Article contents
The physical principles of energy transduction in chloroplast thylakoid membranes
Published online by Cambridge University Press: 17 March 2009
Extract
Photosynthesis in green plants or algae may be represented by an overall equation:
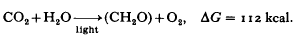
The energy necessary to promote this overall reaction is derived from light through absorption by pigment molecules — chiefly the chlorophylls.
Photosynthesis occurs in chloroplasts - subcellular organelles in which all the chlorophyll pigments are located. The chloroplasts comprise membranous, structures, and can be classified into two types. To the first type belong chloroplasts with appressed stacks of lamellar membranes, termed grana. These chloroplasts occur in mesophyll cells (C3 plants). The second type of chloroplasts are those with lamellar membranes that do not form the grana structures; they occur in bundle sheath cells of maize and other monocotyledons (C4plants, Hatch & Slack, 1970). In algae a greater diversity of structure occurs (Kirk & Tilney-Basset, 1978). Fluorescence microscopy indicates that chlorophyll molecules are localized mainly in the grana membrane regions of mesophyll-type chloroplasts and uniformly throughout the bundle sheath cells (Spencer & Wildman, 1962). Mesophyll chloroplasts are flattened saucer-shaped organelles (20 or more in each cell) of between 5000 and 10000 nm in diameter, and of thickness 1000–2000 nm, whilst the individual grana are each of the order of 300–500 nm in diameter. The available evidence suggests that individual lamellar membranes are arranged to form vesicles, or sacks where the internal space is completely delimited from the external space. These individual closed membrane structures were termed thylakoids (Menke, 1962).
- Type
- Research Article
- Information
- Copyright
- Copyright © Cambridge University Press 1983
References
(VII) REFERENCES
- 28
- Cited by


