Introduction
Febrile seizure (FS) is a frequent cause of emergency attendance in children. Worldwide, the prevalence of FS ranges from 2% to 5% [Reference Verity, Butler and Golding1, Reference Kjeldsen2]. The young age and sudden onset leads FS to be a major cause of parental anxiety, resulting in emergency attendance [Reference Baumer3].
Hand, foot and mouth disease (HFMD) is a highly prevalent paediatric disease in many Asian countries, caused by enteroviruses belonging to the genus of the Picornavirus family. Enterovirus genotypes enterovirus 71 (EV71) and coxsackievirus A16 (CA16) belonging to species A are the most common causes of HFMD worldwide. Other enterovirus types such as CA2, CA4, CA5, CA6, CA8, CA10 and CA12 from species A, CB2 and CB4 from species B and some echoviruses can cause HFMD as well [Reference Xu4]. Most HFMD infections are mild and self-limiting [5]. However, HFMD caused by EV71 infection is relatively severe, leading to occasional neurological involvement and death [Reference Lee6]. The age range for infection with HFMD coincides closely with that for FS: the latter occurring between 6 months and 6 years with a median age of 18 months [Reference Offringa7], while most cases of HFMD occur prior to the start of formal schooling in China [Reference Zhang8], Japan [Reference Momoki9], Singapore [10] and other countries. FS has been more commonly observed in patients infected with EV71 [Reference McMinn11]. However, FS has also been observed among patients with infection caused by coxsackieviruses such as CA2, CA6, CA10 and so on [Reference Francis12].
In addition, FS has been associated with infections with influenza, parainfluenza, adenovirus, rotavirus and human herpes viruses [Reference Chung and Wong13]. Influenza infection is usually self-limiting with recovery within a week in most people, but among people at high risk, including pregnant women, children under the age of 6, the elderly, individuals with chronic medical conditions and individuals with immunosuppressive conditions, and influenza can cause severe illness and even death [Reference Ghebrehewet, MacPherson and Ho14]. It has been estimated that healthy children under the age of 5 have the highest influenza admission rate [Reference Cromer15]. It has previously been demonstrated that influenza A infection is an important cause of FS [Reference Chiu16].
In tropical Singapore, both HFMD and influenza are endemic and year-round transmission occurs [10]. HFMD and influenza outbreaks are irregular, compared to the annual or bi-annual outbreaks in temperate and subtropical countries [Reference Viboud, Alonso and Simonsen17]. HFMD is a notifiable disease, with notification of cases mandatory by both clinicians and preschools; in the latter, children are screened daily for symptoms and isolated for 10 days if infected. Epidemics of HFMD and other respiratory infections are frequent and, as such, Singapore offers a useful opportunity to assess the relative role of EVs and respiratory viruses on FS, which also exhibit temporal fluctuations both within and between years, with less potential for confounding due to seasonal synchronicity of other viruses than in temperate countries.
To assess the magnitude of association between FS and HFMD and respiratory viruses, we conducted a retrospective analysis of paediatric accident and emergency (A&E) attendance for FS at Singapore's largest paediatric hospital, KK Women's and Children's Hospital (KKH) – which admits about 55% of paediatric patients in Singapore, using KKH laboratory respiratory virus data (on influenza A and B, parainfluenza 1, 2 and 3, respiratory syncytial virus (RSV) and adenovirus) and Ministry of Health (MOH) surveillance data on HFMD disease from 2004 to 2012. The objective was to determine the temporal relationship between occurrences of HFMD cases, respiratory virus detection and FS among children.
Materials and methods
This was a retrospective time-series analysis of three sets of existing databases from January 2004 to December 2012: KKH A&E attendance and hospital admissions for FS; KKH Laboratory data of respiratory viruses and cases of HFMD notified to MOH.
Data sources
Three age-structured time-series data sets were created and merged. For the hospitalisation data set, raw, abstracted data from de-identified individual cases was grouped into weekly count data of A&E and hospital admission for FS from KKH records, together with epilepsy as a control condition that should not be affected by circulating viruses. The second data set consists of weekly counts of HFMD cases from the MOH data. The third data set contains weekly counts of specific respiratory viruses from KKH laboratory.
FS: KKH A&E created a database of extracted cases having the following principal or secondary diagnosis based on International Classification of Diseases (ICD) (9th edition) codes: 780.32 (complex FS); 780.31 (simple FS); 345.9 (epilepsy) and 345.8 (other forms of epilepsy and recurrent seizure) from 2004 to 2011. In 2012 where the more recent ICD-10 edition was used to code discharge data, cases were extracted based on the corresponding ICD-10 code: R 56.8 (seizure/fit), R 56.0 (FS (simple or complex)), G 40.90, G 40.91, G 40.10, G 40.40, G 40.80 and G 40.11 (other epilepsy and recurrent seizures). Because the inpatient FS data for 2012 was not consistent with that of previous years, presumably due to the switch to ICD-10, we excluded it from analysis.
HFMD surveillance: HFMD cases were reported by physicians, educational institutions and laboratories. Age-stratified aggregate weekly counts of notified HFMD cases were obtained from the MOH.
Other virus surveillance: KKH laboratory had data on respiratory viruses detected from clinical specimens from children who were admitted to KKH for various conditions. The antigen detection tests identified a panel of respiratory viruses including Influenza A, B, parainfluenza 1,2,3, adenovirus and RSV tested by immunofluorescence and obtained by nasopharyngeal swabs of patients admitted for upper or lower respiratory tract infections. The sensitivity and specificity of immunofluorescence have been estimated to be 85.9% and 87.1%, respectively, when compared to standard viral culture [Reference Matthey18].
Statistical analysis
Four multivariate linear regression analyses were conducted to investigate how much each virus contributed to FS or epilepsy hospital cases. The dependent variables were taken to be weekly counts of FS or epilepsy at A&E or inpatient admissions. Weekly counts of HFMD and other viruses were used as independent variables. The dependent variables were regressed with all potential viruses and significant viruses were identified at a significance level of 0.05. Only the significant viruses were included in the final multivariate regression models to measure the effect size of each virus.
Relative risks of FS and epilepsy by age were calculated using Bayesian approaches [Reference Gelman19]. We approximated the number of children in each age group assuming the population to be constant (the number being unknown: the distribution at exact ages is known for the resident population at census years only, but the non-resident population is large in Singapore). We assigned a non-informative, conjugate Dirichlet prior for the risk of infection, then used Monte Carlo sampling to draw samples from the resulting Dirichlet posterior whereby the relative risk and corresponding 95% credible intervals were obtained.
Statistical analyses were performed using the R Statistical Software (version 3.1.1) [20].
Results
Yearly numbers of A&E attendance and admission for FS and epilepsy, as well as yearly number of HFMD cases, are detailed in Table 1. Among all incidences of FS, a total of 95% were younger than 6 years old, of whom, 12% were in the <1 year age group, 62% in the 1–2 year age group and 21% in the 2–5 year age group.
Table 1. Detailed yearly number of A&E and inpatient FS and epilepsy, and yearly number of HFMD cases from 2004 to 2012
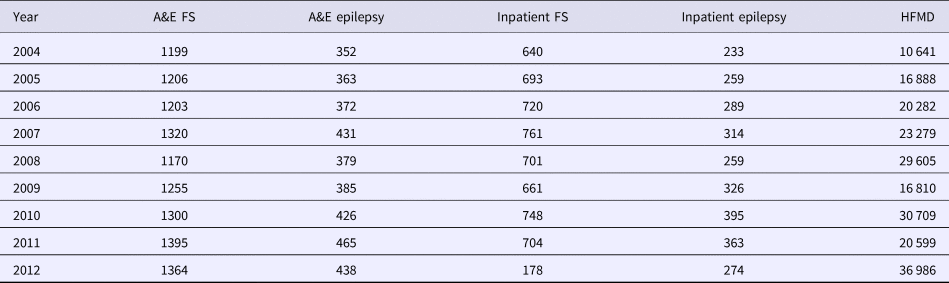
A&E, Paediatric accident and emergency; FS, febrile seizures; HFMD, hand, foot and mouth disease.
Weekly numbers of various respiratory viruses detected during 2004 and 2012 are shown in Figure 1. The bottom right panel shows the total number of viruses detected, whereas all other panels show the number detected for each virus, respectively.
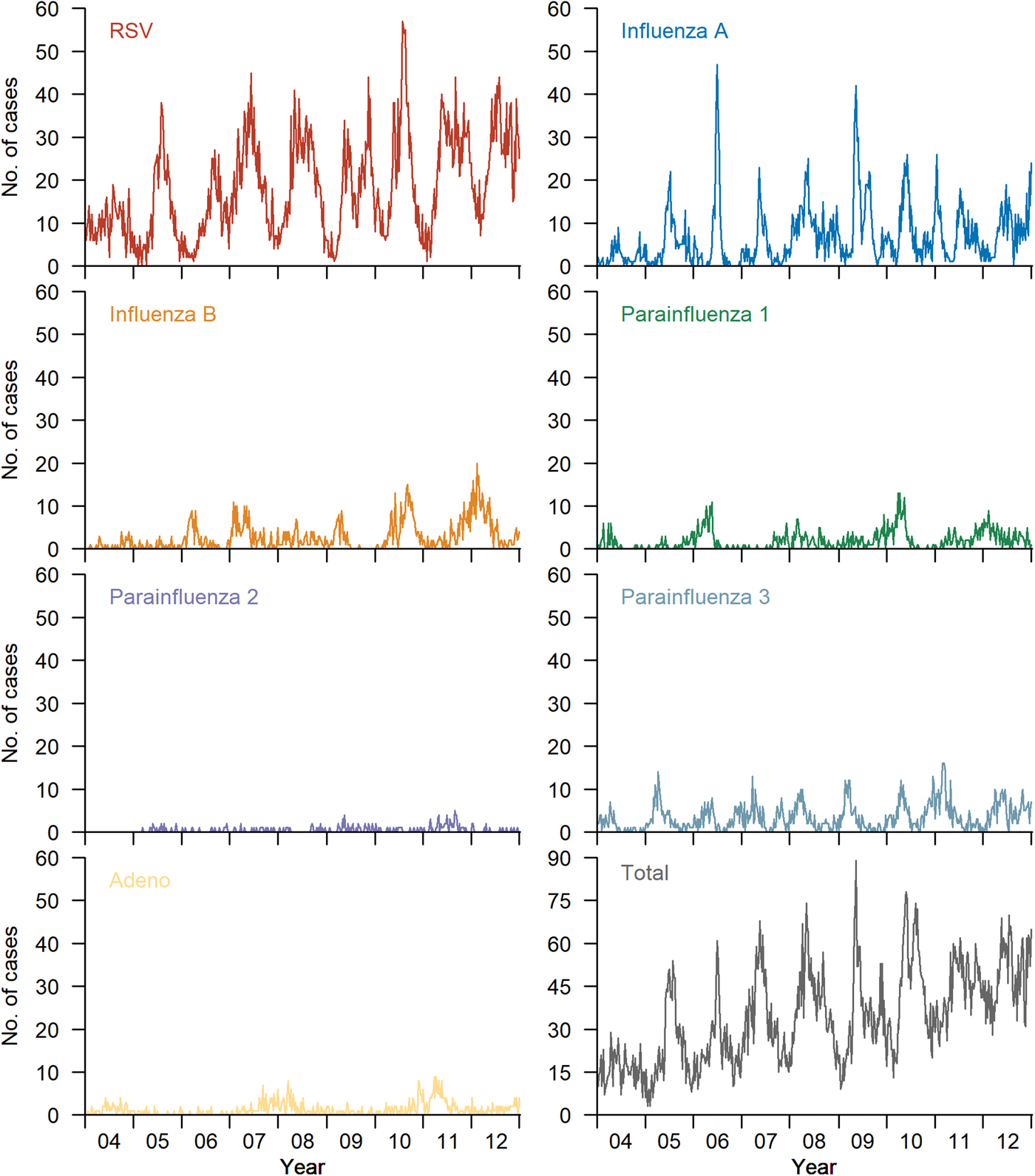
Fig. 1. Weekly number of various respiratory viruses confirmed by KKH laboratory from 2004 to 2012. The bottom right panel shows the total number of viruses detected. All other panels show the number detected for each virus, respectively.
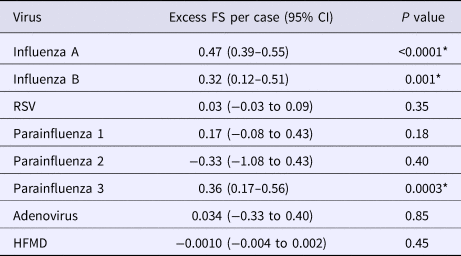
FS was significantly associated with influenza A, influenza B and parainfluenza 3 epidemic activity (Table 2). No association was observed with parainfluenza 1 and 2, RSV, adenovirus and HFMD. The three contributing viruses were then used to form a final multivariate model with similar estimates to the full model containing all the viruses (Table 3). In contrast, HFMD activity was not associated with an increase in A&E FS.
Table 2. Summary of A&E FS cases explained by all viruses, 2004–2012
A&E, Paediatric accident and emergency; FS, febrile seizures; HFMD, hand, foot and mouth disease; RSV, respiratory syncytial virus.
Table 3. Summary of A&E FS cases explained by significant viruses, 2004–2012

A&E, Paediatric accident and emergency; FS, febrile seizures.
The multivariate model using influenza A, B and parainfluenza 3 was able to explain the majority of the excess FS cases above baseline for both inpatient and A&E FS (Fig. 2). Each influenza A case was associated with 0.47 (95% CI (0.40–0.55)) excess A&E FS (P-value<0.001), slightly more than influenza B (0.32, 95% CI (0.16–0.49), P-value = 0.001) and parainfluenza 3 (0.35, 95% CI (0.17–0.52), P-value = 0.001). In contrast, and as expected, there was no association between epilepsy attendance and any of the other viruses.
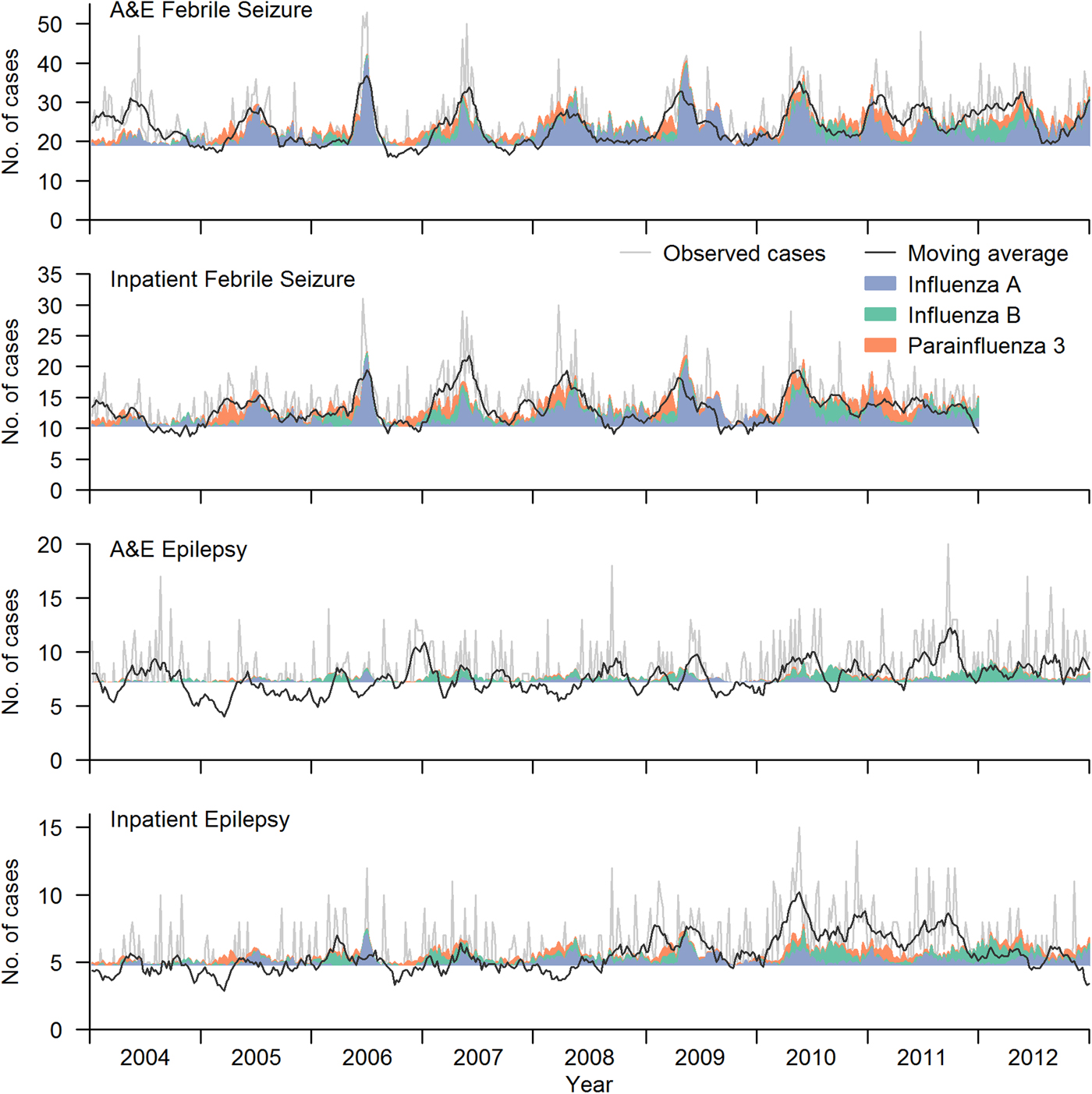
Fig. 2. Number of excess FS cases above baseline that can be explained by one extra case of each virus. Baseline level is indicated by the horizontal line. The excess number explained by influenza virus A, B and parainfluenza virus 3 are shown in blue, green and orange shaded regions, respectively. Observed time series is shown in light grey line in each panel, and a moving average of the number of cases (up to 4 weeks before and after) is overlaid as the black line on top.
Relative risks for children of different ages are shown in Figure 3, using age 0 as baseline group. The relative risk of FS is higher than epilepsy for 1-year-old children, but it decreased rapidly as age increased. The incidence of epilepsy decreased slightly with increasing age and stabilised after 3 years of age.
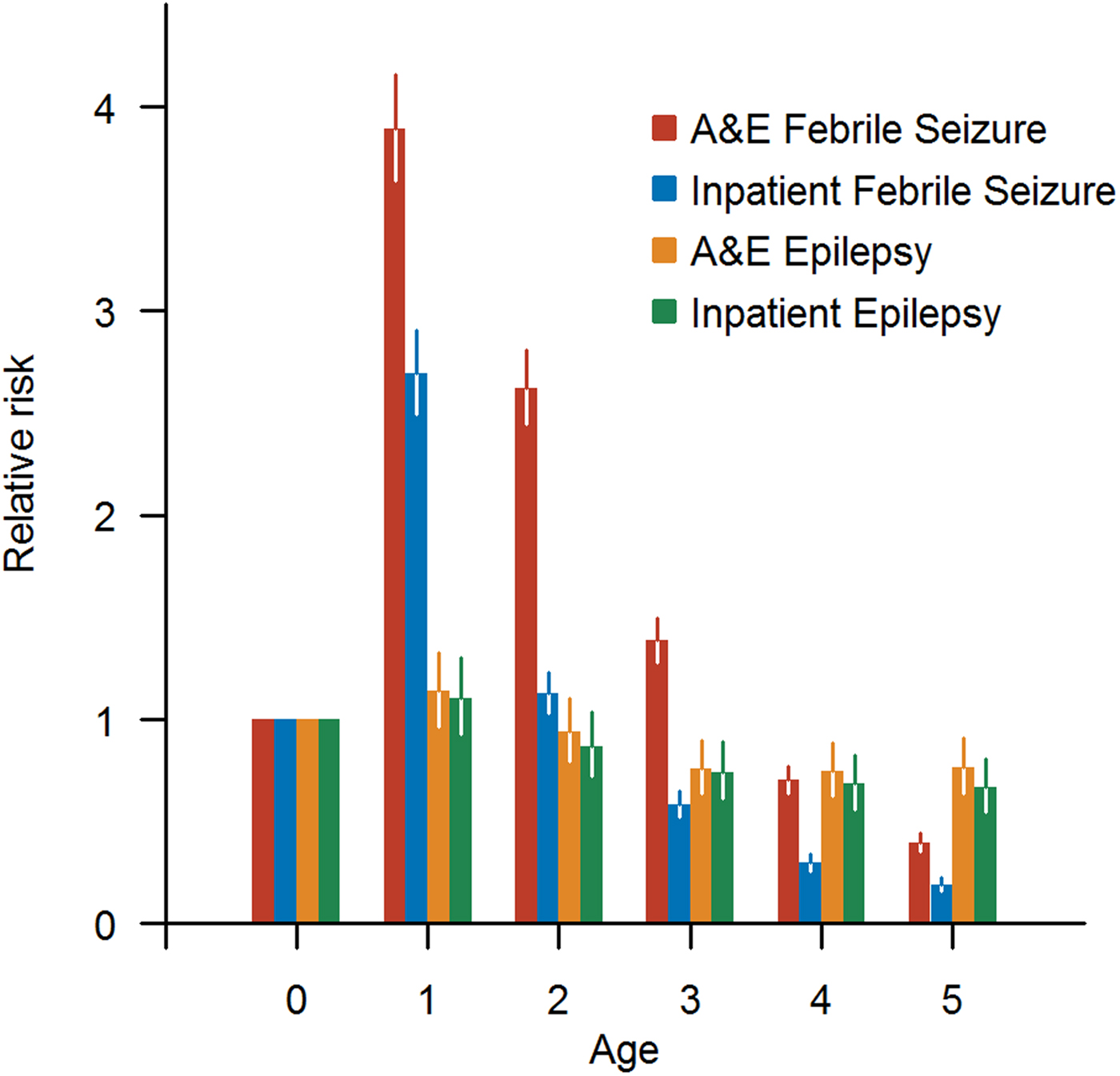
Fig. 3. Relative risk for children of different ages to have A&E/inpatient FS and A&E/inpatient epilepsy, respectively.
Discussion
In contrast to our initial expectations, we did not find an association between HFMD incidence and FS. The data instead revealed a relatively strong association between FS and three respiratory viruses, two of which are vaccine preventable: influenza A and B and parainfluenza 3.
Possible reasons for children developing FS include the structural immaturity of a child's brain which is therefore more susceptible to seizures triggered by minor insults such as systemic viral infections [Reference Jensen, Sanchez, Baram and Shinnar21]. This is supported by the age distribution of FS cases in our data, the incidence of which was the highest among younger patients, in contrast to epilepsy cases for which the relative risk was almost constant with age.
Although neurological involvement is one of the hallmarks of severe EV71 infection [Reference Koh22], and one of the major motivations behind the recent development of a vaccine in China, where more than 15 00 000 children are infected annually [23]. Cases of neurological complications due to EV71 infection have also been reported in Japan, Taiwan, South Korea and Australia [Reference McMinn11, Reference Komatsu24–Reference Ryu26]. Indeed, the risk of severe outcomes has led to the implementation of school closures, and mandatory screening and isolation of infected cases in Singapore [Reference Siegel, Cook and La27]. As a result of this well-established connection, we expected that FS might be associated with HFMD given the high incidence in Singapore, where between 24% and 32% are estimated to be infected with EV71 by the time they reach school age [Reference Ang28], and the ostensible presence of epidemics of FS cases over time, a feature indicative of an infectious aetiology. This association, however, was not established from our data.
On the other hand, for influenza, FS constitutes the majority of neurologic complications [Reference Ekstrand29], followed by encephalopathy, encephalitis, acute necrotising encephalitis, Reye's syndrome, acute disseminated encephalomyelitis, transverse myelitis and Guillain–Barré syndrome [Reference Toovey30]. Both mild and severe neurological complications have been reported to occur in children in other settings during the 2009 influenza A H1N1 pandemic [Reference Blanton31]. In other case series, about 5–19% of children with pandemic H1N1 Influenza presented with neurologic manifestations [Reference Calitri32]. FS is also common among children with seasonal influenza infection, occurring in ~20% of children hospitalised with influenza [Reference Toovey30], while the incidence of A&E presentations for seizures increased by 6.7/1 00 000 among children under 6 years and that of ambulance calls for seizures increased by 3.2/1 00 000 [Reference Polkinghorne33]. Chiu et al. [Reference Chiu16] found in a retrospective hospital-based study in Hong Kong, China, that the risk of FS was high with influenza and not with other respiratory viruses and was associated with repeated seizures in the same febrile episode.
Although less well studied, parainfluenza has also been associated with FS in a historic paper from England [Reference Downham, McQuillin and Gardner34], and more recently [Reference Chung and Wong13] has been demonstrated to have a similar risk profile to influenza and adenovirus for FS, though in our study, adenovirus epidemic activity was not positively associated with FS. Given the lower incidence of parainfluenza 3 (among hospitalised cases), it has relatively lower impact than influenza A and B, both of which are widespread in Singapore and capable of causing epidemics throughout the year [35].
The incidence of FS has previously been estimated to vary across settings, from 0.5 to 1.5% in China, 2.2 to 2.3% in North America and 8.8% in Japan [Reference Hauser36], and while relatively uncommon, the distress caused to families can be substantial. Given the not-insubstantial relationship with influenza epidemic activity demonstrated in this paper, future cost-effectiveness analyses for influenza vaccination or other interventions should consider paediatric FS as an outcome, both for the health and financial impacts. Influenza vaccination is recommended for young children [37] but uptake rates are low – 27% for children between 6 months and 8 years old in the United States [Reference Lu38] and even lower in Singapore [Reference Ang39]. In Singapore, substantial effort is put into surveillance and control of HFMD outbreaks, but rather less on influenza among children. This study did not find any evidence that the former contributed to FS incidence. Given the mismatch between mortality and complications attributed to influenza and due to HFMD, there is an argument for reviewing control policies that include mandatory isolation of infected children for 10 days, daily screening, ‘naming and shaming’ schools with sustained outbreaks online and closing schools with outbreaks for disinfection.
The primary limitation of this study is that the HFMD data were based on notifications to MOH, and thus were not proven to be positive for enterovirus and may have included some cases of herpangina or misidentified herpes simplex stomatitis or other mistaken febrile exanthems. In addition, some HFMD cases may not be accounted for due to misdiagnosis of HFMD. As a retrospective study, the FS diagnoses extracted from ICD coding may potentially have led to some missing or inaccurate diagnoses. Another limitation is that the laboratory method for diagnosis of respiratory viruses was based on antigen detection test, which is less accurate than the gold standard nucleic acid amplification test [Reference Ginocchio and McAdam40]. The analysis was ecological, which limits the strength of inference, but this is countered by the close correlation of the fitted model to the FS data. It is unlikely this is due to overfitting as the same concordance is not observed for epilepsy incidence. Another limitation of this study was our inability to differentiate between first episodes and recurrent FS. We did not look at underlying neurologic disease or structural abnormalities in this cohort, and this would be a useful avenue for further research.
Conclusions
Excess FS was associated with influenza A and B and parainfluenza 3, but neither with HFMD nor other respiratory viruses. There was no association between epilepsy and any of the viruses. This age group is already recommended to receive seasonal vaccination and the association with FS strengthens the argument for routine seasonal influenza vaccination. A valuable extension to the work presented herein would be a prospective study that includes laboratory diagnosis of enterovirus and enterovirus typing to confirm the conclusion that HFMD associated with different genotypes was not associated with FS and to validate the association between influenza, parainfluenza 3 and FS.
Acknowledgements
The work was partly funded by a grant from the Ministry of Health Services Research, Singapore (HSRG12MAY023, to ARC and HHL) and the Singapore Infectious Disease Initiative (to HHL).
Conflict of interest
None.










