Introduction
Fragmentation of the Earth's natural terrestrial ecosystems by humans has resulted in small, isolated populations of many species. Three genetic consequences of these small populations are genetic drift (random loss of alleles from a population and long-term accumulation of recessive deleterious alleles [genetic drift load]), inbreeding (resulting in inbreeding depression) and isolation (resulting in reduced gene flow, or lack thereof, between populations) (Barrett and Kohn, Reference Barrett, Kohn, Falk and Holsinger1991; Ellstrand and Elam, Reference Ellstrand and Elam1993; Young et al., Reference Young, Boyle and Brown1996; Keller and Waller Reference Keller and Waller2002; Lienert, Reference Lienert2004; Honnay et al., Reference Honnay, Jacquemyn, Bossuyt and Hermy2005; Aguilar et al., Reference Aguilar, Quesada, Ashworth, Herrerias-Diego and Lobo2008; Jacquemyn et al., Reference Jacquemyn, De Meester, Jongejans and Honnay2012; Haddad et al., Reference Haddad, Brudvig, Clobert, Davies, Gonzalez, Holt, Lovejoy, Sexton, Austin, Collins, Cook, Damschen, Ewers, Foster, Jenkins, King, Laurance, Levey, Margules, Melbourne, Nicholls, Orrock, Song and Townshend2015). Thus, theoretically, these genetic consequences of fragmentation increase homozygosity, resulting in the loss of fitness. The primary aim of this paper was to review the effect of habitat fragmentation/population size on seed germination, a fitness-related trait (e.g. Reed and Frankham, Reference Reed and Frankham2003; Reed, Reference Reed2005; Angeloni et al., Reference Angeloni, Ouborg and Leimu2011). We hypothesized that seeds of the same species from large populations generally germinate to higher percentages than those from small populations.
Methods
During the past 10 years or so, we have collected information from the scientific literature on the effect of habitat fragmentation/small population size on the fitness trait seed germination. Here, we summarize the results for 119 species (142 species entries). Compared with germination responses of seeds from large populations (‘control’, Wl), we placed the germination responses of seeds from small populations (‘treatments’, Ws) into three categories: (1) negative effect, seeds from small populations (fragments) germinated to lower percentages than those from large populations (continuous vegetation type/large fragments) (Wl > Ws), or percentage of germination was positively correlated (related) to population size; (2) no effect (none), seeds from small populations germinated equally as well as those from large populations (Wl = Ws), or no correlation (relationship) between germination percentage and population size and (3) positive, seeds from small populations germinated better than those form large populations (Wl < Ws), or germination percentage was negatively correlated (related) with population size. To determine to which of the three responses categories (i.e. negative, none or positive effect) seeds of a small population belonged (i.e. the effect of germination of a large population on germination of small population), we used the significant/non-significant results of statistical tests reported by the authors of the papers. Plant nomenclature follows Plants of the World Online.
Results and conclusions
We found information on population size and germination for 119 species in 50 families (Table 1). Sixteen of the species were included in more than one study, making a total of 142 species entries in Table 1. Surprisingly, for 82 of the 142 entries (57.7%) there was no effect (none) on the small population (Wl = Ws), i.e. no difference in germination percentages of seeds from large and small populations (or no relationship between germination percentage and population size). For 50 of the 142 entries (35.2%), the response was negative for the small population (Wl > Ws), i.e. a higher germination percentage for seeds from large than small populations (or germination was positively related to population size). For 10 of the 142 entries (7.0%), the response was positive for the small population (Wl < Ws), i.e. a higher germination percentage for seeds from small than large populations (or germination percentage was negatively related to population size). Eight of the 16 species included in more than one study responded differently to fragmentation (i.e. same species, different effect); seven species none and negative and one species positive and negative (Table 1).
Table 1. Effect of habitat fragmentation (larger → smaller population size) on seed germination
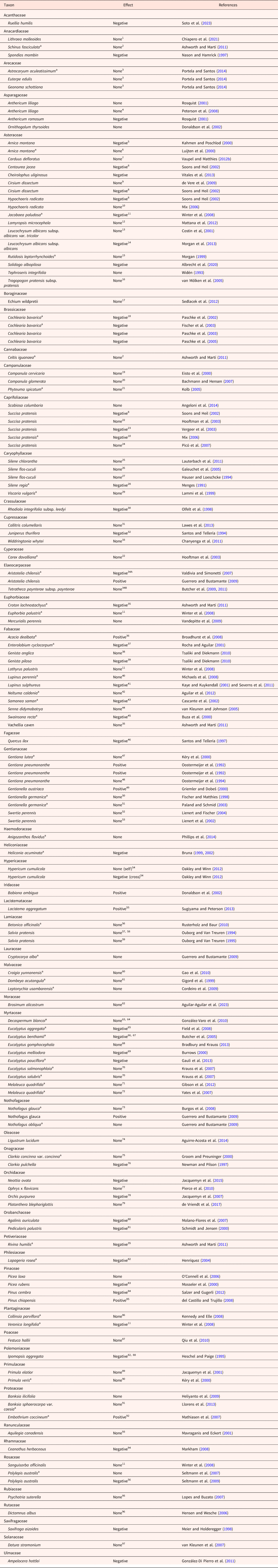
1 There was no effect of fragmentation on progeny performance.
2 Seed mass was not significantly different between continuous forest and fragments.
3 Population structure, i.e. proportions of seedling, infant, juvenile, immature and reproductive stages, was not affected in the smaller fragments.
4 There was a significant correlation between log population size and the Shannon index of gene diversity.
5 There was no significant relationship between population size and genetic variation. Percent germination was correlated with seed size and percent viable seeds.
6 Neither percentage nor rate (speed) of germination was correlated with population size. Germination in nearly all populations was 100%. Neither fruit mass nor seedling characteristics was correlated with population size.
7 Seed germination was not influenced by population size, density or centrality, i.e. small peripheral populations did not differ from large central populations. Seed mass was higher in large than in small populations.
8 Seed germination percentage decreased with a decrease in population size (Ne), but time to germination was not affected by population or by site productivity.
9 There was a positive relationship between population size (number of rosettes) and genetic diversity. Seedling survival was used as the measure of fitness.
10 Germination percentage was not related to population isolation.
11 Germination of Euphorbia palustris and Senecio paludosus was negatively affected by population isolation, but apparently isolation had no effect on germination of Lathyrus palustris, Sanguisorba officinalis or Veronica longifolium. Mean seed mass was significantly higher in small than in large populations of L. palustris, but apparently population size had no effect on mean seed mass of the other four species. In all five species, germination percentage was positively related to seed size.
12 All seeds from both large and small populations germinated when sown in the field. Seeds germinated as soon as the snow melted in spring.
13 Germination percentages were very high, and germination rate was rapid.
14 Mean germination percentage was >65 in all 19 study populations, but there was a significant positive relationship between log population size and mean germination percentage in laboratory trials. Some measures of genetic variation were positively correlated with population size. The relationship between measures of isolation and final germination percentage was not significant.
15 In two years of the three-year study, seed set was positively associated with population size.
16 There was no effect of population size or degree of isolation on germination.
17 There was no effect of population size on seed mass.
18 Seed mass was greater in large than in small populations.
19 Seed size was not related to germination percentage.
20 There was no relationship between population genetic diversity and germination percentage.
21 There was no effect of seed mass on germination.
22 There was no effect of size or degree of isolation of local habitat islands on seed germination percentage.
23 Seed mass was positively correlated with population size.
24 Seed mass and germination percentage were not significantly affected by inbreeding levels.
25 There was no significant correlation between population size and genetic diversity.
26 Seed germination percentage increased with heterozygosity, i.e. seeds from more inbred populations germinated to lower percentages. Population of origin significantly affected germination percentage.
27 There was no clear relationship between seed germination proportion (number of germinated seeds/total number of developed seeds) and population size or degree of isolation. There also was no relationship between seed mass and population size.
28 Seed germination percentages were higher in large than in small populations but were unrelated to population isolation. Non-germinated seeds were assumed to be dead, not dormant. If this assumption is correct, then 100% of the viable seeds germinated across all population sizes, and there were many non-viable seeds in the smallest populations, which the author thought might be due to inbreeding depression. The high percentage of (presumably) non-viable seeds from the smallest populations is surprising because the author says that he used ‘Full-sized, healthy-looking seeds … ’ in his germination studies.
29 There was no correlation between seed germination percentage and genetic diversity; however, population size was positively related to genetic diversity.
30 Seed germination percentage was significantly lower in population MN1 than in populations MN2, MN3 and MN4. MN1 had a much lower Ne/N ratio [number of genetically effective individuals (Ne)/total number of individuals (N)] than did the other three populations. Germination percentage was positively correlated with the Ne/N ratio.
31 Seed germination percentage was significantly higher in large than in small (monospecific) stands due to a higher proportion of seeds with developed embryos in large than in small stands. However, the proportions of seeds that were viable and that germinated were almost identical, regardless of stand size.
32 The lower density of seedlings in small forest fragments than in large forests was the result of much higher seed consumption by wood mice in the small fragments.
33 There was no relationship between seed germination percentage/seed viability (seed viability per cone = seed germination percentage + positive results with tetrazolium test for non-germinated seeds). The authors concluded that ‘ … the proportion of viable seeds per cone in W. whytei is not affected by population fragmentation, tree diameter and crown position in the forest canopy.’
34A Frugivory was 2.4 times higher in continuous forest than in forest fragments. Seeds eaten by birds germinated 1.7 and 3.7 times higher percentages than non-eaten seeds from continuous forest and fragment, respectively.
34B Genetic diversity was higher in the large than in small populations, but germination of was not related to population size.
35 Seed mass was not significantly different between continuous forests and fragments.
36 Genetic diversity was higher in large than in small populations.
37 Trees in continuous forest were more likely to set seeds than isolated trees in pastures. Seed mass and seedling vigour also were higher for trees in primary forest than in isolated trees.
38 Seed mass increased significantly with population size.
39 Seed mass did not increase significantly with population size.
40 Strength of inbreeding depression did not differ with population size.
41 Genetic diversity did not differ between very small, small, medium and large populations.
42 Seed mass did not differ between fragment sizes.
43 Germination of (scarified) seeds from continuous forest (75%) was significantly higher than that of (scarified) seeds from trees in isolation (58%). However, there was no significant difference in days to emergence between seeds of continuous forest and isolated trees. Undamaged seeds were planted for the germination tests; thus, differences in germination percentages were not due to inviable seeds. Genetic diversity was comparable for seeds from trees in continuous forest and isolated trees.
44 There was no relationship between population size and seed mass. However, there was a positive relationship between seed mass and seed germination percentage.
45 Seed germination percentages were high for all populations, but there were significant differences among the three inbred classes, with fewer seeds germinating in the most highly inbred population than in the other two populations.
46 Abundance of normal acorns was the same (or perhaps even higher in small than in large populations). However, acorn consumption by mice was much higher in the small than in large populations, thus accounting for the lower seedling establishment in small than in large populations.
47 There was a significant negative correlation between population size and seed mass.
48 There was no correlation between seed germination percentage and either population size or genetic variation.
49 Seed germination percentage was highest in the smallest population, which also had the highest genetic diversity.
50 Seed mass was independent of population size.
51 Total fitness of selfed progeny in small populations was 19% higher than that of selfed progeny in large populations.
52 Seed germination percentage did not differ between island population types, i.e. considering size of population and distance (degree of isolation) from other populations.
53 Seed germination percentage was not affected by either area or isolation (i.e. size or distance of island).
54 Compared to large populations, small populations had lower individual fitness, and crosses between them produced offspring with greater heterosis (hybrid vigour); however, there was no difference in inbreeding depression between small and large populations. The 68% lower individual fitness of within-population outcrosses in small than in large populations is consistent with fixation of deleterious alleles by genetic drift.
55 Seeds were larger in small than in large fragments.
56 Six years after fragmentation, seed mass was higher in the fragments than in the continuous population.
57 Mean seed mass was significantly correlated with seed germination percentage.
58 Inbreeding load was not significantly different among populations, but it did differ among maternal families.
59 Seed mass did not differ among populations.
60 Seed mass differed significantly among populations and was highest in the largest population.
61 The large population produced more seeds per fruit than the small populations.
62 Vigour of progeny from continuous large forests was higher than that of progeny from fragmented forests, which the authors thought was associated with reduced number of sires in the fragments. Genetic diversity of adult trees and their progeny did not differ between continuous forests and fragments. Seed mass had a positive effect on germination and seedling emergence.
63 Genetic diversity of the adult population was not associated with seed germination.
64 There was no difference in seed mass between large and small populations.
65 Genetic diversity was negatively correlated with relative population size (RPS) in Eucalyptus aggregata. RPS of E. aggregata = Actual population size (APS) values for E. aggregata/APS of E. aggregata + E. rubicola + E. viminalis + E. dalympleana. Seed germination percentage of E. aggregata increased with RPS.
66 There was no clear relationship between genetic diversity and population size.
67 Seed size was smaller in large than in small populations.
68 There was no significant difference in genetic diversity among the six populations.
69 There was no difference in mean mass of seeds from trees in woodland and of those from isolated trees.
70 Seed mass was independent of population size.
71 There was no relationship between population size, degree of isolation or fragment size and seed germination percentage.
72 There was a significant positive correlation between number of seeds produced per fruit and an increase in population size for each of the three study years.
73 Seed germination percentage was low (<3%) and did not vary between seeds from continuous forest and fragment.
74 Ligustrum lucidum is a non-native invasive evergreen tree in the Argentinian Chaco Serrano phytogeographical region, the study area. Reproductive success of this species was much lower in fragments than in a continuous forest.
75 This species is naturally patchily distributed. We considered central populations as large and isolated populations as small. Inbreeding depression of seed germination was not influenced by population type, i.e. central versus isolated.
76 Seed germination percentage (proportion of seeds planted that germinated and survived through the winter) was significantly higher in populations with high genetic effective population size (21.1%) than in populations with low genetic effective population size (8.7%).
77 Athough the relative performance index (RP) was –0.16, indicating that seeds from the small population germinated better than those from the large population (see Baskin and Baskin, Reference Baskin and Baskin2015), the germination percentages for seeds from large and small populations were not statistically different.
78 Fruiting success and seedling recruitment were not related to genetic diversity of the populations.
79 Seed germination percentage decreased with increase in population isolation.
80 The smallest and most isolated population in the study had the lowest seed germination percentage.
81 Number of seedlings per flowering plant was significantly higher in populations with a high amount of genetic variation.
82 Seed size was greater in large than in small populations.
83 Although seed germination percentages between large (75) and small (72) populations were statistically significant, relative performance index was only 0.04, indicating that there was no difference in germination of seeds from large and small populations (see Baskin and Baskin, Reference Baskin and Baskin2015).
84 There was significantly lower seed production, lower seed mass, higher embryo abortion and lower seed germination percentages in the small fragmented than in the large continuous population. Seed germination percentages was positively related to seed mass, and the differences between the large and small populations were still significant after accounting for seed mass.
85 Seed germination percentage was higher in small than in medium or large populations.
86 Large-flowered plants produced seeds with greater mass than small-flowered plants.
87 Seed germination percentage was not associated with population size, population isolation or genetic diversity.
88 Mean seed germination percentage was positively correlated with seed size.
89 In the smallest population (N = 11), there was a positive relationship between seed size and germination percentages. However, in the other three populations (N = 40, 1235 and 2291) there was no relationship between seed size and germination percentage.
90 There was a significant negative correlation between population size and seed mass.
91 Both mean seed mass and number of fathers per seed crop influenced the proportion of seeds that germinated.
92 There was no significant correlation between genetic variation of adult plants and population size.
93 Seed mass in this naturally patchily distributed species did not differ significantly between islands in the St. Lawrence River and the mainland in eastern Ontario, Canada. Although there was a negative correlation between population isolation and seed germination percentage, it was not significant.
94 Although there was a significant positive exponential relationship between population size and seed germination percentage, germination was <20% in all populations (small → large), and it was ≤ca. 6% in all populations except the largest one.
95 Neither seed germination percentage nor seed mass differed between non-fragments (NF), fragments (F) and fragments connected by corridors (F + C), i.e. WNF = WF = WF + C.
96 There was no relationship between seed germination percentage and genetic diversity.
97 Populations differed significantly in seed germination percentage, but population size was not related to germination percentage.
a The species was included in the meta-analysis by Aguilar et al. (Reference Aguilar, Cristóbal-Pérez, Balvino-Olvera, Aguilar-Aguilar, Aguirre-Acosta, Ashworth, Lobo, Martén-Rodríguez, Fuchs, Sanchez-Montoya, Bernardello and Quesada2019). See text for explanation of ‘effect’.
Thirty-three of the 142 species entries contained useful information on seed mass of plants from large (Wl) and small (Ws) populations: 9, Wl > Ws; 18, Wl = Ws and 6, Wl < Ws. Thus, in 24 of the 33 entries (72.7%) seed mass of small populations was equal to or greater than that of large populations (see footnotes of Table 1). Various other aspects related to population size of the 142 species entries are included in the footnotes of Table 1. These include population genetic diversity and population size (5, Wl > Ws; 9, Wl = Ws; 0, Wl < Ws), seed germination percentage and genetic diversity (3, Wl > Ws; 9, Wl = Ws; 0, Wl < Ws) and seed germination percentage and seed mass (10,Wl > Ws; 7, Wl = Ws; 0, Wl < Ws). Furthermore, except in one study in which germination percentage decreased with an increase in population isolation (footnote 79) and in another study in which germination percentage decreased with isolation for two species and did not change for three species (footnote 11), seed germination percentage showed no significant relationship to degree of population isolation (footnotes 10, 14, 16, 22, 27, 28, 43, 52, 53, 71, 87 and 93 for Table 1). Thus, the great majority of these 14 studies (18 species) showed that population isolation had no effect on seed germination.
Here, we also report the results (not in Table 1 or footnotes) of 15 studies (12 species) on germination of seeds from species at the centre (Wc) versus the margin (Wm) of their geographical range: 3, Wc > Wm (Summerfield, Reference Summerfield1973; Cerabolini et al., Reference Cerabolini, Andreis, Ceriani, Pierce and Raimondi2004; Giménez-Benavides et al., Reference Giménez-Benavides, Escudero and Iriondo2007, Reference Giménez-Benavides, Escudero and Iriondo2008; Tsaliki and Diekmann, Reference Tsaliki and Diekmann2009); 7, Wc = Wm (Lammi et al., Reference Lammi, Siikamäki and Mustajärvi1999; Groom and Preuninger, Reference Groom and Preuninger2000; Mosseler et al., Reference Mosseler, Major, Simpson, Daigle, Lange, Park, Johnsen and Rajora2000; Castro et al., Reference Castro, Zamora, Hódar and Gómez2004, Reference Castro, Zamora, Hódar and Gómez2005; Vaupel and Matthies, Reference Vaupel and Matthies2012; Tabassum and Leishman, Reference Tabassum and Leishman2018; Pelletier and de Lafontaine, Reference Pelletier and de Lafontaine2023) and 2, Wc < Wm (Yakimowski and Eckert, Reference Yakimowski and Eckert2007; Bartle et al., Reference Bartle, Moles and Bonser2013). Thus, in nine of the 12 (75%) entries seeds of plants at the range margin germinated equally well or better than those at the centre of the range. Finally, we report the results (not in Table 1 or footnotes) of seven papers (10 species) on germination of seeds of species from the forest (or other vegetation type) interior (Wi) versus those from the edge of the forest or other vegetation type (We): 1, Wi > We (Piechowski, Reference Piechowski2007); 5, Wi = We (Restrepo and Vargas, Reference Restrepo and Vargas1999; Ramos et al., Reference Ramos, José, Solferini and Santos2007; Schmucki and de Blois, Reference Schmucki and de Blois2009; Christianini and Oliveira, Reference Christianini and Oliveira2012) and 4, Wi < We (López-Barrera and Newton, Reference López-Barrera and Newton2005; Suzán-Azpiri et al., Reference Suzán-Azpiri, Ponce-González, Malda-Barrera, Cambrón-Sandoval and Carrillo-Angeles2017). Thus, for nine of the 10 (90%) entries seeds of plants at the edge of the population germinated equally well or better than those of plants in the centre of the population.
Creation of edge effects via forest fragmentation undoubtedly will have negative effects on seed germination of recalcitrant species, especially in the tropics (Wen and Cai, Reference Wen and Cai2014; also see Wen, Reference Wen2011), where many of the non-pioneer tree species have recalcitrant seeds (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003; Yu et al., Reference Yu, Baskin, Baskin, Tang and Cao2008; Pritchard et al., Reference Pritchard, Sershen, Tsan, Wen, Jaganathan, Calvi, Pence, Mattana, Ferraz, Seal, Baskin and Baskin2022).
Our hypothesis that seeds from large populations generally germinate better than those from small populations is not supported. Seed germination percentage did not differ in the majority of cases (57.7%) in which seeds from large and small populations were compared, and in 7.0% of the comparisons seeds from small populations actually germinated better than those from large populations. Thus, population size is not consistently and positively related to seed germination percentage, i.e. not a reliable predictor of seed germination. Neither was there an overall positive relationship between seed germination and either seed mass or genetic diversity. In 12 of 14 studies that included population isolation and germination, population isolation had no effect on germination; in a 13th study isolation had a negative effect on germination and in a 14th study isolation had a negative effect on two species and no effect on three species. Our limited information suggests that in the majority of species seeds from marginal populations germinate about equally well or better than those from central populations and that seeds from the edge of a forest germinate about equally well or better than those from the forest interior.
The results of our ‘vote-counting’ method (see Gurevitch et al., Reference Gurevitch, Curtis and Jones2001) to determine the relationship between population size and seed germination percentage do not agree with those of a meta-analysis (M-A) by Aguilar et al. (Reference Aguilar, Cristóbal-Pérez, Balvino-Olvera, Aguilar-Aguilar, Aguirre-Acosta, Ashworth, Lobo, Martén-Rodríguez, Fuchs, Sanchez-Montoya, Bernardello and Quesada2019), who found an overall negative habitat fragmentation effect (Hedges’ d about −0.6) on seed germination. We think that an M-A may not be an appropriate way to get a reliable conclusion from our global dataset on population size versus seed germination for two reasons (e.g. Bailar, Reference Bailar1997; Lee, Reference Lee2019). First, one of the statistical advantages of M-A is that it increases the number of replicates in a study, thereby increasing statistical power. Thus, to be used correctly in an M-A the individual experiments (studies) that are pooled in an M-A need to be similar (i.e. replicates of each other). In doing an M-A of seed germination studies on a global scale, the so-called replicates include different kinds of seed dormancy and experimental procedures using seeds from plants that grow in different climates and vegetation types.
A second concern about M-A is that one number (effect size) summarizes the results of the whole field of research, in our case the effect of fragmentation/population size on seed germination. It seems to us that using a single number based on variable methodology (inconsistent protocol and context-dependent source experiments and different classes and degrees of dormancy) to represent germination responses of numerous plant taxa may convey the wrong impression to conservationists, ecologists and seed biologists.
For the 49 species included in the Aguilar et al. M-A that we include in our review, we tallied our designations of (1) no effect (none), (2) positive effect and (3) negative effect of fragmentation/population size on seed germination. For 31 of the 49 (63.3%) species, we recorded no effect (none) of fragmentation/population size on seed germination, and for 2 (4.1%) and 16 (32.7%) species there was a positive and negative effect of fragmentation/population size on germination, respectively. The percentages for the three categories based on the 49 species are similar to those reported for these three categories based on 119 species (142 species entries), namely 57.7, 35.2 and 7.0% for none, negative and positive, respectively.
We wonder if it is possible to get a reliable conclusion on seed germination in relation to anything on a global scale via M-A when there is wide variation in methodology in the individual studies used in the M-A.
Competing interest
The authors declare that they have no competing interests.






