Assisted reproductive technologies (ARTs) have become widely accepted therapies for certain types of infertility and account for a large proportion (18%) of multiple births in Canada and the United States (Cook et al., Reference Cook, Collins, Buckett, Racowsky, Hughes and Jarvi2011; Sunderam et al., Reference Sunderam, Chang, Flowers, Kulkarni, Sentelle, Jeng and Macaluso2009). Increased rates of twin and higher order multiple pregnancies following ART are a serious public health concern due to higher perinatal mortality and morbidities in multiple compared with singleton pregnancies (Bissonnette et al., Reference Bissonnette, Cohen, Collins, Cowan, Dale, Dill and Yuzpe2007; Braude, Reference Braude2006; Rydhstroem & Heraib, Reference Richardson, Corcoran, Escobar and Lee2001; Wimalasundera et al., Reference Wimalasundera, Trew and Fisk2003). Studies examining the association between mode of conception and neonatal mortality/morbidities among twins have produced conflicting results (Boulet et al., Reference Boulet, Schieve, Nannini, Ferre, Devine, Cohen and Macaluso2008; Hansen et al., Reference Hansen, Colvin, Petterson, Kurinczuk, de and Bower2009; Helmerhorst et al., Reference Helmerhorst, Perquin, Donker and Keirse2004; Joy et al., Reference Joy, McClure and Cooke2008; McDonald et al., Reference McDonald, Murphy, Beyene and Ohlsson2005; Rossi & D'Addario, Reference Rossi and D'Addario2011). These contradictory findings may be due to inadequate control of effect modification, confounding factors, and/or low statistical power.
Male sex is an established risk factor for preterm birth and subsequent adverse neonatal outcomes (Cooperstock et al., Reference Cooperstock, Bakewell, Herman and Schramm1998; Mulla et al., Reference Mulla, Plavsic, Ortiz, Nuwayhid and Ananth2013; Peacock et al., Reference Peacock, Marston, Marlow, Calvert and Greenough2012), and in twins, sex concordance has been associated with prematurity (Dailey et al., Reference Dailey, Jayakrishnan, Phipps, Raker and Chien2009; Rydhstroem & Heraib, Reference Rydhstroem and Heraib2001; Tan et al., Reference Tan, Wen, Walker, Fung, Demissie and Rhoads2004; Weghofer et al., Reference Weghofer, Klein, Stammler-Safar, Worda, Barad, Husslein and Gleicher2010) and neonatal mortality/morbidities (Melamed et al., Reference Melamed, Yogev and Glezerman2009; Mulla et al., Reference Mulla, Plavsic, Ortiz, Nuwayhid and Ananth2013). Due to its correlation with zygosity (shared genetic material) and chorionicity (shared in utero environment), the mode of conception may confound or modify the effect of sex concordance on neonatal mortality/morbidities; however, the association of sex concordance and neonatal outcomes accounting for mode of conception has not been studied.
The objective of this study was to examine the association of mode of conception and sex concordance with neonatal mortality and severe morbidities in very preterm (≤32 weeks gestation) twins admitted to a network of neonatal intensive care units (NICUs). We hypothesized that higher mortality/morbidities would be present in (1) opposite-sex infants conceived using ART versus spontaneously (SP); (2) infants conceived spontaneously from same-sex versus opposite-sex pairs; and (3) infants from male–male versus opposite-sex or female–female pairs following either ART or SP conception.
Subjects and Methods
Twin pairs born at ≤32 weeks gestation with both co-twins admitted to a Level 3 NICU between January 1, 2010, and December 31, 2011, were retrospectively identified from the Canadian Neonatal Network (CNN) database, which captures >95% of admissions to Level 3 NICUs in Canada (Canadian Neonatal Network, 2012). All CNN NICUs are tertiary-level referral centers, ranging in size from 12 to 73 beds, with 103–1,576 admissions annually during 2010–2011. At each site, trained abstractors collected data for each infant from admission until discharge from the NICU according to a standard manual of protocols and definitions (Canadian Neonatal Network, 2012). Data were entered electronically from patient charts using a customized data-entry program with built-in error checking algorithms. Institutional approval for each site was provided either by a local research ethics board or through an institutional quality improvement process. Informed consent from individual patients was not required as the study included only collection of de-identified data from patient records.
Infants from twin births admitted to the same NICU were identified as a pair. Twin pairs were excluded if the mode of conception was unavailable, or if either twin was declared moribund at admission, had major congenital anomalies, information on sex was missing, or sex was recorded as ambiguous. For each co-twin, a binary composite outcome was derived indicating mortality (all causes during NICU hospitalization) or any of the following severe neonatal morbidities: intraventricular hemorrhage (IVH) grades ≥3 or periventricular leukomalacia (Papile et al., Reference Papile, Burstein, Burstein and Koffler1978; Synnes et al., Reference Synnes, Chien, Peliowski, Baboolal and Lee2001), retinopathy of prematurity (ROP) stages ≥3 (International Committee for the Classification of Retinopathy of Prematurity, 2005), bronchopulmonary dysplasia (BPD; operationally defined as need for oxygen at 36 weeks post-menstrual age, or at the time of discharge or transfer to a Level 2 NICU; Shennan et al., Reference Shennan, Dunn, Ohlsson, Lennox and Hoskins1988), or necrotizing enterocolitis (NEC) stages ≥2 (Bell et al., Reference Bell, Ternberg, Feigin, Keating, Marshall, Barton and Brotherton1978; Walsh & Kliegman, Reference Walsh and Kliegman1986). Subsequently, the presence of the composite outcome in both, only one, or neither co-twin was defined at the pair level; however, due to low counts, multivariable analyses were performed by combining pairs with the composite outcome present in one and both co-twins.
Conception using ART included in vitro fertilization (IVF), intracytoplasmic sperm injection, embryo transfer, intrauterine insemination, and any other mechanical method in which both the oocytes and sperm are manipulated to facilitate pregnancy and implantation, as defined in the CNN database (Canadian Neonatal Network, 2012). Data on the use of ovulation-inducing agents were not available. Infants were classified according to conception mode (ART or SP) and sex concordance (male–male [MM], opposite-sex [MF], and female–female [FF]), generating a six-category conception-sex predictor variable (ART-MM, ART-MF, ART-FF, SP-MM, SP-MF, and SP-FF). Additional risk factors and potential confounding variables included gestational age (GA), small for gestational age (SGA) defined as birth weight below the 10th percentile for GA, inborn/outborn status (born in hospital with a Level 3 NICU/transferred after birth to a Level 3 NICU), severity of illness at admission to NICU quantified by the score for neonatal acute physiology version II (SNAPII) >20 (Richardson et al., Reference Richardson, Corcoran, Escobar and Lee2001), Apgar score at 5 minutes <5, maternal age, nulliparity, gestational diabetes, hypertensive disorder of pregnancy, receipt of antenatal corticosteroids, presence/absence of chorioamnionitis and smoking during pregnancy. Analogous pair-wise covariates were derived as the difference in birth weight and the number of co-twins (zero, one, or both) with SGA, SNAPII score >20 and 5-minute Apgar score <5.
Statistical Analyses
Preliminary individual-level and pair-wise analyses applied χ2 tests to examine differences in the distribution of sex (male, female) and sex concordance (MM, MF, FF) among the ART and SP conception groups. Within each ART and SP group, independent male/female pairing was assessed using a χ2 test. To identify potential confounders, the distribution of each covariate was examined between ART and SP twins, and among sex-concordance groups according to mode of conception, using χ2 or Fisher exact tests for categorical variables, and one-way ANOVA F tests for continuous measures. Similar univariate analyses examined the association of mode of conception and sex concordance with the composite outcome and each of its mortality/morbidity components.
Effects of conception mode and sex concordance were assessed using multivariable logistic regression models adjusted for potential confounders and important risk factors (1) at the individual level (examining the composite outcome of each twin infant) and (2) pair wise (comparing pairs with the composite outcome present in one or both twins vs. pairs with neither twin affected). Individual-level analyses were adjusted for individual sex (male or female) and employed generalized estimating equations (unstructured correlation) to adjust for residual familial correlation between co-twins. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from both individual-level and pair-wise analyses. Comparisons of ART versus SP were performed using all twin pairs, but these analyses are subject to confounding by zygosity/chorionicity. To eliminate possible confounding, additional analyses were restricted to opposite-sex pairs, who are all dizygotic/dichorionic. Furthermore, comparisons of same-sex (MM and FF combined and separately) versus opposite-sex (MF) were performed using all twin pairs, and then stratified by mode of conception to account for differences in the distribution of sex concordance due to zygosity/chorionicity, which are expected between ART and SP twin pairs. All statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., 2008), and significance was evaluated using two-sided p values at the 5% testing level.
Results
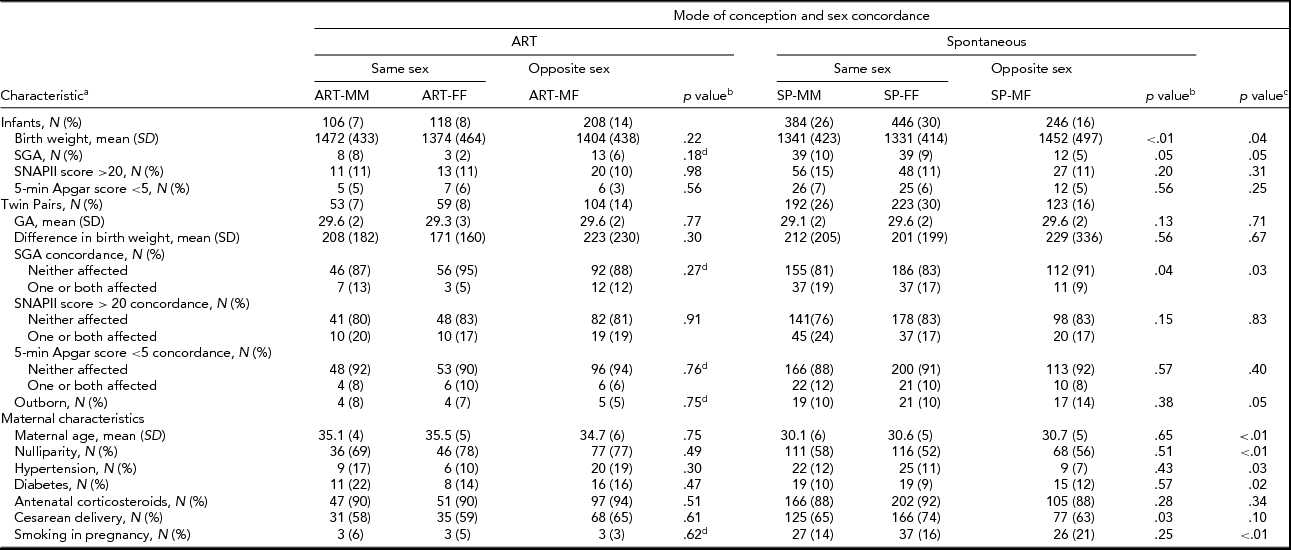
Of the 2,034 twins born at ≤32 weeks gestation and admitted to CNN NICUs during 2010–2011, 1,508 (74%) infants from 754 complete pairs were included in this study. We excluded 10 infants with missing/ambiguous sex, 81 with missing mode of conception, 3 who were moribund, 47 with major congenital anomalies, and 385 single co-twins. The final sample size comprised of 432 (29%) ART and 1,076 (71%) SP twins from 754 complete pairs. Individual sex (male and female) was not associated with mode of conception (Table 1). However, the distribution of sex pairing was significantly different (p value < .01) between ART and SP twins, with a larger proportion of opposite-sex pairs among ART (48%) than SP (23%) twins (Table 1).
TABLE 1 Distribution of Sex Among Individual Twins, and Sex Concordance Among Corresponding Twin Pairs

ART = assisted reproductive technology; SP = spontaneous.
aSignificance of the Pearson χ2 test; bsignificance of the χ2 test assessing independent male/female pairing: p value = .59 for ART, and p value <.01 for SP twin pairs.
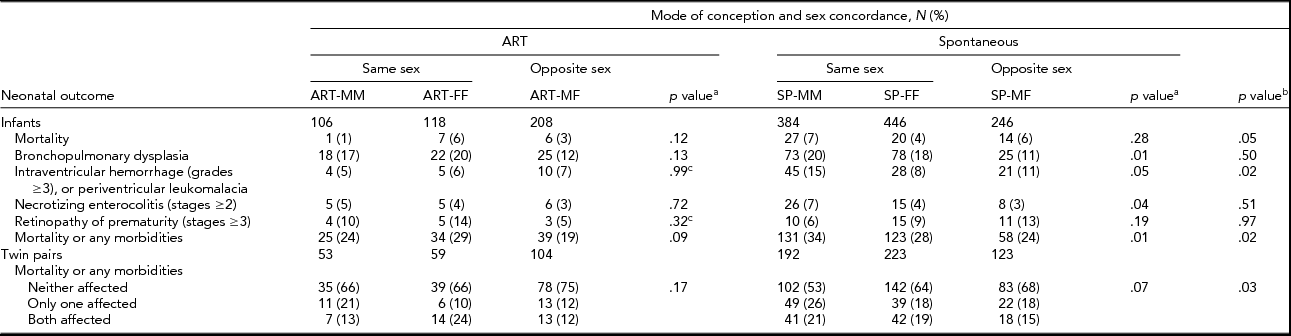
Significant differences were detected in mean birth weight, both individual-level and pair-wise SGA, inborn/outborn status, maternal age, nulliparity, hypertension, diabetes, and smoking during pregnancy between ART and SP twins (Table 2). Among ART twins, no association was detected between sex concordance and the infant and maternal characteristics examined. However, among SP twins, mean birth weight, individual-level and pair-wise SGA, and cesarean delivery varied according to sex concordance (Table 2). The composite mortality/morbidities outcome was significantly lower for ART than for SP twins in individual-level unadjusted analyses (23% vs. 29%). Pair-wise analyses confirmed differences in the distribution of the composite mortality/morbidities outcome between ART and SP twins (Table 3). IVH (6% vs. 11%) and mortality (3% vs. 6%) were lower in ART versus SP twins, although the latter was at borderline of statistical significance (Table 3). Notably, twins from opposite-sex pairs, after either ART or SP conception, had the lowest rates of the composite outcome (Table 3). No significant differences in the composite outcome or its components were detected among sex-concordance groups of ART twins. For SP twins, BPD, NEC, and the composite outcome varied significantly among sex-concordance groups, with highest rates in MM, intermediate rates in FF, and lowest rates in MF pairs (Table 3).
TABLE 2 Distribution of Individual-Level and Pair-Wise Characteristics Across Mode of Conception and Sex Concordance

ART = assisted reproductive technology; SP = spontaneous conception; MM = male–male; FF = female–female; MF = male–female; SD = standard deviation.
aCategorical variables are summarized as count and percent; continuous variables are summarized as mean and standard deviation.
bComparisons of MM, FF and MF groups within ART and SP twins.
cComparisons of ART versus SP twins.
dSignificance was assessed using the Fisher exact test; otherwise significance was assessed using the Pearson χ2 test for categorical variables and the ANOVA F test for continuous measures.
TABLE 3 Distribution (Count and Percent) of Neonatal Mortality, Each Morbidity, and the Composite Mortality/Morbidity Outcome Across Mode of Conception and Sex Concordance

ART = assisted reproductive technology; SP = spontaneous conception; MM = male–male; FF = female–female; MF = male–female. Numbers may not add up to the column total due to missing values or lack of assessment.
aComparisons of MM, FF, and MF groups within ART and SP twins; bcomparisons of ART versus SP twins; csignificance was assessed using the Fisher exact test; otherwise, significance was assessed using the Pearson χ2 test for categorical variables and the ANOVA F test for continuous measures.
Comparing ART Versus SP in All Twins and Opposite-Sex Pairs
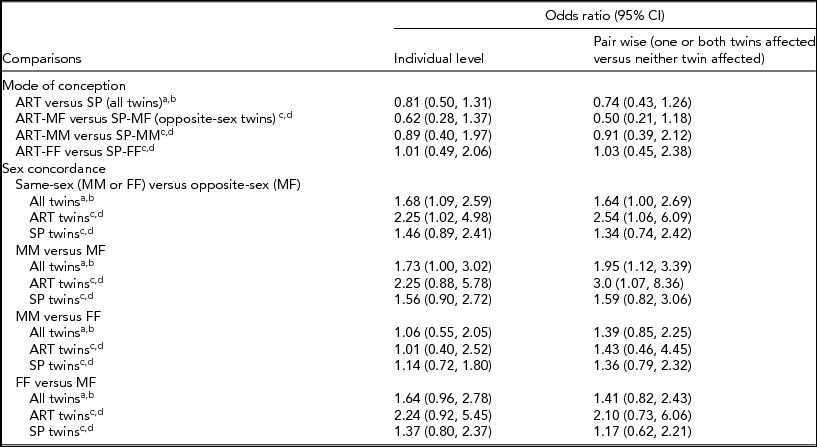
After adjustments, no significant association between mode of conception and the composite outcome was detected in any multivariable analyses using all twins or when restricted to opposite-sex pairs (Table 4).
TABLE 4 Adjusted Odds Ratios and 95% Confidence Intervals for Association of the Composite Mortality/Morbidities Outcome With Mode of Conception and Sex Concordance

ART = assisted reproductive technology; SP = spontaneous conception; MM = male–male; MF = male–female; FF = female–female; CI = confidence interval.
aIndividual-level multivariable model included mode of conception and sex concordance as risk factors, and was adjusted for individual-level sex (M, F), GA, SGA, SNAPII score >20, and maternal age.
bPair-wise multivariable model included mode of conception and sex concordance as risk factors, and was adjusted for GA, SGA concordance, SNAPII score >20 concordance, and maternal age.
cIndividual-level multivariable model was adjusted for GA, SGA, SNAPII score >20, and maternal age.
dPair-wise multivariable model was adjusted for GA, SGA concordance, SNAP-II score >20 concordance, and maternal age.
Comparing Same-Sex Versus Opposite-Sex in All, ART, and SP Twins
Significantly higher odds of mortality/morbidities were detected by individual-level multivariable analyses comparing twins from same-sex versus opposite-sex pairs, while pair-wise multivariable analyses yielded a similar odds ratio estimate with borderline statistical significance (Table 4). Among ART twins, higher odds of the composite outcome were estimated in same-sex compared than in opposite-sex twin pairs by both individual-level and pair-wise multivariable analyses. Despite elevated odds ratio estimates, no significant association between sex concordance and the composite outcome was detected in SP twin pairs (Table 4).
Comparing Male–Male Versus Opposite-Sex in All, ART, and SP Twins
More specific comparisons of male–male versus opposite-sex pairs detected significantly higher mortality/morbidities in pair-wise multivariable analyses using all twin pairs and among ART pairs. No significant association with the composite outcome was detected between male–male versus opposite-sex twin pairs from SP conception (Table 4).
Comparing Male–Male Versus Female–Female in All, ART, and SP Twins
No significant associations were detected between male–male versus female–female twin pairs with the composite outcome after adjustment (Table 4).
Comparing Female–Female Versus Opposite-Sex in All, ART, and SP Twins
Odds ratio estimates were elevated, but statistical significance was not reached in any multivariable analyses comparing the composite outcome between female–female versus opposite-sex twin pairs (Table 4).
Discussion
In our national, multicenter cohort of very preterm twins, the mode of conception was not associated with the composite outcome of mortality/morbidities. Sex concordance and the composite outcome were associated, with higher odds of the composite outcome in same-sex versus opposite-sex pairs and in one or both co-twins from male–male versus opposite-sex pairs. The effect of sex concordance on the composite outcome was more pronounced in ART than SP twins, but the difference between odds ratio estimates was not statistically significant, and mode of conception could not be established as an effect modifier.
The study is unique in that it examines the impact of sex concordance on a composite outcome of mortality/morbidities in a cohort representing the population of very preterm twins with highest risk for mortality/morbidities who receive treatment in a Level 3 NICU. Previously, sex concordance has been linked to preterm birth by several studies showing higher risk of preterm birth in twins from same-sex versus opposite-sex pairs (Melamed et al., Reference Melamed, Yogev and Glezerman2009; Rydhstroem & Heraib, Reference Rydhstroem and Heraib2001; Tan et al., Reference Tan, Wen, Walker, Fung, Demissie and Rhoads2004). In contrast, a study of dichorionic twin pairs conceived after IVF showed shorter gestational age for twins from opposite-sex versus same-sex pairs (Weghofer et al., Reference Weghofer, Klein, Stammler-Safar, Worda, Barad, Husslein and Gleicher2010), while another earlier study demonstrated a linear relationship between the number of male fetuses and the likelihood of preterm birth <35 weeks gestation (Cooperstock et al., Reference Cooperstock, Bakewell, Herman and Schramm1998). Results from our analyses using all twins indicate an impact of sex concordance beyond preterm birth, with the composite mortality/morbidities outcome significantly increased in twins from same-sex versus opposite-sex pairs. Similar lines of evidence from previous twin studies examining sex concordance showed an increased likelihood of mechanical ventilation and respiratory distress syndrome in male–male twins compared with females from opposite-sex pairs (Mulla et al., Reference Mulla, Plavsic, Ortiz, Nuwayhid and Ananth2013), and higher IVH in female–female twins than in females from opposite-sex pairs (Melamed et al., Reference Melamed, Yogev and Glezerman2009). Notably, our estimated odds ratio comparing the composite mortality/morbidities outcome between same-sex versus opposite-sex twins born very preterm is the exact reciprocal to the relative risk comparing hospital mortality between opposite-sex versus same-sex twin pairs estimated by Mulla et al. (Reference Mulla, Plavsic, Ortiz, Nuwayhid and Ananth2013) in a population study.
Possible biological mechanisms underlying higher risk of adverse outcomes in same-sex versus opposite-sex twins may relate to complications arising in monochorionic and/or monoamnionic twins (same-sex obligatory), including twin–twin transfusion, cord entanglement, and possible early induced delivery. It is also possible that the rate of gestational growth of the male and female twin in opposite-sex pairs may vary, yielding reduced competition for nutrients in utero (James, Reference James2002; Melamed et al., Reference Melamed, Yogev and Glezerman2009).
The majority (>95%) of ART twins result from the implantation of multiple embryos and are dizygotic/dichorionic irrespective of sex similarity. Accordingly, the distribution of sex concordance among ART twins in our study was consistent with random male/female pairing. By contrast, in SP twins there were significantly more same-sex (both male–male and female–female) pairs than expected under random pairing, likely due to monozygosity. To account for differences in the distribution of sex pairing, the association between sex concordance and the composite outcome was assessed separately in ART and SP twin pairs. Although odds ratio estimates comparing the composite outcome between same-sex (male–male and/or female–female) and opposite-sex pairs were notably higher in ART than SP twin pairs, the interaction between sex concordance and mode of conception was not statistically significant in our data (results not shown). Higher composite outcome in same-sex versus opposite-sex pairs conceived using ART suggests a mechanism independent of zygosity/chorionicity. Further study is warranted to tease out the relative effects of sex concordance, zygosity, and chorionicity on neonatal outcomes in ART twins.
Findings from our analyses are consistent with previous studies reporting no overall difference between ART or SP conception on outcomes in preterm or low-birth-weight twins (Messerschmidt et al., Reference Messerschmidt, Olischar, Birnbacher, Weber, Pollak and Leitich2010; Schimmel et al., Reference Schimmel, Hammerman, Lusky and Reichman2006; Shah et al., Reference Shah, Alwassia, Shah, Yoon and Shah2011). We recognize that an increased risk of very preterm birth (≤32 weeks GA) of twins after ART conception has been demonstrated by several investigators (Hansen et al., Reference Hansen, Colvin, Petterson, Kurinczuk, de and Bower2009; Kallen et al., Reference Kallen, Finnstrom, Lindam, Nilsson, Nygren and Olausson2010), including a recent meta-analysis (McDonald et al., Reference McDonald, Han, Mulla, Ohlsson, Beyene and Murphy2010). Furthermore, we note that the risk of preterm birth and subsequent mortality/morbidities after ART can be reduced through the practice of single embryo transfer. However, as ART twin pregnancies and preterm birth remain prevalent, our study was intended to assess the impact of the mode of conception on outcomes within the population of high-risk twins who are born very preterm and require acute care in a Level 3 NICU. We examined mode of conception as an independent predictor of the composite outcome with adjustment for potential confounders including sex concordance. As explicit data on zygosity/chorionicity were not available for confounding adjustment, analyses of conception mode and the composite outcome were restricted to opposite-sex pairs ensuring comparisons of ART and SP twins from similar dizygotic/dichorionic pairs.
Strengths of our study include the use of a cohort of twin pairs born very preterm and treated in Canadian Level 3 NICUs that is recent (2010–2011) and nationally representative (multicenter). Data on mortality and morbidities used to derive the composite outcome, and the covariates used for adjustment, were obtained and processed in a standardized manner that included a number of error-checking algorithms. We did not have explicit data on twin–twin transfusion; however, birth weight difference was examined, and pair-wise analyses were adjusted for pair-level SGA and SNAPII score variables. Because a ‘male disadvantage’ with respect to adverse neonatal outcomes has been established (Brothwood et al., Reference Brothwood, Wolke, Gamsu, Benson and Cooper1986), all individual-level analyses were adjusted for sex.
A limitation of our study is that 343 twins were excluded as data for their co-twins were unavailable in the CNN database. However, selection bias is unlikely as no significant differences were detected in the distribution of mode of conception and male/female sex between the 343 single twins who were excluded and the 1,508 twins from complete pairs included in our analyses. Co-twin death in utero is the most likely possibility for only one co-twin being included in our dataset, as the vast majority (>85%) of preterm infants born ≤32 weeks gestation are admitted to Level 3 NICUs in Canada. The alternative possibility (for the remaining <15%) is management in a Level 2 NICU, which is common for infants born after 30 weeks gestation who are not seriously ill on admission and do not require respiratory support. Confounding due to unavailable data for zygosity/chorionicity is possible in analyses examining all twin pairs, but not in the subset of opposite-sex pairs. We acknowledge that some misclassification of conception mode is possible as data on history of infertility, indication for ART, and the use of ovulation inducing agents were unavailable. Based on our total study sample, the minimal odds ratio detectable with 80% power was 1.40 (or 0.71) and 1.45 (or 0.69) for association of the composite outcome with mode of conception and sex concordance, respectively. Therefore, non-significant results in subgroup analyses with odds ratio estimates exceeding these values are most likely due to reduced sample size, and associations cannot be excluded.
In conclusion, mode of conception was not associated with a composite outcome of mortality/morbidities in very preterm twins. Sex concordance was a significant predictor of neonatal mortality/morbidities in very preterm twins. Clinical prediction models, used by physicians to identify infants with greatest risk of adverse neonatal outcomes, select treatments, and counsel families, can be developed specifically for twins to include sex concordance, thereby yielding improved prediction ability. It remains important to tease out the underlying effects of genetics including zygosity, sex concordance, and epigenetic mechanisms impacted by ART, from shared maternal in utero factors.
Acknowledgments
We thank the Canadian Institutes of Health Research-funded Team in Maternal-Infant Care (MiCare) for providing organizational support to the Canadian Neonatal Network™. The Maternal-Infant Care Research Centre is supported by the Ontario Ministry of Health and Long-Term Care. We thank Dr. Ruth Warre, Scientific Writer/Editor, from the Maternal-Infant Care Research Centre, Mount Sinai Hospital, Toronto, for editorial assistance with this manuscript.
Site Investigators of the Canadian Neonatal Network
Shoo K. Lee (Director, Canadian Neonatal Network); Prakesh S. Shah (Associate Director, Canadian Neonatal Network); Ruben Alvaro (St. Boniface General Hospital, Winnipeg, MB); Wayne Andrews (Janeway Children's Health and Rehabilitation Centre, St John's, NL); Keith Barrington (Sainte Justine Hospital, Montreal, QC); Valerie Bertelle (Centre Hospitalier Universitaire de Sherbrooke, Fleurimont, QC); Barbara Bullied (Everett Chalmers Hospital, Fredericton, NB); Rody Canning (Moncton Hospital, Moncton, NB); Zenon Cieslak (Royal Columbian Hospital, New Westminster, BC); Orlando da Silva (London Health Sciences Centre; London, ON); Kimberly Dow (Kingston General Hospital, Kingston, ON); Michael Dunn (Sunnybrook Health Sciences Centre, Toronto, ON); Daniel Faucher (Royal Victoria Hospital, Montreal, QC); Adele Harrison (Victoria General Hospital, Victoria, BC); Andrzej Kajetanowicz (Cape Breton Regional Hospital, NS); Zarin Kalapesi (Regina General Hospital, Regina, SK); Lajos Kovacs (Jewish General Hospital, Montreal, QC); Kyong-Soon Lee (The Hospital for Sick Children, Toronto, ON); Douglas D. McMillan (IWK Health Centre, Halifax, NS); Chuks Nwaesei and Mohammed Adie (Windsor Regional Hospital, ON); Cecil Ojah (St. John Regional Hospital, St. John, NB); Abraham Peliowski and Khalid Aziz (Royal Alexandra Hospital, Edmonton, AB); Bruno Piedboeuf (Centre Hospitalier Universitaire de Québec, Sainte Foy, QC); Patricia Riley (Montreal Children's Hospital, Montreal, QC); Nicole Rouvinez-Bouali (Children's Hospital of Eastern Ontario, Ottawa, ON); Koravangattu Sankaran (Royal University Hospital, Saskatoon, SK); Mary Seshia (Health Sciences Centre, Winnipeg, MB); Prakesh S. Shah (Mount Sinai Hospital, Toronto, ON); Sandesh Shivananda (Hamilton Health Sciences Centre, Hamilton, ON); Anne Synnes (Children's and Women's Health Centre of British Columbia, Vancouver, BC); Wendy Yee (Foothills Medical Centre, Calgary, AB).