Recently, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (ESBL-E) have been detected in livestock animals including poultry, pigs and cattle [Reference Hille1]. In Germany, ESBL-E, mostly belonging to the species Escherichia coli and Klebsiella pneumoniae, were detected on 44% and 56% of pig fattening and breeding farms, respectively [Reference Hille1]. Moreover, ESBL-E were found in samples from 100% of broiler farms [Reference Hille1]. Of note, the occurrence of enterobacterial isolates that were able to produce carbapenemases in addition to ESBL was recently reported from a German poultry farm [Reference Guerra, Fischer and Helmuth2].
The emergence of ESBL-E in livestock may have important implications for humans. In the past years, intestinal carriage of ESBL-E has increased in the general population [Reference Valenza3]. In Germany, the prevalence of rectal ESBL-E carriage reached 6·3% in healthy persons [Reference Valenza3]. As enterobacteria (including ESBL-producing E. coli and Klebsiella spp.) are among the most important causes of community-acquired and endogenous healthcare-associated infections of humans, this development raises concern. However, it is still a matter of discussion if and to what extent increasing intestinal ESBL carriage in humans is due to ingestion of contaminated food items or animals in general [Reference Valentin4].
For methicillin-resistant Staphylococcus aureus (MRSA), another facultative pathogen that has emerged in livestock husbandries during the past 10 years [Reference Köck5], multiple investigations found that persons with direct livestock exposure or frequent exposure to dust from livestock holdings are very often colonized with MRSA in the nares. Hence, in European countries, 24–100% of pig farmers, 37% of poultry farmers and 30–38% of cattle farmers are nasally colonized with the same MRSA genotypes that are also predominant in the animals [Reference Köck5]. Moreover, it was shown that short-time exposure to dust, only leads to transient nasal MRSA carriage, while regular exposure leads to stable colonization which persists when contact to the animals is interrupted during holidays [Reference Köck5].
Interestingly, there is little data assessing occupational contact with livestock as a risk factor for ESBL-E carriage. In a Dutch investigation, it was found that 33% of chicken farmers exhibited rectal ESBL-E carriage [Reference Dierikx6]. Although it is well known that Enterobacteriaceae are found not only in the intestinal tract, there is currently no information on nasal carriage of Enterobacteriaceae (and ESBL-E in particular) in farmers. Since this might be a very relevant factor facilitating transmission of ESBL-E in the general population (possibly even more relevant than rectal colonization) via hands and contaminated surfaces, we performed a nasal screening of persons with regular occupational pig exposure. We assessed the prevalence of nasal Enterobacteriaceae carriage, associated species and antimicrobial susceptibility patterns of these isolates.
The investigation was performed between July and December 2014. Pig-exposed persons were sampled (i) at educational training courses for pig farmers in the federal state of North Rhine-Westphalia, Germany and (ii) within the framework of an ongoing cross-sectional study on livestock farms in this federal state. The area (districts DEA33–38 according to the European Nomenclature for Territorial Units) is one of the regions with the highest densities of pig production in Europe.
Swabs (Transwab, Check Diagnostics, Germany) from the anterior nares were obtained from persons having contact with pigs. For every participant, we assessed profession, age, sex, hours of weekly animal contact and farm type using a standardized questionnaire.
Swabs were enriched in nutrient broth (Heipha Diagnostics, Germany, without antibiotic pre-selection) for 24 h at 36 °C and then streaked on MacConkey agar (Oxoid, Germany) and a colorimetric medium for ESBL-screening (bioMérieux, France), which were both incubated for 24 h. Phenotypically different colonies from MacConkey agar and all colonies from ESBL-screening agar were subcultured on Columbia blood agar. Species identification was performed using matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-ToF MS, Bruker Daltonik, Germany). For all Enterobacteriaceae, antimicrobial susceptibility testing was done by agar disc diffusion as recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) applying EUCAST clinical breakpoints for categorization of susceptible, intermediate and resistant isolates. If an isolate tested intermediate or resistant to either cefotaxime or ceftazidime, a double-disk diffusion test (MAST Laboratories, UK) including third-generation cephalosporins (cefpodoxime, ceftazidime, cefotaxime) with and without combination of clavulanic acid was performed to confirm ESBL production.
Pearson's χ 2 test and Mann–Whitney U test were used as a first description of statistical differences (SPSS v. 22, SPSS Inc., USA). Ethical agreement was obtained from the Chamber of Physicians of Westfalen-Lippe and the Medical Faculty of the University of Münster (2014-143-f-S). Written informed consent was obtained from each participant prior to enrolment.
Nasal swabs from 114 persons were analysed. Of these, 110 (96·5%) were men. Median age was 42 years (mean 42 years, range 18–77 years). Of all participants, 114/114 (100%) had contact with pigs, and 18/114 (15·8%) had contact with cattle in addition. The average exposure time to pigs was as follows: <20 h/week (n = 40 persons, 35·1%), 20–39 h/week (n = 49, 43·0%), ⩾40 h/week (n = 25, 21·9%). For all participants pig contact was due to work on pig fattening farms (n = 54, 47·4%), on farrow-to-finishing farms (n = 47, 41·2%) or on farrow-to-weaning farms (n = 7, 6·1%). Six (5·3%) participants were not farmers, but worked as pig-care veterinarians or farm consultants for the optimization of the pig production system.
Enterobacteriaceae were detected in the nares of 76/114 participants (66·7%). Weekly pig contact time did not influence the nasal prevalence of Enterobacteriaceae with a prevalence of 0·7 for <20 h/week (28 persons colonized vs. 12 persons not colonized), 0·64 for 20–39 h/week (32 vs. 17 persons), and 0·65 for ⩾40 h/week (16 vs. 9 persons, P = 0·85). Carriage of Enterobacteriaceae was 72% in persons working on farrow-to-finishing farms, 61% on fattening farms and 57% on farrow-to-weaning farms, and was 83% in the six persons who were not farmers (P = 0·48). The median age of persons with Enterobacteriaceae carriage vs. persons without carriage (44 years vs. 38 years) did not differ significantly (P = 0·11). Farmers who had contact with cattle in addition to pigs had enterobacterial carriage rates similar to those who were only pig-exposed (13/18 vs. 63/96, P = 0·79).
Seventeen (14·9%) of the 114 different participants carried Proteus mirabilis, 13 (11·4%) Pantoea agglomerans, nine (7·9%) Morganella morganii, nine (7·9%) Citrobacter koseri, eight (7·0%) K. pneumoniae, eight (7·0%) E. coli, eight (7·0%) Proteus vulgaris, four (3·5%) Enterobacter aerogenes, three Enterobacter cloacae complex (2·6%), three Citrobacter freundii (2·6%), three Klebsiella oxytoca (2·6%), two Raoultella ornithinolytica (1·8%) and one participant Citrobacter braakii (0·9%).
Twelve of the 76 persons (15·8%) carrying Enterobacteriaceae in the anterior nares, carried two different enterobacterial species [P. mirabilis/K. pneumoniae (n = 3), P. vulgaris/E. coli (n = 2), P. mirabilis/E. coli (n = 2), and E. aerogenes/C. braakii, M. morganii/K. oxytoca, M. morganii/K. pneumoniae, P. mirabilis/C. koseri, P. vulgaris/K. pneumoniae (each n = 1)].
Overall, we retrieved 88 nasal bacterial isolates with the species distribution shown in Figure 1. The antimicrobial susceptibility pattern of all isolates belonging to different species is shown in Table 1. Resistance against carbapenems was not detected. Of all Enterobacteriaceae, only one isolate of M. morganii and one isolate of C. koseri grew on ESBL screening agar. These isolates were intermediate or resistant to third-generation cephalosporins indicative of ESBL production. However, ESBL production was not confirmed by double-disk diffusion test for these two isolates. The overall rates of resistance of all isolates tested were: ciprofloxacin (3·4%), gentamicin (2·3%), trimethoprim-sulfamethoxazole (23·9%) and tigecycline (44·3%), respectively. However, tigecycline was considered intrinsically resistant for all Morganella spp. and Proteus spp. isolates according to EUCAST test standards. Tigecycline resistance in isolates of enterobacterial species other than Proteus spp. and Morganella spp. was only 9·3% (5/54).
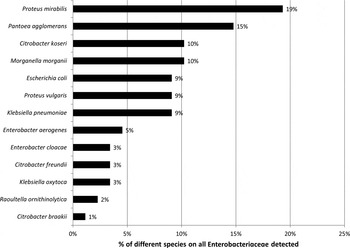
Fig. 1. Proportion of different species in Enterobacteriaceae (n = 88 isolates) from the anterior nares of 76 pig-exposed individuals.
Table 1. Antimicrobial susceptibility of Enterobacteriaceae isolated from the anterior nares of pig-exposed persons

CTX, Cefotaxime; CTZ, ceftazidime; MER, meropenem; CIP, ciprofloxacin; SXT, trimethoprim-sulfamethoxazole; GEN, gentamicin; TGC, tigecycline.
Percentage of susceptible isolates for the respective antibiotic. Intermediate test results are counted as non-susceptible. Table sorted by numbers of isolates (n) tested per species.
Data on the occurrence of Enterobacteriaceae in the anterior nares is rare. Single reports found that this affects 4–38% of the population [Reference Glück and Gebbers7–Reference Frank9]. We are not aware of any reports on occupation-related ESBL-E carriage of the nares. Hence, this study was planned to evaluate whether the nares represent a potential reservoir for ESBL-E, which is neglected by current investigations. As multiple studies found ESBL-E in dust samples from livestock farms [Reference Hering10], we hypothesized that inhalation of dust directly exposes the anterior nares, which could lead to colonization or contamination with ESBL-E. Besides the classically considered oral transmission route facilitating intestinal colonization this may represent an additional route of transmission, which has not yet been studied.
Indeed, we found that 67% of farmers and other pig-exposed persons carried Enterobacteriaceae in their nares. This prevalence was higher than expected compared to the few data assessments elucidating this issue: In Switzerland, Klebsiella spp., E. coli, E. cloacae and Citrobacter diversus were isolated from nasal specimens in 38% of healthy men [Reference Glück and Gebbers7].
However, when considering the antibiotic susceptibility profiles of the nasal isolates, we did not identify any person who carried ESBL-E. This was surprising, because ESBL-E was detected in high frequencies in the environment of pig farms in Germany [Reference Hille1] and rectal ESBL-E carriage involves up to 6·3% of persons in the human general population [Reference Valenza3]. However, ESBL-E distributed both on farms and in faecal samples from the general population are mostly E. coli or K. pneumonia or K. oxytoca [Reference Hille1, Reference Valenza3]. These species were also detected in the nares of participants included in this study, but represented only 22% (n = 19 isolates) of the nasal Enterobacteriaceae. The majority of isolates belonged to other species such as Proteus spp., Pantoea sp., Citrobacter spp. or Morganella spp. Hence, it can be concluded that ESBLs were not found in these four predominating species and that ESBLs were not detected in a limited number of isolates associated with ‘classical’ ESBL-bearing species tested. This argues against a wide distribution of ESBL-E as nasal colonizers, which is in contrast to one study suggesting rather high (33%) rectal ESBL-E carriage in Dutch poultry farmers [Reference Dierikx6]. For the two isolates in which cefotaxime and ceftazidime resistance was detected (Morganella spp. and Citrobacter spp.), phenotypic confirmatory tests indicated that this resistance was most likely not due to ESBL production, but related to other mechanisms such as ampC β-lactamase production, which is known to be frequent in isolates of these two species [Reference Harris and Ferguson11].
Resistance to antibiotics other than third-generation cephalosporins also differed from clinical data: nasal Enterobacteriaceae showed very low resistance rates to ciprofloxacin (3·4% of all isolates) and to gentamicin (2·3%). This is in contrast to isolates from diagnostic specimens related to infections. German national surveillance data demonstrated that ciprofloxacin and gentamicin resistance involved 9·0% and 4·1% of isolates in 2013 in K. pneumoniae, or 16·5% and 5·4% in E. coli, respectively (http://ars.rki; ARS.RKI reference data for ambulatory care, 2013, data assessed 16 December 2014). Resistance to trimethoprim-sulfamethoxazole was more frequent in nasal enterobacterial isolates (23·9%) and was comparable to susceptibility levels of clinical isolates included in the reference database (11·5% of K. pneumoniae, 25·3% for E. coli). Of note, carbapenem resistance was not detected, which is important considering the first report of carbapenemase-producing Enterobacteriaceae in German livestock holdings [Reference Guerra, Fischer and Helmuth2].
This study has three important limitations. First, we have no information on the occurrence of ESBL-E in animals, dust or manure samples from the farms, where the persons included in this study were working. However, other recent studies performed in the same geographical area have demonstrated that almost all (85%) pig farms were affected by ESBL-E [Reference Hering10]. Second, we did not simultaneously assess rectal carriage of ESBL-E in the farmers. We focused on nasal carriage since we considered this body site as highly relevant for the transmission to other persons (not working with livestock) via the hands or indirectly via contamination of surfaces (in households, public transport, healthcare facilities, etc.). However, there is a need to address the topic of occupation-related rectal ESBL-E carriage in farmers or veterinarians in further studies, as there is only very limited data regarding this issue and rectal colonization can be a source for endogenous infections. The third limitation is that the study design does not allow for differentiating between persistent colonization and short-term contamination or carriage of the nares. To answer this question consecutive sampling in a cohort of pig-exposed persons over time is needed.
In conclusion, we found high rates of nasal carriage of Enterobacteriaceae in German pig farmers. However, ESBL-E was not detected. This data suggests that, in contrast to MRSA, the nares are not a major reservoir for carriage of ESBL-E in pig-exposed persons.
ACKNOWLEDGEMENTS
This study was performed as a cooperative effort of the research consortia RESET (no. 01Kl1013C) and MedVet-Staph (no. 01KI1301A) both funded by the German Ministry of Education and Research (BMBF).
DECLARATION OF INTEREST
None.







