Introduction
Weedy rice (Oryza sativa L.), also commonly referred to as “red rice” due to its red grain pericarp, is a noxious weed classified as a rice (Oryza sativa L.) companion weed worldwide (Fogliatto et al. Reference Fogliatto, Ferrero and Vidotto2020; Nadir et al. Reference Nadir, Xiong, Zhu, Zhang, Xu, Li, Dongchen, Henry, Guo, Khan, Suh, Lee and Chen2017). Weedy rice infestation began its significant global spread after the shift from rice transplanting to direct seeding, which was fueled by water scarcity and the high labor costs associated with hand or mechanical weeding (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan and Menezes2015). Weedy rice is characterized by a high genetic divergence that results in morphological and phenological differences across populations with specific traits which contribute to their weediness (Fogliatto et al. Reference Fogliatto, Vidotto and Ferrero2011, Reference Fogliatto, Vidotto and Ferrero2012; Grimm et al. Reference Grimm, Sahi, Amann, Vidotto, Fogliatto, Devos, Ferrero and Nick2020; Kanapeckas et al. Reference Kanapeckas, Tseng, Vigueira, Ortiz, Bridges, Burgos, Fischer and Lawton-Rauh2018).
Several effective weed control tools are currently available for irrigated rice. However, some weeds, such as weedy rice, are particularly recalcitrant and can escape control and produce seeds (Davis et al. Reference Davis, Scott and Dickson2012; Kraehmer et al. Reference Kraehmer, Jabran, Mennan and Chauhan2016; Zhang et al. Reference Zhang, Linscombe, Webster, Tan and Oard2006). In rice, more than 70% to 80% of seeds are easily shed before and during rice harvest, with shedding also facilitated by rain and wind (Ferrero and Vidotto Reference Ferrero and Vidotto1998; Nadir et al. Reference Nadir, Xiong, Zhu, Zhang, Xu, Li, Dongchen, Henry, Guo, Khan, Suh, Lee and Chen2017). A high weedy rice seed load forms a persistent soil seedbank, resulting in increased weedy rice infestation across many years (Chen et al. Reference Chen, Ge, Chu, Xu, Yan, Zhang and Wang2017; Kraehmer et al. Reference Kraehmer, Jabran, Mennan and Chauhan2016). Weedy rice seeds are also added to the soil seedbank through contaminated commercial or saved seed (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020; Fogliatto et al. Reference Fogliatto, Vidotto and Ferrero2012; Rao et al. Reference Rao, Brainard, Kumar, Ladha and Johnson2017).
Weedy rice seed load in fields may exceed 1,000 seeds m−2 (Delouche et al. Reference Delouche, Burgos, Gealy, Zorrilla and Labrada2007; Marchezan et al. Reference Marchezan, Oliveira, Avila and Bundt2003; Zhang et al. Reference Zhang, Dai, Song and Qiang2014). Normally, seeds remain on the soil surface after shattering until the subsequent season (Fogliatto et al. Reference Fogliatto, Ferrero and Vidotto2020). Avoiding fall tillage may reduce the weedy rice soil seedbank, as it creates more opportunities for predation (birds, rodents, arthropods) and exposes seeds to harsh winter temperatures, resulting in loss of seed viability (Fogliatto et al. Reference Fogliatto, Vidotto and Ferrero2010, Reference Fogliatto, Vidotto and Ferrero2011; Rao et al. Reference Rao, Brainard, Kumar, Ladha and Johnson2017; Zhang et al. Reference Zhang, Gao, Dai, Song, Hu and Qiang2019).
Weed seed burial into the soil profile can prolong seedbank viability, as temperature variations and water and gas exchanges are reduced beneath the surface (Chauhan Reference Chauhan2012; Ghosh et al. Reference Ghosh, Rathore, Brahmachari, Singh and Kumar2017; Rao et al. Reference Rao, Brainard, Kumar, Ladha and Johnson2017). An inverse relationship between weedy rice emergence rate and depth of seed burial has been found: seeds lying on the soil surface showed an emergence rate of about 51% as opposed to only about 13% at a depth of 10 cm (Vidotto and Ferrero Reference Vidotto and Ferrero2000). Crop rotation has proved to be one of the most effective systems to reduce the evolution of a specialized, highly competitive weed flora and to control overall infestation levels (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020; Kanatas Reference Kanatas2020; Stein and Steinmann Reference Stein and Steinmann2018). Previous studies found a significant decline in the weedy rice seedbank with rice rotation with non-flooded crops and application of an effective herbicide (Scherner et al. Reference Scherner, Schreiber, Andres, Concenço, Martins and Pitol2018; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan and Menezes2015). Davis et al. (Reference Davis, Scott and Dickson2012) have suggested that fallow programs coupled with soybean [Glycine max (L.) Merr.] rotation could provide 100% weedy rice control. However, in many rice areas, such as in Italy, crop rotation is seldom practiced because of poorly drained soils (Fogliatto et al. Reference Fogliatto, Vidotto and Ferrero2011). A recent survey conducted in the Italian rice area revealed that crop rotation was practiced only in 14% of the surveyed farms (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020).
The introduction of imidazolinone (IMI)-resistant rice varieties and the use of IMI herbicides—patented as the Clearfield® rice technology (CL)—have resulted in good weedy rice control in rice POST (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020). The herbicide for IMI-resistant rice varieties in Italy is imazamox; in other rice-growing areas of the world, imazethapyr, imazapyr, and imazapic are also labeled for CL rice (Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). Acetolactate synthase (ALS)-inhibiting herbicides are a group of broad-spectrum herbicides that are also effective on weedy rice. This mechanism of action in association with the limited rotation of rice with other crops exerts a strong pressure for selection of ALS inhibitor–resistant weed populations (Durand-Morat and Nalley Reference Durand-Morat and Nalley2019). Indeed, cases of resistance have been confirmed in weedy rice (Fogliatto et al. Reference Fogliatto, Serra, Patrucco, Milan and Vidotto2019; Singh et al. Reference Singh, Singh, Black, Boyett, Basu, Gealy, Gbur, Pereira, Scott, Caicedo and Burgos2017) and Echinochloa spp. (Dalazen et al. Reference Dalazen, Pisoni, Bredemeier, de Avila and Merotto2020; Serra et al. Reference Serra, Fogliatto and Vidotto2018; Vidotto et al. Reference Vidotto, Dalla Valle, Fogliatto, Milan, De Palo, Tabacchi and Ferrero2020). To mitigate this risk, IMI-resistant varieties have been introduced with restrictive stewardship guidelines, such as not planting CL rice in the same field for two consecutive years and practicing crop rotation (Lamichhane et al. Reference Lamichhane, Devos, Beckie, Owen, Tillie, Messéan and Kudsk2017; Scarabel et al. Reference Scarabel, Cenghialta, Manuello and Sattin2012). A recent survey conducted in Italy revealed that about 63% of respondents used imazamox coupled with imazamox-resistant rice varieties (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020). In the same study, about 45% of the respondents reported the occurrence of IMI-resistant weedy rice (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020).
Recently, rice varieties resistant to the acetyl-CoA carboxylase–inhibiting herbicides (i.e., cycloxidim in Italy), patented as the Provisia® technology, have been introduced to better control weedy rice populations, in particular those that became resistant to the ALS-inhibiting herbicides (Dauer et al. Reference Dauer, Hulting, Carlson, Mankin, Harden and Mallory-Smith2018). However, the occurrence of weeds resistant to these herbicides can be anticipated. This study had two primary objectives: (1) to measure the weedy rice seed density in the soil seedbanks of rice fields with different histories of CL rice varieties and planting systems (drill seeding in dry fields or broadcast seeding in flooded fields) and (2) to predict weedy rice seedbank dynamics under different weed management scenarios over time, using a modeling approach. In particular, we determined the number of years required to obtain zero weedy rice seeds in the soil seedbank under various weed management systems using CL rice. The model simulated the evolution of the weedy rice seedbank in both the absence and presence of IMI-resistant weedy rice populations.
Materials and Methods
Weedy Rice in the Soil Seedbank
The study was conducted during spring 2011 in rice production areas of five Italian provinces (Alessandria, Biella, Novara, Pavia, and Vercelli). The rice fields were chosen based on the number of years that CL rice varieties were planted consecutively (0, 1, 2, or 3 yr). The system of sowing rice for each field was also recorded. More than 50 fields of at least 1 ha each were sampled. Soil samples were collected in spring (April to May) after soil preparation and before field flooding and rice seeding. In each field, soil samples were taken from 15 different randomly selected locations using an 11-cm-diameter soil corer to a depth of 20 cm. Individual soil core samples from each zone were stored in plastic bags and kept in a 5 C refrigerated room to prevent seed germination before sample processing. Weedy rice seeds were extracted from each soil core independently, using equipment specifically constructed for this purpose (Figure 1). Weedy rice seeds were separated from soil by a high-pressure water wash. The seeds were then floated and separated from the water stream, first by a 2-mm-mesh sieve, and then by a 1-mm-mesh sieve. Seeds were counted in the laboratory. Seeds that were firm when pressed between the fingers were considered filled, while those that were soft or hollow were discarded. Each filled seed was de-hulled to determine pericarp coloration; red- or brown-colored seeds were classified as weedy rice seeds. The weedy rice seedbank density was expressed as number of seeds per square meter at a 20-cm depth.
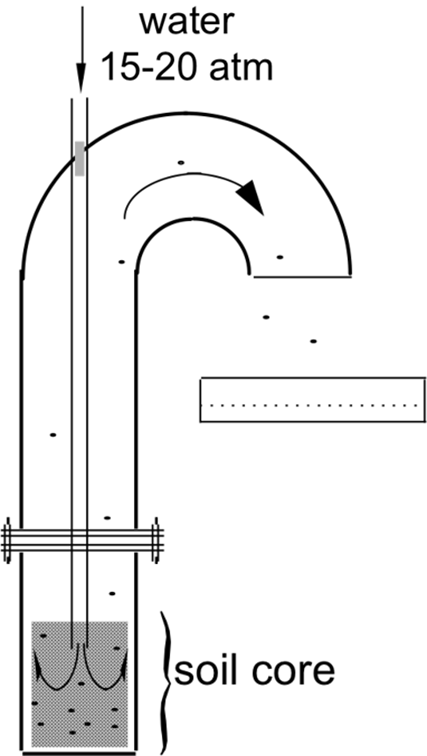
Figure 1. Seed extractor used to remove weedy rice seeds from the soil samples.
Statistical Analyses
The weedy rice soil seedbank data showed a nonnormal distribution according to the Shapiro-Wilk test; therefore, the statistical analysis was based on nonparametric models. The fields were first grouped according to: (1) rice planting method (drill seeding in dry fields, broadcast seeding in flooded fields) and (2) number of consecutive years of CL rice cultivar (0, 1, 2, or 3 yr). The Mann-Whitney rank U-test, a nonparametric test that measures the differences between two independent groups of nonnormally distributed data, was used to compare the planting classes (P ≤ 0.05). Classes of fields cultivated consecutively with different CL varieties were compared using the Kruskal-Wallis test, a nonparametric test that measures the differences among three or more independent samples of nonnormally distributed data. After the Kruskal-Wallis test, the medians were separated using the Mann-Whitney U-test with a Bonferroni adjustment. Statistical analyses were conducted using IBM SPSS Statistics v. 26 (IBM Corp., Armonk, NY, USA).
Prediction of Weedy Rice Seed Dynamics in the Soil Seedbank under Different Weed Management Strategies
To estimate the change in abundance of weedy rice in the rice field soil seedbank under different weed management scenarios, a mathematical model proposed by Spitters et al. (Reference Spitters, Kropff and Groot1989) was adopted. This competition model is based on hyperbolic regression equations and was proposed to describe maize (Zea mays L.) yield losses in relation to weed density. The model was chosen as it is suitable for predicting changes in weed seed populations in the soil (Spitters et al. Reference Spitters, Kropff and Groot1989). This model was modified to predict weedy rice seedbank dynamics over time by considering estimated growth parameters of rice and weedy rice for plants both in pure stand and in competition. The model was applied by simulating separately the absence of resistance (all weedy rice plants are IMI susceptible) or presence of IMI-resistant weedy rice plants due to mutation and outcrossing between CL rice and weedy rice. In the second case, a mutation rate of 1 × 10−6 (Diggle and Neve Reference Diggle and Neve2001) was included every year in the model to calculate the number of IMI-resistant weedy rice seeds; an outcrossing rate of 5 × 10−5 (Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007) due to gene flow from CL rice to weedy rice was added to the mutation rate only in the years in which CL rice was planted. Gene flow from IMI-resistant weedy rice to IMI-susceptible weedy rice was not considered in the model.
The parameter values used as input in the model were determined from several sources: our studies, available literature, and personal experience. The model considered the following scenarios:
-
Scenario A simulated rice monoculture with CL rice variety and non-CL rice variety (NCL rice) in alternate years. In CL rice, weedy rice control is based on the labeled IMI herbicide for CL rice, which had an estimated average efficacy of 98.5% against susceptible weedy rice (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Bzour et al. Reference Bzour, Zuki and Mispan2018; Marchesan et al. Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011; Rainbolt et al. Reference Rainbolt, Thill, Yenish and Ball2004; Webster and Masson Reference Webster and Masson2001), while a 0% efficacy against IMI-resistant seedlings was assumed. For NCL rice, it was assumed that the use of traditional practices, such as stale seedbed and preplant herbicides, had an average efficacy against weedy rice (regardless of whether it was susceptible or resistant to ALS inhibitors) of 90% (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Ferrero et al. Reference Ferrero, Vidotto, Balsari and Airoldi1999).
-
Scenario B simulated a 3-yr cycle of rice monoculture with two consecutive years of CL rice, followed by 1 yr with NCL rice. Herbicides and efficacy when CL and NCL rice were planted were the same as in scenario A.
-
Scenario C simulated a 4-yr cycle of rice monoculture: three consecutive years of CL rice were followed by 1 yr of NCL rice. Herbicides and efficacy when CL and NCL rice were used were the same as in scenario A.
-
Scenario D simulated rice monoculture with only CL rice. Weedy rice control was based on POST use of an ALS inhibitor registered for this technology, with the imazamox efficacy used in scenario A.
-
Scenario E simulated rice monoculture with only NCL rice and the use of traditional practices for weedy rice control (average efficacy 90%, as in scenario A).
-
Scenario F simulated introduction of crop rotation on a 4-yr cycle. Two consecutive years of CL rice were followed by 1 yr of a different crop (crop rotation: CR), and then by an additional year with NCL rice. For CL and NCL rice, the control strategy and simulated efficacy values were the same as in scenario A. Weedy rice control efficacy in CR was assumed to be 100%, both toward susceptible and IMI-resistant weedy rice (Davis et al. Reference Davis, Scott and Dickson2012; Scherner et al. Reference Scherner, Schreiber, Andres, Concenço, Martins and Pitol2018).
-
Scenario G was similar to the 4-yr cycle of scenario F, but with different timing. A first year of CR was followed by 2 yr of CL rice, and then by 1 yr of NCL rice. Average efficacy values of weedy rice control methods were identical to those in scenario F.
-
Scenario H simulated a 4-yr cropping cycle: 2 yr of CR were followed by 2 yr of CL rice. The parameters of weedy rice control were the same as in scenario F.
The parameters used in the model are listed in Table 1. The parameter values used in the simulations are defined in the following sections.
Table 1. Parameters used in the simulations of the dynamics of the weedy rice seedbank and their values.
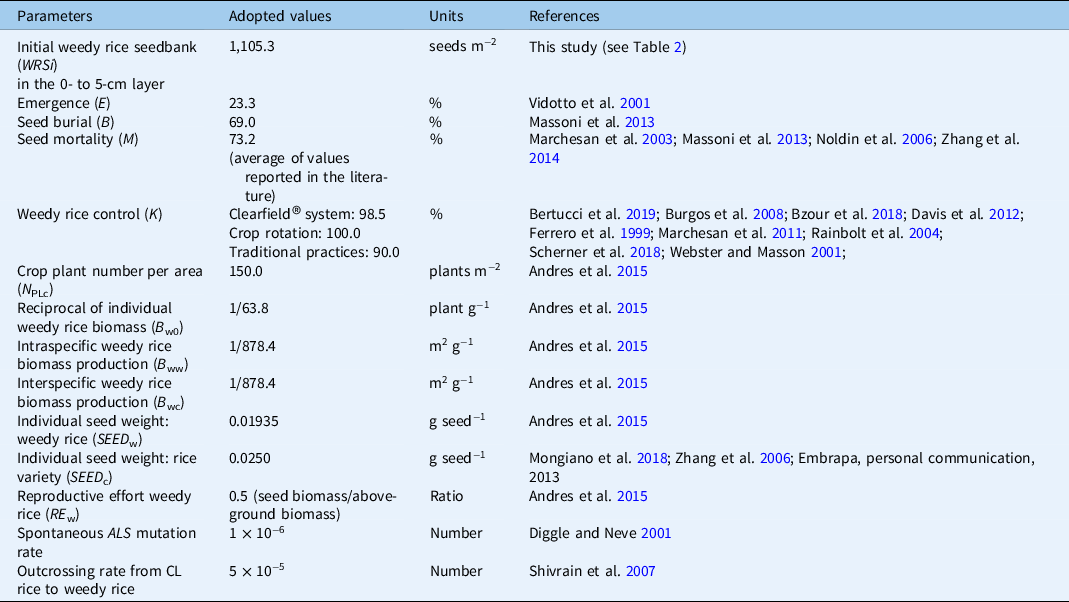
Initial Weedy Rice Seedbank (WRSi)
In this study we estimated that weedy rice soil seedbank ranged between 0 and 4,421 seeds m−2 for the soil layer from 0- to 20-cm deep. For the purpose of this simulation, the initial weedy rice seedbank was assumed to be at the highest level actually found in the field. Weedy rice seed emergence from 5 cm below the soil surface is negligible (Vidotto et al. Reference Vidotto, Ferrero and Ducco2001). The initial quantity of 4,421 seeds m−2 was, therefore, divided into four 5-cm depth segments, assuming that the seeds were evenly distributed across the 0- to 20-cm soil layer. It was also assumed that rice was seeded in no-tillage conditions, which means minimum seed movement down the soil profile.
Weedy Rice Emergence (E)
The mean emergence weedy rice rate was assumed to be 23.3% of the seeds present in the top 5-cm soil layer, as indicated by Vidotto et al. (Reference Vidotto, Ferrero and Ducco2001). The number of seedlings per square meter (N seedlings) was obtained by multiplying the weed seedbank (WRSi) and E (Equation 1):
The weedy rice plants estimated to reach maturity (N weeds) was calculated by multiplying the seedling number (N seedlings) by weed control efficacy (K) (Equation 2). The parameter K was set according to the adopted weed control program: K = 0.985 (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Bzour et al. Reference Bzour, Zuki and Mispan2018; Marchesan et al. Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011; Rainbolt et al. Reference Rainbolt, Thill, Yenish and Ball2004; Webster and Masson Reference Webster and Masson2001) in the CL system, K = 1 (Davis et al. Reference Davis, Scott and Dickson2012; Scherner et al. Reference Scherner, Schreiber, Andres, Concenço, Martins and Pitol2018) in CR, and K = 0.90 (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Ferrero et al. Reference Ferrero, Vidotto, Balsari and Airoldi1999) in fields of traditional weedy rice control.
Weedy rice biomass m−2 (Y weed) was calculated by applying the model proposed by Spitters et al. (Reference Spitters, Kropff and Groot1989) (Equation 3):
Where N weeds was the number of plants per sqare meter, B w0 the reciprocal of individual weedy rice biomass (plant g−1), B ww the intraspecific weedy rice biomass production expressed in terms of per-plant weight decrease with any plant added to the population (m2 g−1), B wc the interspecific competition of the crop on the weed (m2 g−1), and N PLc the crop plant number per area (plants m−2) as determined in our previous study conducted on different Italian weedy rice populations (Andres et al. Reference Andres, Fogliatto, Ferrero and Vidotto2015). The number of weedy rice seeds produced per square meter (N prod_seed) was determined as (Equation 4):
where the weedy rice reproductive effort (RE w) value was set to 0.5, and the individual weedy rice seed weight (SEED w) was set to 0.01935 g on the basis of our previous study (Andres et al. Reference Andres, Fogliatto, Ferrero and Vidotto2015). RE w and SEED w were the average values of 10 Italian weedy rice biotypes (5 awnless and 5 awned), which produced about 1,300 seeds plant−1 at an infestation level of 16 plants m−2 (Andres et al. Reference Andres, Fogliatto, Ferrero and Vidotto2015).
The annual input of seed (as number of seeds m−2) was calculated by multiplying weedy rice seed production (N prod_seed) by seed burial (B) (Equation 5):
Seed burial (B) represents the proportion of seeds produced by weedy rice that actually becomes part of the seedbank. It also considers the weedy rice seeds that are removed from the fields during rice harvesting. For this study, B was assumed to be 0.69 (Massoni et al. Reference Massoni, Marchesan, Grohs, Roso, Coelho, Machado, Teló and Dal’Col Lúcio2013).
The annual output of weedy rice seed was determined by the product of the initial seedbank (WRSi) and the sum of weedy rice emergence (E) and mortality (M) (Equation 6).
Mean seed mortality (M) was set as 0.732 in accordance with the findings of Marchezan et al. (Reference Marchezan, Oliveira, Avila and Bundt2003), Noldin et al. (Reference Noldin, Chandler and McCauley2006), Massoni et al. (Reference Massoni, Marchesan, Grohs, Roso, Coelho, Machado, Teló and Dal’Col Lúcio2013), and Zhang et al. (Reference Zhang, Dai, Song and Qiang2014).
The balance of weedy rice seed production after every growing season (ΔN seed) was calculated as (Equation 7):
Results and Discussion
Weedy Rice in the Soil Seedbank
A high weedy rice seed density of more than 1,000 seeds m−2 was found in the seedbank of the fields surveyed. The weedy rice in the soil differed widely across sampled fields, ranging from 0 to 4,421 seeds m−2 (data not shown). An average of 2.1 and 2.2 weedy rice seeds per soil sample (soil sample volume: 1,899.7 cm3), corresponding to a seedbank density of about 199 seeds m−2 and 214 seeds m−2, were found in drill-seeded dry fields and in broadcast-seeded flooded fields, respectively (Table 2). The weedy rice seedbank did not differ between drill-seeded flooded fields based on the Mann-Whitney test (P-value = 0.1425) (Table 2). However, in the weedy rice soil seedbank across years of consecutive cultivation of CL varieties, significant differences were found (Kruskal-Wallis test, P-value = 0.000) (Table 3). In particular, the highest weedy rice seed density was obtained in fields planted with CL rice for the first time, which contained about 3.7 seeds per soil sample, equivalent to about 351 seeds m−2 (Table 3). We learned that many farmers plant CL rice in fields with a high infestation of weedy rice to control the weed. Thus, the soil seedbank would be high after only one season of planting CL rice. A similar result was found in a survey conducted on Italian rice fields in which the interviewed farmers declared that they decided to cultivate CL varieties in the fields with high weedy rice infestations because this technique was specifically developed to control this weed (Ferrero et al. Reference Ferrero, Fogliatto, Barberi and Vidotto2020).
Table 2. Weedy rice seedbank density of surveyed rice fields (n = 50) averaged between drill-seeded rice in dry fields and broadcast-seeded rice in flooded fields.
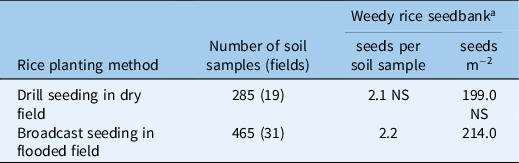
a Mann-Whitney U-test of the effect of rice planting method on the weedy rice seed number in Italian rice fields. NS: weedy rice seedbank means between the two rice planting methods were not significantly different as determined by Mann-Whitney U-test (P = 0.1425).
Table 3. The effect of the number of Clearfield® (CL) rice sequences (0–3) on weedy rice seed density in the soil seedbank of Italian rice fields surveyed (n = 50).
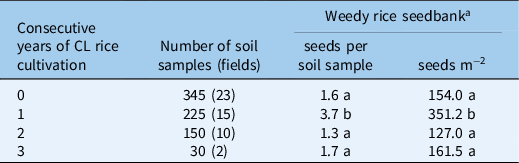
a Weedy rice seedbank values between the years sharing the same letter are not significantly different as determined by the Kruskal-Wallis test (P = 0.000).
In fields planted with CL rice for two or three consecutive years, the average weedy rice seedbank was reduced roughly 50% and thus was similar to that of fields with no history of IMI-resistant rice cultivation (Table 2). Even though only a few farmers cultivated CL varieties for more than 2 yr, this result supported those of other studies, wherein the weedy rice seedbank was not depleted even after three consecutive years of IMI-resistant rice cultivation (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Marchesan et al. Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011).
Prediction of Weedy Rice Dynamics in the Soil Seedbank under Different Weed Management Strategies
Monoculture Rice (scenarios A–E)
IMI-susceptible weedy rice
The CL rice system is estimated to have an average efficacy on weedy rice of 98.5% (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Bzour et al. Reference Bzour, Zuki and Mispan2018; Marchesan et al. Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011; Rainbolt et al. Reference Rainbolt, Thill, Yenish and Ball2004; Webster and Masson Reference Webster and Masson2001), while the efficacy of traditional NCL strategies is considered to be about 90% (Bertucci et al. Reference Bertucci, Fogleman and Norsworthy2019; Ferrero et al. Reference Ferrero, Vidotto, Balsari and Airoldi1999). Although these values might not seem extremely different, the simulations clearly show that the higher frequency of use of CL rice would result in larger reduction of the weedy rice seedbank (Table 4).
Table 4. Simulated weedy rice seedbank density after 10 yr of application of different management scenarios using Clearfield® (CL) rice (imidazolinone [IMI]-resistant CL rice variety), non-Clearfield® (NCL) rice (IMI-susceptible rice variety), traditional practices (mechanical and chemical control, without CL system), and rotation with a crop different from rice (CR).
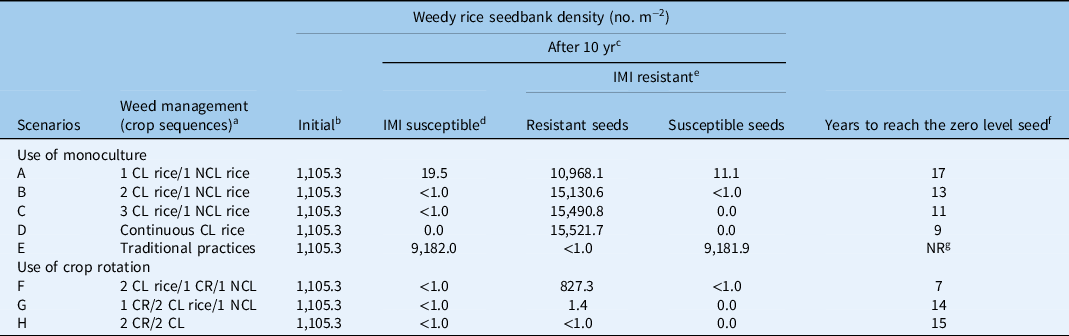
a 1, 2, or 3 yr with CL rice variety followed by 1 yr traditional rice variety or crop rotation.
b Initial weedy rice seedbank assessed in the present study.
c Time of crop sequence.
d IMI-susceptible: estimation of weedy rice seed density in absence of resistance occurrence due to mutation or outcrossing.
e IMI-resistant: estimation of weedy rice seed density in case of occurrence of mutation and outcrossing.
f number years to reach zero weedy rice seeds in soil seedbank in in absence of resistance occurrence due to mutation or outcrossing.
g NR, zero level is never reached.
In scenarios A and B, which meet the stewardship for use of the CL rice system, the model estimates a fluctuation in the presence of weedy rice in the soil seedbank (Figure 2). During a period of 10 consecutive years of rice monoculture, in the case of weedy rice populations susceptible to imazamox, the model predicted a reduction of the number of weedy rice seeds, even though the seedbank was not fully depleted (weedy rice seed density after 10 yr: 19.5 seeds m−2 and <1 seed m−2 in A and B, respectively) (Figure 2). These results demonstrated that even when less restrictive stewardship guidelines are adopted in Italy, allowing for the use of the CL rice variety for up to two consecutive years, a substantial reduction of the weedy rice seedbank can be achieved, even though they are insufficient to guarantee eradication of the weed (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008). The low levels of weedy rice in the seedbank estimated by the model were, in fact, sufficient to cause reinfestation, as suggested by Marchesan et al. (Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011). In scenarios A and B, complete elimination of weedy rice seeds in the superficial soil layer (0 to 5 cm) would require 17 and 13 yr, respectively (Table 4; Figure 2). Scenario C, which also simulates a rice monoculture with three successive years of CL rice followed by a year of a conventional NCL variety, predicted a strong reduction in the weedy rice soil seedbank after a 10-yr period (a decline from 1,105.3 to <1.0 seeds m−2). Marchesan et al. (Reference Marchesan, Massoni, Villa, Grohs, Avila, Sartori and Bruck2011) and Burgos et al. (Reference Burgos, Norsworthy, Scott and Smith2008) reported similar trends. To achieve complete elimination of the weedy rice seedbank, the model predicts 11 yr of cultivation would be required in this scenario (with 3 yr of CL and 1 yr of NCL) (Table 4; Figure 2). Scenario D estimated the elimination of susceptible weedy rice in the seedbank after 9 yr of monoculture rice (Table 4). This scenario included the following conditions: continuous cultivation with only CL rice for all consecutive years at a 98.5% level of weedy rice control, no introduction of weedy rice seeds in commercial rice seeds, and essentially no provision for development of weedy rice plants resistant to ALS-inhibiting herbicides. Scenario E, in which traditional practices are applied to control weedy rice (e.g., stale seedbed, preseeding residual herbicides), the simulation failed to show any significant reduction in the IMI-susceptible weedy rice seedbank. This system reached equilibrium at a density of about 9,180 seeds m−2 (Table 4). This high seed density was predicted based on a quite low efficacy level (90%), which can vary greatly between lower or higher values according to the chosen control techniques and the initial seedbank density in the field.
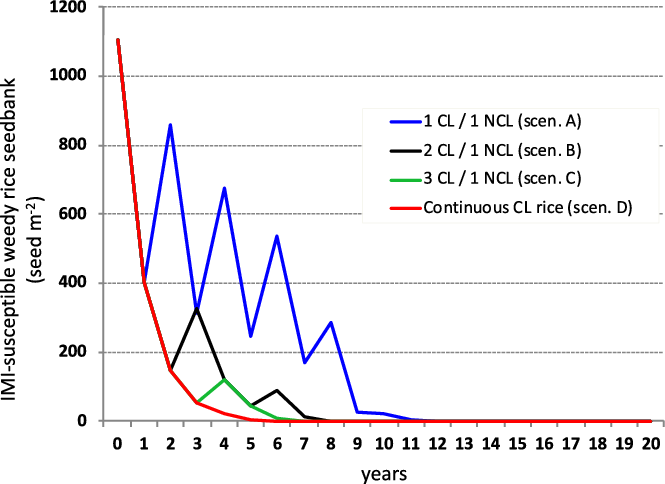
Figure 2. Simulated imidazolinone (IMI)-susceptible weedy rice seedbank dynamics under different weed control scenarios (A–D) in rice monocultures. Simulations assumed an initial seedbank density of 1,105 seeds m−2. CL, Clearfield® rice varieties; NCL, non-Clearfield® varieties.
IMI-resistant weedy rice
When the simulations assumed an initial presence of resistance alleles in an unselected weed population (spontaneous mutations of the ALS gene) with a 1 × 10−6 frequency (Diggle and Neve Reference Diggle and Neve2001) and an outcrossing rate of a 5 × 10−5 between CL rice and weedy rice (Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007), the model predicted a significant growth of a resistant weedy rice population within 4 to 5 yr (Figure 3). In particular, in scenario D, this population can no longer be controlled with IMIs and will thus be responsible for high rice yield losses. Even though the species is mostly autogamous, repeated use of ALS inhibitors in the CL rice system in the same fields may contribute to development of IMI-resistant weedy rice, even at a small rate, as a result of outcrossing between the CL rice and susceptible weedy rice (Dauer et al. Reference Dauer, Hulting, Carlson, Mankin, Harden and Mallory-Smith2018; Goulart et al. Reference Goulart, Matzenbacher and Merotto2012).
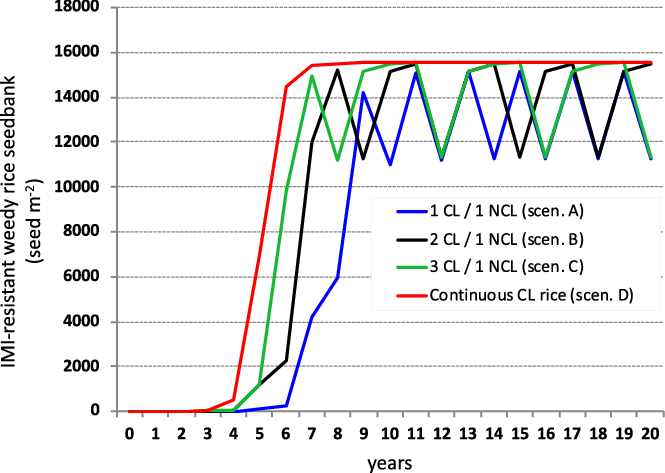
Figure 3. Simulated imidazolinone (IMI)-resistant weedy rice seedbank dynamics under different weed control scenarios (A–D) in rice monocultures. Simulations assumed an initial seedbank density of 1,105 seeds m−2 with an initial frequency of resistant individuals of 1 × 10−6 and an outcrossing rate between Clearfield® (CL) rice and weedy rice of 5 × 10−5 only in the years in which CL rice was planted. NCL, non-Clearfield® varieties.
In Scenario A, in which CL rice was cultivated every other year, the IMI-resistant weedy rice increase was delayed, as the biggest increase in the number of seeds was observed after 6 yr, as opposed to the other scenarios, in which a strong seed increase was already predicted to occur between the fourth and fifth years (Figure 3). In scenario E, when an initial resistance level is assumed, no further increase of the resistant population is observed. The CL system is not used in traditional practices, resulting in an absence of selection pressure, as the control strategies are equally effective against plants susceptible and resistant to ALS-inhibiting herbicides (Table 4).
Plant density estimation
Based on earlier research, it has been established that a weedy rice density of about 3 to 4 plants m−2 can cause a yield loss of about 10% (Vidotto et al. Reference Vidotto, Ferrero and Ducco2001). All scenarios with the CL system in rice monoculture (A–C) highlighted that weedy rice seed density decreased gradually throughout the years in the presence of IMI-susceptible populations. In particular, scenario A required more years for weedy rice seed density reduction compared with the other scenarios that included CL and NCL rice planting. In fact, at an initial weedy rice seed density of 1,105.3 plants m−2, it would take 11, 7, and 6 yr to reduce the seed density to 3 plants m−2 (10% rice yield loss) under scenarios A, B, and C, respectively (data not shown).
In the case of IMI-susceptible weedy rice, in scenario A, after an 11-yr cropping sequence, fewer than 2 weedy rice seeds m−2 were found in the soil seedbank; such a number of potentially emerged plants would have a small impact on crop yield (<4%). In scenario B, a 7-yr cultivation cycle of 2 yr of CL varieties and 1 yr of NCL are required to reach the point at which rice yield losses are kept to 10%. In scenario C, the simulation showed that a 6-yr cropping sequence, with 3 yr of CL varieties and 1 yr of NCL, were required to reduce the level of weedy rice infestation from 1,105.3 to 9.4 seeds m−2, which leaves just 2.2 weedy rice seedlings m−2 in the rice field (Figure 2). At this rate, rice yield losses could reach 8%. Scenario D, with successive monoculture of CL rice, showed that the weedy rice density in the soil seedbank was reduced to 5.4 seeds m−2 after 5 yr. This result equates to less than 2 new weedy rice seedlings m−2 emerging from the soil seedbank and causes a far smaller impact on crop yield. Finally, in scenario E, which used traditional techniques to control weedy rice, there was no estimated weedy rice reduction in the soil seedbank. On the contrary, the seedling density grew following a sigmoidal curve, and density equilibrium was achieved at about 2,150 seedlings m−2 (data not shown). In this simulation, even in the first year of the cropping sequence, more than 258 weedy rice seedlings m−2 are estimated to emerge. The model predicted a seedling density of IMI-resistant weedy rice of about 3 plants m−2 after 5 yr of cultivation of CL and non-CL rice every other year (scenario A), while the same level of resistant weedy rice seedlings was attained after 3 yr in scenarios B, C, and D.
Rice and Crop Rotation (Scenarios F–H)
IMI-susceptible weedy rice
The inclusion of CR confers a great advantage to the CL rice system, because it not only eliminates weedy rice seedlings but also reduces the risk of occurrence of plants resistant to ALS-inhibiting herbicides (Singh et al. Reference Singh, Singh, Black, Boyett, Basu, Gealy, Gbur, Pereira, Scott, Caicedo and Burgos2017; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). In simulations with CR strategies, in which 100% weedy rice control was set (Davis et al. Reference Davis, Scott and Dickson2012; Scherner et al. Reference Scherner, Schreiber, Andres, Concenço, Martins and Pitol2018), soil seedbank density was strongly reduced, in particular in the case of IMI-susceptible weedy rice (Table 4; Figure 4). Simulations inclusive of CR strategies showed strong IMI-susceptible weedy rice density reductions in the soil, reaching values after 3 yr from the start of the cropping sequence of about 5 seeds m−2 in scenarios F and G and even lower (less than 1 seed m−2) in scenario H (Figure 4). However, complete depletion of susceptible weedy rice in the seedbank was not detected until about 7 yr of sequencing had been performed in the simulation with the best result (scenario F), while scenarios G and H necessitated about 14 and 15 yr, respectively, to completely deplete the seedbank (Table 4). Furthermore, the results highlighted that the crop sequence options that started with 1 or 2 yr of CR before CL rice (scenarios G and H) would best kill emerged seedlings and avoid replenishment of the soil seedbank compared with scenario F (Figure 4). This was particularly evident in the second and the third years of scenario G, in which the seedbank density was reduced approximately 10 times more than in scenario F. In scenario H, after the simulation of two successive years of CR, weedy rice presence in the soil seedbank was reduced from 1,105.3 to 1.3 seed m−2 in the superficial soil layer. The estimated emergence rate (23.3%) used in this simulation (Vidotto et al. Reference Vidotto, Ferrero and Ducco2001), calculated a seedling density of about 0.3 plants m−2 after 2 yr of rotation; such an infestation would result in yield loss below 1.5%. Additionally, the few seeds left in the soil seedbank could be controlled in the next crop sequences with 2 yr of CL rice. Under this scenario, the selection of resistant individuals (assuming in this case that there is no outcrossing between the CL cultivar and weedy rice) has low probability of occurrence, as both susceptible and resistant weedy rice populations are easily controlled in 2 out of 3 yr. A cautionary note came from comparison of scenarios G and H. While 2 yr of CR left 1.3 seed m−2 on the soil surface of the seedbank (scenario H), 1 yr left 14.2 seed m−2 on the soil surface (scenario G). This result is important in weedy rice seed dynamics, because it suggests a new potential for seed infestation in the next rice-growing season.
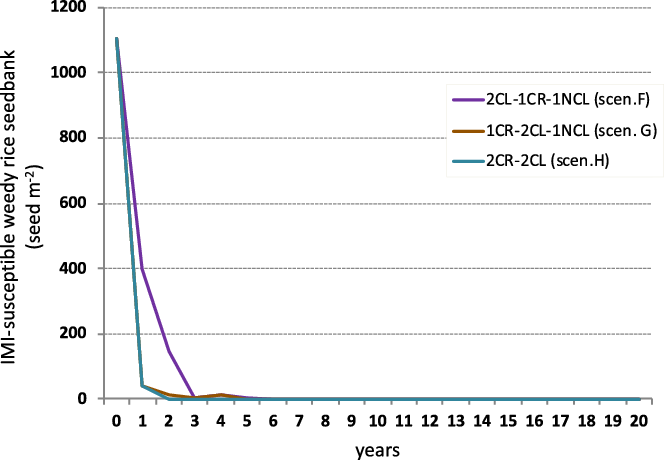
Figure 4. Simulated imidazolinone (IMI)-susceptible weedy rice seedbank dynamics under different weed control scenarios (F–H) of rice in crop rotation. Simulations assumed an initial seedbank density of 1,105 seeds m−2. CL, Clearfield® rice varieties; NCL, non-Clearfield® varieties.
IMI-resistant weedy rice
In the case of IMI-resistant weedy rice, the number of weedy rice seeds using different CR sequences increased later compared with scenarios in which rice was cultivated in monoculture (Figures 2 and 5). In particular, in the case of rice monocropping, best results were obtained with scenario A, in which 1 yr of CL rice was followed by 1 yr of NCL rice (weedy rice began to increase in the seedbank after about 5 yr). Adopting CR, the worst result was obtained with scenario F, in which the weedy rice seed density started to increase significantly after about 10 yr. Starting the sequencing with 1 yr of CR (scenario G), the rise of IMI-resistant weedy rice was strong only after 14 yr from the beginning of the simulation (Figure 5). Scenario H, in which 2 yr of CR were alternated with 2 yr of CL rice, showed the best results, as already observed for IMI-susceptible weedy rice. In this case, an increase of resistant weedy rice was not observed (Figure 5).
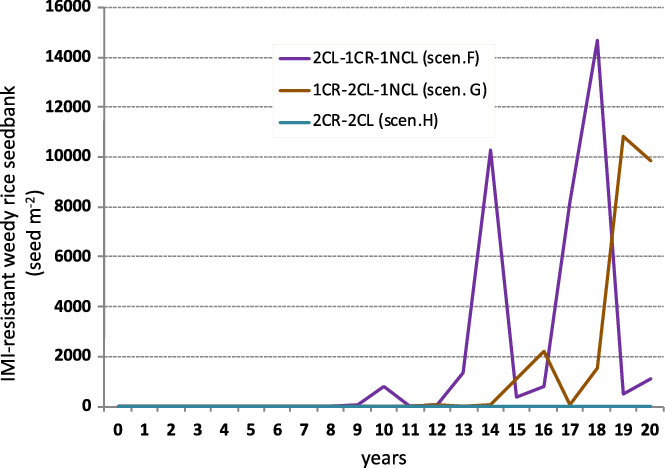
Figure 5. Simulated imidazolinone (IMI)-resistant weedy rice seedbank dynamics under different weed control scenarios (F–H) for rice in crop rotation. Simulations assumed an initial seedbank density of 1,105 seeds m−2 with an initial frequency of resistant individuals of 1 × 10−6 and an outcrossing rate between Clearfield® (CL) rice and weedy rice of 5 × 10−5 only in the years in which CL rice was planted. NCL, non-Clearfield® varieties.
Limitations of the Weedy Rice Seedbank Simulation
Our simulations did not consider weedy rice seedlings emerging from below 5 cm in the soil. These plants may, however, be capable of emerging later in the growing season and escaping herbicide activity, which will complicate the dynamics of emergence and survival of any particular weedy rice population (Olajumoke et al. Reference Olajumoke, Juraimi, Uddin, Husni and Alam2016). It should be noted that our simulations assume that the efficacy of each control method adopted is constant over the years. In general, models dealing with seedbank dynamics are particularly sensitive to variations of efficacy-related parameters (Bagavathiannan et al. Reference Bagavathiannan, Beckie, Chantre, Gonzalez-Andujar, Leon, Neve, Poggio, Schutte, Somerville, Werle and Acker2020; Vidotto et al. Reference Vidotto, Ferrero and Ducco2001; Zhang et al. Reference Zhang, Dai, Song and Qiang2014). In particular, slight variations around high values of efficacy (e.g., above 95%) may result in different patterns of seedbank evolution (Zhang et al. Reference Zhang, Dai, Song and Qiang2014). Inclusion of stochasticity, rather than using fixed values, might overcome such problems. However, in this study, rather than exact predictions, simulations were mainly used for ranking the different control strategies, thereby providing insights that might help farmers to make decisions. Moreover, the efficacy of the control methods against different weedy rice populations can vary because of the high variability of the species, which makes some biotypes more adaptable to different environments and more competitive with the crop and less sensitive to control tools such as to herbicides (Fogliatto et al. Reference Fogliatto, Ferrero and Vidotto2020, Reference Fogliatto, Patrucco, Milan and Vidotto2021; Mohd Hanafiah et al. Reference Mohd Hanafiah, Mispan, Lim, Baisakh and Cheng2020; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan and Menezes2015). Frequent use of ALS-inhibiting herbicides in CL rice varieties can contribute to selection of resistant weedy rice populations (Bzour et al. Reference Bzour, Zuki and Mispan2018; Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Mazlan2020; Scarabel et al. Reference Scarabel, Cenghialta, Manuello and Sattin2012; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). This selection pressure may lead to a higher number of IMI-resistant weedy rice plants as a consequence of outcrossing between the CL rice varieties and susceptible weedy rice or from repeated use of these herbicides on biotypes with a natural tolerance (Busconi et al. Reference Busconi, Rossi, Lorenzoni, Baldi and Fogher2012; Bzour et al. Reference Bzour, Zuki and Mispan2018; Shivrain et al. Reference Shivrain, Burgos, Sales, Mauromoustakos, Gealy, Smith, Black and Jia2009).
Implications for Weedy Rice Management
Rice monoculture simulations or crop rotation sequence effects on weedy rice seedbank density have practical implications for management of weedy rice in the soil seedbank (Zhang et al. Reference Zhang, Dai, Song and Qiang2014). The scenarios that estimate a lower incidence of weedy rice in the soil seedbank might result in higher rice yields. In contrast, the use of traditional techniques to control IMI-susceptible weedy rice might maintain high levels of weedy rice in the soil seedbank and negatively interfere with rice production.
Some weedy rice management scenarios did strongly affect weed presence, which in turn likely improved rice yield when crop rotation sequences were adopted. The sharp decrease in the weedy rice seedling numbers occurred only when crop rotation was inserted in the simulations. The estimates showed that, after adoption of crop rotation, a time period of 2 or 3 yr can be sufficient to contain rice yield losses to less than 10%. Observed changes in weedy rice seedbank abundance due to different strategies caused decreased seedling numbers in only those cases that included crop rotation. As noted earlier, the potential evolution and spread of weed herbicide resistance, in this case in weedy rice, might be avoided or delayed, but this will only occur with crop rotation (Scarabel et al. Reference Scarabel, Cenghialta, Manuello and Sattin2012; Zhang et al. Reference Zhang, Dai, Song and Qiang2014). In fact, our simulations showed that adoption of crop rotation for 2 yr before the use of CL rice varieties reduced weedy rice abundance. Previous studies have already confirmed linkages in the weedy rice soil seedbank density to crop systems and crop rotations (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Davis et al. Reference Davis, Scott and Dickson2012; Gao et al. Reference Gao, Zhang, Shen, Mao, Wei, Wei, Zuo, Li, Song and Qiang2020).
A previous simulation of weedy rice seedbank dynamics linked to different population densities in Chinese rice fields also highlighted that crop rotation is a key factor for weedy rice management and seedbank suppression (Dauer et al. Reference Dauer, Hulting, Carlson, Mankin, Harden and Mallory-Smith2018; Zhang et al. Reference Zhang, Dai, Song and Qiang2014). A study from Brazil underlined the importance of crop rotation to delay and reduce the occurrence of IMI-resistant weedy rice in CL rice (Kalsing et al. Reference Kalsing2019). The study showed that IMI-resistant weedy rice was detected within 3 seasons following the cultivation of CL rice, while in the United States this occurred after 5 yr (Kalsing et al. Reference Kalsing2019; Ziska et al. Reference Ziska, Gealy, Burgos, Caicedo, Gressel, Lawton-Rauh, Avila, Theisen, Norsworthy, Ferrero, Vidotto, Johnson, Ferreira, Marchesan and Menezes2015). The later occurrence of resistant populations in the United States was explained by the widespread adoption of crop rotation (Kalsing et al. Reference Kalsing2019).
These findings confirm the risk of applying a weed management strategy based on a single tool (as in the case of CL rice), as its success is strictly related to the accuracy and efficacy of herbicide application, which in turn can be achieved only through the knowledge of the biological behavior and dynamics of weed growth in the field. As suggested by the CL stewardship guidelines, crop rotation should be applied in alternation with CL rice varieties, and great attention should be paid to those weeds escaping herbicide application, which should be removed to prevent gene flow from rice to weedy rice and to reduce seedbank input (Kalsing et al. Reference Kalsing2019; Zhang et al. Reference Zhang, Linscombe, Webster, Tan and Oard2006).
From the results of the simulations, it appears that rotation with a different crop can be crucial for obtaining a satisfactory reduction of the weedy rice seedbank while hampering the selection of populations resistant to ALS-inhibiting herbicides. Further studies are needed to validate the used model for weedy rice seedbank dynamic prediction with data from different management conditions, including the alternation of IMI-resistant (CL) and graminicide-resistant (Provisia®) rice varieties.
Acknowledgments
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors. No conflicts of interest have been declared.














