease progression. They were traditionally divided into cholesterol and pigment (bilirubin) stones (Schafmayer et al., Reference Schafmayer, Hartleb and Tepel2006; Cariati, Reference Cariati2015); however, our previous research overturned this view, as we found that gallbladder stones could be classified into eight types and more than ten subtypes, including cholesterol stones, pigment stones, CaCO3 stones, phosphate stones, calcium stearate stones, protein stones, cystine stones and mixed stones (Qiao et al., Reference Qiao, Ma, Luo, Yang, Luo and Zheng2013b). Further research implied that different types of stones may expose a distinct formation mechanism (Qiao et al., Reference Qiao, Ma, Luo, Luo, Zheng and Yang2013a, Reference Qiao, Ma, Luo, Yang, Luo and Zheng2014; Ma et al., Reference Ma, Luo, Wang, Qiao, Huang and Zhong2017).
According to previous research, gallbladder stones, especially CaCO3 and bilirubinate stones, are associated with C. sinensis infection, but the role of C. sinensis adult worms has not been clarified. In the present study, 33 gallbladder stones resembling adult C. sinensis worms were obtained from cholecystolithiasis patients. The morphology of these stones exhibited a shape and size similar to those of C. sinensis adult worms. These stones aroused our interest, and we intended to explore whether they were stones, worms or even calcified worms. Based on the clinical experience of hepatobiliary surgeons, live C. sinensis adult worms could enter the cystic duct or gallbladder cavity, which is a normal phenomenon under endoscopy, implying that C. sinensis adult worms enter the gallbladder with bile.
Histopathological examination revealed parasite tissues in 12 gallbladder stones resembling C. sinensis adult worms, the main component of which was CaCO3 and bilirubinate according to FTIR analysis. Parasite tissues surrounded with stone particles or CaCO3 crystals could be seen under the optical microscope with a lower magnification, while a large number of C. sinensis eggs in clusters could be seen in the parasite with a higher magnification. Mapping analysis of element distribution in general stones revealed that calcium ions wrapping the core (parasite tissue) were mainly distributed in the outer layer of the stones. Furthermore, SEM revealed a large number of C. sinensis eggs in clusters surrounded by calcified particles. These data suggested that C. sinensis adults may be prone to absorb calcium salts to form CaCO3 stones or bilirubinate–CaCO3 mixed stones.
Tissue analysis studies showed that samples with parasite tissue are derived from early calcification of dead worms, providing strong evidence that calcification and packaging occurred after C. sinensis adults entered the gallbladder, which led to the formation of CaCO3 or bilirubinate–CaCO3 mixed gallbladder stones. On the other hand, those without parasite tissue may result from calcification of C. sinensis eggs or dead worms during long-term formation, while the parasite tissues may be dehydrated and further calcified and degraded due to nutritional deficiency in the stone (Qiao et al., Reference Qiao, Ma, Luo, Zheng, Luo and Yang2013c).
We have developed a sensitive and specific real-time polymerase chain reaction assay for the detection of C. sinensis DNA in adult worms, gallbladder bile and stone samples of patients with cholecystolithiasis (Qiao et al., Reference Qiao, Zheng, Ma, Luo and Luo2012c). Our previous research confirmed that C. sinensis DNA genes could be detected in all egg-positive samples and some egg-negative stones (Qiao et al., Reference Qiao, Zheng, Ma, Luo and Luo2012c, Reference Qiao, Ma, Luo, Zheng, Luo and Yang2013c), which provides strong evidence for our current finding.
A systematic analysis of the stones demonstrated that the main components were CaCO3 and bilirubinate. Clonorchis sinensis eggs were found in 30 of these stones, including all CaCO3 and mixed stones. Moreover, C. sinensis eggs have also been found in the corresponding bile sediment, with a relatively fresh appearance, but the detection rate in bile sediment was lower than that in stones, which was consistent with our previous research (Qiao et al., Reference Qiao, Ma, Luo, Zheng, Luo and Yang2013c). Adult worms parasitize the hepatobiliary duct and ovulate eggs in an intermittent process, most of which enter the gallbladder along with the bile and are not easily eliminated because of hindrance by the valves of Heister. Stone formation is a long process from adherence or wrapping with mucus and granules to dehydration and calcification. Consequently, the detection rate of C. sinensis eggs in stones was higher than that in bile sediments; additionally, the eggs were morphologically ‘older’ in the stone than those found in the bile.
Further analysis demonstrated that CaCO3 was mainly calcite, which was consistent with our previous research. CaCO3 can form three homogeneous polycrystals in nature, including calcite, aragonite and vaterite, among which calcite and aragonite are the main types found in CaCO3 gallstone (Ma et al., Reference Ma, Luo, Wang, Qiao, Huang and Zhong2017). It was supposed that C. sinensis eggs could stimulate mucin secretion, which contributes to the specific appearance of these stones. The released mucin and the muskmelon wrinkles on the surface of eggs easily adhere to CaCO3 crystals and bilirubinate granules, which are mainly calcite CaCO3 (Ma et al., Reference Ma, Luo, Wang, Qiao, Huang and Zhong2017). In addition, C. sinensis adult worms stimulate much more mucin metabolite production, which could absorb bilirubinate particles and calcium salts, thus leading to calcification and the formation of CaCO3 stones or bilirubinate–CaCO3 mixed stones morphologically similar to worms.
In general, we provided straightforward evidence here that entrance of C. sinensis adult worms into the gallbladder would lead to the formation of stones, during which they went through aging and dead, being wrapped with stone particles or crystals enriched in calcium ions. During a long-term process of stone formation, some of the parasite tissues might be destroyed and degraded; thus, only some of the stones in the early stage of formation could be detected by histopathological examination. We further presumed that stones with diverse appearances, such as black melon seed-like or coralliform, were derived from wrapped C. sinensis eggs and other nucleation factors.
Conclusion
Our current data provide evidence of direct calcification and packaging occurring after the entrance of C. sinensis adults into the gallbladder, which leads to the formation of CaCO3 or bilirubinate–CaCO3 mixed gallbladder stones resembling adult worms in the early stage. This finding highlights strong evidence for C. sinensis infection causing gallbladder stones.
Acknowledgements
We are grateful to Yu-yang Feng and Jie-liang Wang in the General Surgery Department of our hospital for kindly providing the specimens. We thank our colleagues in the General Surgery Department and the operating room for their enthusiastic help and the leaders of our hospital for their support.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethics statement
The operation and sample collection procedures were explained to all patients. Informed consent was signed by each patient. The principles outlined in the Declaration of Helsinki of 1975 (revised in 1983) were followed throughout the study period. This research was approved by the Ethics Committee of The Sixth People's Hospital of Nansha, Guangzhou, China.