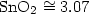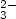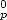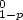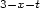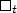The paper presents two large-scale observations of magnetic treatment of industrial water,
aimed at investigating changes in the formation of deposits. First, a four-month experiment is
described with two identical 25 kW heat exchangers, where in one case the inlet water was treated
by a magneto-hydrodynamic method. Deposits recovered from both exchangers were analyzed
chemically, by X-ray diffraction, infrared spectroscopy and PIXE. The amount of deposit for
untreated water, composed mostly of calcite, increased exponentially with temperature reaching
20 g/m of tube at the warm end of the heat exchanger. The mass of the deposit for magnetically
treated water did not depend on temperature and was only ca. 0.5 g/m of tube. It was composed of
mainly noncrystalline silica-rich material. Further results were obtained from the practical
installation at three blocks of a 1 GW power plant. The soft, amorphous deposit for magnetically
treated water had a specific surface area of 80 m2/g and an infrared spectrum similar to that of a
silicate hydrogel. Therefore, it appeared that, as a result of the passage through the magnetic
device, crystallization of carbonates in water was blocked due to initiation of another, competitive
process. This process is the activation of the colloidal silica, which will adsorb calcium,
magnesium or other metal ions and then precipitate from the solution as the coagulated
agglomerate. The most probable mechanism responsible for silica activation is a Lorentz-force
induced deformation of the diffuse layer leading to the increased counterion concentration in the
adsorption layer of the negatively charged silica.