The impact of nutrition on the methylome has received much attention over the past years( Reference Parle-McDermott and Ozaki 1 ). The methylome describes the distribution of methylated sequences within a given genome without changes to the DNA sequence itself. In man, DNA methylation takes place at the C-5 position of cytosine in CpG dinucleotides( Reference Parle-McDermott and Ozaki 1 ). One-carbon metabolism is central to the methylation of DNA (Fig. 1). The main nutrients implicated are methyl-group donors and cofactors including methionine, choline, betaine, folate, vitamin B6 and vitamin B12, which are all derived from the diet. Consequently, any dietary factor influencing this pathway may affect DNA methylation( Reference McKay and Mathers 2 , Reference Chmurzynska 3 ). The transfer of a methyl group to DNA depends on the availability of S-adenosylmethionine. S-Adenosylmethionine is derived from methionine, which is de-methylated through the action of DNA methyltransferases to S-adenosylhomocysteine( Reference Dominguez-Salas, Cox and Prentice 4 ). S-Adenosylhomocysteine is hydrolysed to homocysteine in a reversible reaction that strongly favours S-adenosylhomocysteine synthesis compared with hydrolysis. Homocysteine can leave the methionine cycle through the transsulfuration pathway or can be re-methylated back to methionine with vitamin B12 acting as a cofactor and 5-methyltetrahydrofolate acting as substrate( Reference King, Ho and Dodds 5 ).

Fig. 1 Simplified scheme of one-carbon metabolism. Nutrients included in the present study appear in bold. 5-Methyl THF, 5-methyl tetrahydrofolate; 5,10-methylene THF, 5,10-methylene tetrahydrofolate; DHF, dihydrofolate; DMG, dimethylglycine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; THF, tetrahydrofolate
In the context of a prospective observational study investigating the relationship between the intake of maternal methyl-group donors during each trimester of pregnancy and offspring DNA methylation, an instrument assessing the daily intake of methyl-group donors was needed. At study onset, no such tool was available since food composition databases for betaine and choline only recently became available( 6 ). It is preferred to use FFQ over diet records or 24 h recalls, because they have shown to be more suitable and accurate in assessing long-term or habitual exposure to specific nutrients involved in one-carbon metabolism (e.g. folate)( Reference Mason 7 ).
Studies on the effect of maternal diet and the methylome of the offspring are showing the importance of maternal nutrition. Heijmans et al.( Reference Heijmans, Tobi and Stein 8 ) explained the long-term effects of prenatal famine on the offspring methylome. They found an average decrease of 5·2 % in DNA methylation on the insulin-like growth factor 2 (IGF2) gene locus, showing that maternal undernutrition (low intake of methyl-group donors) can indeed have persistent effects on the offspring throughout adulthood. A similar study by Tobi et al.( Reference Tobi, Slagboom and van Dongen 9 ) suggested that famine exposure (in utero) only held a long-term effect on the offspring IGF2 gene when the exposure was periconceptional. On the other hand, Steegers-Theunissen et al.( Reference Steegers-Theunissen, Obermann-Borst and Kremer 10 ) found that periconceptional supplementation of mothers with 400 μg of folic acid (methyl-group donor) daily was associated with epigenetic changes (4·5 % higher DNA methylation) in the same IGF2 locus in the child.
A novel, self-administered, semi-quantitative FFQ was designed to estimate the daily intake of methionine, choline, betaine, folate and the sum of methyl-group donors in women of reproductive age (18–35 years). The aim of the present study was to assess the validity and reproducibility of the FFQ using the 7 d estimated diet record (7 d EDR) as reference method. The reproducibility of the FFQ was determined by administering the FFQ at two time points (6 weeks apart) to the same group of women, so that the association between the two responses could be assessed. Also the validity of the FFQ was determined to assess the degree to which the FFQ agreed with the reference method (7 d EDR). Even a subtle change in the design of the FFQ or the use of the FFQ in different demographic groups and cultures might affect its performance, so each new FFQ should be validated( Reference Cade, Thompson and Burley 11 ).
Materials and methods
Study design
A total of 296 women of reproductive age (18–35 years) were invited to participate in the study. Female students or employees of the Catholic University of Leuven were asked to take part in the study. An invitation letter, sent through mail or personally delivered, informed the participants about the aim of the study. They were asked to return their first completed FFQ (FFQ1) and their written consent in a sealed envelope. Six weeks later the second FFQ (FFQ2) together with the 7 d EDR was distributed to only those participants from whom we received FFQ1. Participants were instructed to fill in the FFQ at day one, to start the 7 d EDR at day two, and to put the dates of completion on the documents. Detailed instructions for filling in the FFQ and EDR were given. The field work of the validation and reproducibility study was carried out from February to March 2012.
Of the 296 women invited to participate in the study, 161 women (54 %) returned FFQ1. The 161 women were again invited to take part in the second phase of the study and of them ninety-eight (61 %) completed FFQ2 and the 7 d EDR. Only FFQ without missing values were included in the reproducibility study, so after exclusion of incomplete FFQ and outliers ninety-two women were included in the analysis. Similarly, only complete 7 d EDR including seven completed record days and containing sufficiently detailed descriptions of the food products (including brand names, the food type (e.g. the use of whole, semi-skimmed or skimmed milk, the type of bread used, etc.)) and portion sizes (expressed as household measures, standard units (e.g. a medium-sized apple) or units like grams or litres) consumed could be used in the validation study; so after exclusion of incomplete 7 d EDR eighty women were included in the analysis.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the UZ Leuven-Committee for Medical Ethics (reference number: ML7975). Written informed consent was obtained from all participants.
FFQ
The Flemish, self-administered, semi-quantitative FFQ contained questions on the average consumption of fifty-one food items during the past 3 months (see online supplementary material, Part 1). The time frame of 3 months was chosen because this FFQ will be used to assess the intake of maternal methyl-group donors during each trimester of pregnancy. The concept and structure of the FFQ were based on a previously developed and validated FFQ estimating usual daily fat, fibre, alcohol, caffeine and theobromine intakes among Belgian postmenopausal women( Reference Bolca, Huybrechts and Verschraegen 12 ). However, the food list included in our newly developed FFQ (fifty-one food items) was adopted considering our study aims, namely to estimate the daily intake of methionine, choline, betaine, folate and the sum of methyl-group donors. Food items included in an FFQ should be eaten reasonably often by the population and/or contain a substantial amount of one or more nutrients of interest( Reference Cade, Thompson and Burley 11 ).
The fifty-one food items included in our FFQ were food items that are part of the Belgian diet (National Food Consumption Survey) and/or had a high nutrient content (>90th percentile) of one the four methyl-group donors. All food items were listed according to their nutrient content (from high to low) and the top 10 % food items were selected. Participants were asked to indicate their answer from a list of frequencies: ‘never or less than once per month’; ‘1–3 days per month’; ‘1 day per week’; ‘2–4 days per week’; ‘5–6 days per week’; or ‘every day’( Reference Willett 13 ). The FFQ also contained three to five daily portion size categories per food item and a list of common standard measures as examples. For some food groups, additional questions were asked regarding the type, such as specific type of fruit juice. All returned FFQ were reviewed for completeness and written comments. Frequency data from the FFQ were converted to servings per day (e.g. 1 serving/week=0·14 serving/d) to standardize the different frequency categories by means of a common unit (servings/d). Portion size categories were converted to a portion (grams) per serving by considering the mean of the portion size range that was chosen by the participant. The usual food intakes were computed by multiplying the servings per day with the portion size per serving. Usual nutrient (methionine, choline, betaine and folate) intakes were calculated by multiplying the usual food intakes with the nutritional value per 100 g of product. The food composition data were based on the Dutch NEVO food composition database( 14 ) for folate, the German BLS nutrient database( Reference Dehne, Klemm and Henseler 15 ) for methionine and the US Department of Agriculture (USDA) database for the Choline Content of Common Foods( 6 ) for choline and betaine. The USDA database was also used for the nutrient content of folate and methionine if not found in the NEVO and BLS database, respectively. Databases of neighbouring countries or the USDA database for choline and betaine content were used since the Belgian food composition database NUBEL( 16 ) does not contain the four nutrients of interest.
Estimated diet record (reference method)
In the present relative validation study the 7 d EDR was chosen as the reference method. The participants were given guidelines for filling in their diary and we also provided one correctly filled in day as an example. The participants were asked to report all consumed foods and drinks over seven consecutive days. In the 7 d EDR, days are subdivided into six eating occasions (breakfast, morning snacks, lunch, afternoon snacks, dinner and evening snacks). Detailed information on the type (including brand names, the food type (e.g. use of whole, semi-skimmed or skimmed milk, the type of bread used, etc.)) and portion size (expressed as household measures, standard units (e.g. a medium-sized apple) or units like grams or litres) of the foods consumed was collected using an open entry format. Only complete food diaries, including seven completed record days and containing sufficiently detailed descriptions of the food products and portion sizes consumed, were taken into consideration. In total seventeen EDR had to be excluded because of incomplete data. The complete 7 d EDR were coded and entered into a diet entry and storage program (NUBEL Voedingsplanner( Reference v.z.w. 17 )) using a manual on food portions and household measures( 18 ). Methionine, choline, betaine and folate are not included in the Belgian food composition table NUBEL( 16 ), so the diet records were linked to the same food composition databases as for the FFQ (Dutch NEVO food composition database( 14 ), the USDA database for the Choline Content of Common Foods( 6 ) and the German BLS nutrient database( Reference Dehne, Klemm and Henseler 15 )). Some typically Belgian food products like ‘speculaas’, a type of shortcrust biscuit, are not included in the USDA database. For these products, the choline and betaine content of the American variety was entered in the database. For example, a ginger biscuit was used for the choline and betaine content of ‘speculaas’.
Statistical analysis
Statistical analyses were carried out using the statistical software package IBM SPSS for Windows version 20·0. Results were considered statistically significant at a two-tailed α level of 0·05. Tests for normality of the data were performed using the Kolmogorov–Smirnov test. Data were normally distributed for the validation study and reproducibility study (after exclusion of three outliers with unusual eating behaviours); consequently only parametric tests were used during analysis. Means and standard deviations of the four methyl-group donating nutrients and the sum of all were estimated from the FFQ and 7 d EDR. Paired Student’s t test was used to determine significant mean differences. Associations were described using intra-class correlations. Correlations ranging from 0·00 to 0·25 indicate weak or no relationship; those from 0·25 to 0·50 suggest a fair degree of relationship; values of 0·50 to 0·75 are moderate to good; and values above 0·75 are considered good to excellent( Reference Portney and Watkins 19 ). In the validity study, the de-attenuated intra-class correlation coefficients were calculated to correct for within-person variation in the 7 d EDR. This de-attenuated correlation is calculated as the ratio of the inter-individual (true between-subject variation in usual intake) CV to the intra-individual (day-by-day variation in intake, day-of-the-week variation in females) CV( Reference Beaton, Milner and Corey 20 ). Agreement between the 7 d EDR and the FFQ at an individual level was assessed using mean difference, standard deviation and limits of agreement of the difference, and visually represented in a Bland–Altman plot( Reference Bland and Altman 21 ). Individual results for nutrient intake estimated by the 7 d EDR and FFQ were classified into tertiles to assess the questionnaire’s ability to rank individuals according to the intake as in the 7 d EDR( Reference Willett 13 ). The percentage classified into the same and opposite tertile was calculated. Agreement between both methods was assessed using weighted kappa (κ w) statistics, calculated with a linear set of weights( Reference Altman 22 ). The measurement error of the FFQ was analysed using ‘actual values for surrogate categories’( Reference Willett 13 ). One-way ANOVA test was used to determine significant differences in means between tertiles. Specificity was defined as the proportion of those with a daily intake above the guidelines( 23 – 25 ) on the basis of the 7 d EDR who also fell above the guidelines on the FFQ (true negatives). Sensitivity was the proportion of those with a daily intake below the guidelines on the basis of the 7 d EDR who also fell below the guidelines on the FFQ (true positives). The positive predictive value was the proportion of those identified by the FFQ as having an inadequate intake who actually had an inadequate intake (according to the 7 d EDR). The negative predictive value was the proportion of those identified by the FFQ as having an adequate intake who actually had an adequate intake (according to the 7 d EDR; see online supplementary material, Part 2).
Results
Validation study
In total, eighty women were included in the validation study. Table 1 presents the characteristics of the study participants. Women included in the validation study had a mean age of 23 (sd 4·1) years (range 18–35 years), a mean weight of 60 (sd 9·6) kg, a mean height of 1·68 (sd 0·05) m and a mean BMI of 21·7 (sd 3·2) kg/m2. Twenty-six women (32·5 %) were employed at the time of the study, so fifty-four participants (67·5 %) were students. Only two women (2·5 %) smoked at the time of the study. Mean intakes of methionine, choline, betaine, folate and the sum of methyl-group donors (SUM) estimated with the 7 d EDR and the FFQ, mean differences, and raw and de-attenuated intra-class correlation coefficients are presented in Table 2. The FFQ overestimated the intake of folate and betaine in comparison with the 7 d EDR. The mean intakes of folate and betaine were significantly different, but fair to good de-attenuated correlation coefficients ranged from 0·32 (betaine) to 0·68 (folate). The mean difference in methionine intake between FFQ1 and the 7 d EDR was −33·3 (sd 600·6) mg/d, demonstrating that the FFQ underestimated methionine intake. The mean differences in choline, betaine, folate and SUM intakes between the two methods were 2·3 (sd 104·2) mg/d, 32·1 (sd 78·0) mg/d, 68·6 (sd 88·0) μg/d and 1·27 (sd 733·0) mg/d, respectively, demonstrating that the FFQ overestimated choline, betaine, folate and SUM intakes compared with the 7 d EDR. This is shown graphically in Bland–Altman plots (Fig. 2), which reveal that outliers widened the limits of agreement and made the plots more divergent. This divergent pattern could indicate increasing bias with increasing intakes. Cross-classification analysis indicated that about half of the participants (40–54 %) were classified correctly, while 9 % (methionine) to 20 % (choline) of them were grossly misclassified (Table 3). Results from the κ w statistics showed fair agreement between the FFQ and 7 d EDR (0·21 for betaine to 0·35 for methionine and SUM), but a poor agreement for choline (0·10). Actual values for surrogate FFQ tertiles showed a progressive increase in 7 d EDR intakes of methionine, betaine, folate and SUM between the first and third FFQ tertile (Table 4) with statistically significant differences in mean intake between the tertiles for all nutrients except for choline. The specificity and sensitivity of the FFQ, for indicating women with lower nutrient intake than the recommended daily intake, were 87 % and 37 % for folate, 33 % and 97 % for choline, and 100 % and 100 % for methionine, respectively. The positive and negative predictive values were 76 % and 55 % for folate, 97 % and 33 % for choline, and 100 % and 100 % for methionine, respectively. The specificity and sensitivity of the FFQ for betaine could not be calculated since there is no recommended daily intake for betaine.

Fig. 2 Bland–Altman plots evaluating the relative validity of the novel FFQ to assess the daily intake of four methyl-group donors among Flemish women (n 80) of reproductive age (18–35 years), February–March 2012. The difference between the intake estimated by the FFQ and the intake from the 7 d estimated diet record (7 d EDR) is plotted against the mean intake by the two methods for each participant and nutrient: (a) methionine; (b) choline; (c) betaine; (d) folate; and (e) the sum of methyl-group donors (SUM). The mean difference (———) and the limits of agreement (mean difference±2 sd; - - - - - -) are shown
Table 1 Characteristics of the study participants: Flemish women of reproductive age, female students or employees of the Catholic University of Leuven, February–March 2012

Table 2 Mean intakes of methionine, choline, betaine, folate and SUM estimated with the 7 d EDR and FFQ1 among Flemish women (n 80) of reproductive age (18–35 years), February–March 2012
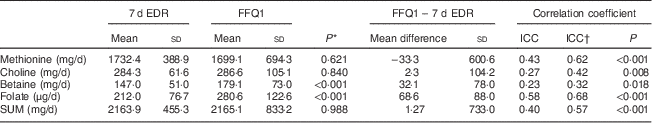
SUM, sum of methyl-group donors; 7 d EDR, 7 d estimated diet record; FFQ1, first administration of the FFQ; ICC, intra-class correlation coefficient.
* Paired Student’s t test was used to determine significant differences between means.
† De-attenuated ICC.
Table 3 Cross-classification analysis and κ w statistics for the 7 d EDR and FFQ tertiles of usual daily intakes of methyl-group donors among Flemish women (n 80) of reproductive age (18–35 years), February–March 2012
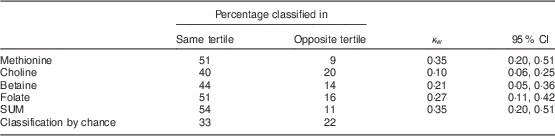
κ w, weighted kappa; 7 d EDR, 7 d estimated diet record; SUM, sum of methyl-group donors.
Table 4 Use of actual values for surrogate tertiles to compare the usual daily intakes of methyl-group donors from the FFQ with those from the 7 d EDR among Flemish women (n 80) of reproductive age (18–35 years), February–March 2012
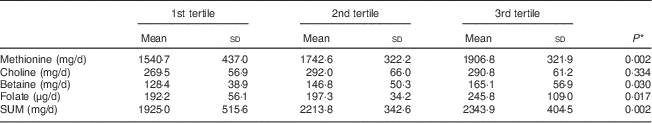
7 d EDR, 7 d estimated diet record; SUM, sum of methyl-group donors.
* One-way ANOVA was used to determine significant differences in means between tertiles.
Reproducibility study
In the reproducibility study, ninety-two women were included. Table 1 presents the characteristics of the study participants. Women included in the reproducibility study had a mean age of 23 (sd 3·9) years (range 18–35 years), a mean weight of 60·2 (sd 9·8) kg, a mean height of 1·68 (sd 0·06) m and a mean BMI of 21·7 (sd 3·1) kg/m2. Twenty-seven women (33·7 %) were employed at the time of the study, so sixty-five participants (66·3 %) were students. Only two women (2·1 %) smoked at the time of the study. Mean intakes of methionine, choline, betaine, folate and SUM from FFQ1 and FFQ2 were significantly different and correlation coefficients ranged from 0·62 (betaine) to 0·83 (folate; Table 5). Cross-classification analysis indicated that 1 % (methionine) to 7 % (betaine) of the participants were grossly misclassified, while more than half of them (57–71 %) were classified correctly (Table 6). Results from the κ w statistics showed moderate to good agreement (0·44 for betaine to 0·66 for methionine).
Table 5 Mean intakes of methionine, choline, betaine, folate and SUM estimated with FFQ1 and FFQ2 among Flemish women (n 92) of reproductive age (18–35 years), February–March 2012
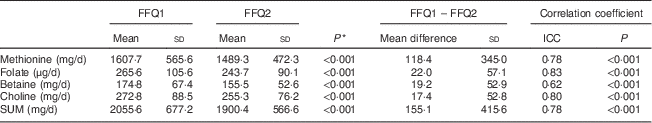
SUM, sum of methyl-group donors; FFQ1, first administration of the FFQ; FFQ2, second administration of the FFQ; ICC, intra-class correlation coefficient.
* Paired Student’s t test was used to determine significant differences between means.
Table 6 Cross-classification analysis and κ w statistics for the FFQ1 and FFQ2 tertiles of usual daily intakes of methyl-group donors among Flemish women (n 92) of reproductive age (18–35 years), February–March 2012
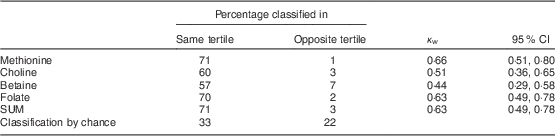
κ w, weighted kappa; FFQ1, first administration of the FFQ; FFQ2, second administration of the FFQ; SUM, sum of methyl-group donors.
Discussion
Validation study
The FFQ has an acceptable ranking ability (r=0·32–0·68; κ w=0·10–0·35), but in general overestimates the daily intake of folate (280·6 μg v. 212·0 μg) and betaine (179·1 mg v. 147·0 mg) compared with the 7 d EDR. Cross-classification analysis indicated that 20 % (choline) of the women were grossly misclassified in the validation study.
Based on the comparison of means, the FFQ seems to overestimate the mean intakes of folate (which has also been reported in previous studies( Reference Longnecker, Lissner and Holden 26 – Reference Pufulete, Emery and Nelson 28 )) and betaine compared with the 7 d EDR. Unfortunately, we could not determine whether this was due to over-reporting, because our FFQ was not designed to estimate energy intake. A possible explanation is the contribution of vegetables to total folate intake. In our FFQ, vegetables contributed 34 % of total folate intake, which is comparable to the 36 % found in another FFQ( Reference Pufulete, Emery and Nelson 28 ). Fruit and vegetables are most often over-reported in FFQ( Reference Feskanich, Rimm and Giovannucci 29 ), possibly because these are socially desirable foods( Reference Salvini, Hunter and Sampson 30 ). Another explanation for this overestimation of folate is the long list of vegetables in the FFQ contributing to the discrepancy in intake between the FFQ and 7 d EDR, since estimates of vegetable intake have been shown to be related to the number of questions asked( Reference Krebs-Smith, Heimendinger and Subar 31 ). A possible reason for the overestimation of betaine by the FFQ is that the betaine values assigned to the food items in the 7 d EDR might be more accurate (because of higher level of detail in the food descriptions) than the aggregated values assigned to the food groups in the FFQ. Another potential explanation might be that some of the food groups high in betaine have been overestimated by the women due to several possible reasons: difficulties in assessing the frequency of consumption of such large food groups, socially desirable behaviour, etc. These possible reasons should be investigated further in future research.
Associations between dietary instruments (FFQ and EDR) measured by correlation coefficients should be at least 0·40 and optimally in the range of 0·50–0·70 in order to rank persons reliably( Reference Willett 13 ). Because the de-attenuated correlation coefficients observed in the present study are between 0·40 and 0·68, we can conclude that the FFQ has a good ranking ability (except for betaine (0·32)). In the present study we found a de-attenuated correlation of 0·68 for folate. Longnecker et al.( Reference Longnecker, Lissner and Holden 26 ) compared nutrient intakes assessed with diet records and an FFQ with 116 food items in 138 men and women. For folate, they found a de-attenuated Pearson correlation coefficient of 0·42, which is lower than the de-attenuated correlation coefficient found in the present study (0·68). Pufelete et al.( Reference Pufulete, Emery and Nelson 28 ) developed a short FFQ to assess folate intake and validated it against a 7 d weighed diet record. For women, the correlation coefficient between the two methods was 0·30. A higher de-attenuated Pearson correlation coefficient for folate (0·77) was found by Sevak et al.( Reference Sevak, Mangtani and McCormack 27 ). They validated an FFQ with 207 food items against 24 h recalls in 100 women. No correlation coefficients for betaine and choline were found in the literature, since a food composition database only became available a few years ago( 6 , Reference Zeisel, Mar and Howe 32 ). Also, no validation papers that assess the intake of methionine using an FFQ were found in the literature.
The present cross-classification analysis showed that the FFQ classified 40 % of the women for choline and 54 % for the sum of methyl-group donors in the same tertile, and that there was a maximal misclassification of 20 % for choline. The κ w values showed a fair agreement between the FFQ and 7 d EDR, but a poor agreement for choline. Sevak et al.( Reference Sevak, Mangtani and McCormack 27 ) reported a moderate agreement (κ w=0·44) for folate between their FFQ and multiple 24 h recalls. They found that the FFQ classified 41 % of the women in the same quartile for folate, as opposed to the 51 % found in the present study. A lower misclassification of 5 % (16 % in the present study) and a similar classification of 45 % (41 % in the present study) in the same tertile for folate (in women) was found by Pufulete et al.( Reference Pufulete, Emery and Nelson 28 ), who developed a short FFQ to assess folate intake (in men and women) and validated it against a 7 d weighed diet record.
Since the actual values for surrogate tertiles showed the expected significant stepwise increase in nutrient intake, we concluded that the FFQ could reliably distinguish extreme nutrient intakes (except for choline).
The Bland–Altman plots showed large limits of agreement (mean difference±2 sd) between the two methods, indicating the limited use of the FFQ to estimate nutrient intake for individuals. The observed divergence in these plots suggests a greater difficulty in estimating nutrient intake with higher mean nutrient intakes. However, the FFQ was developed to classify and rank participants according to their intake in an epidemiological setting, not to assess intake at an individual level.
Also, the FFQ is not a good tool to use at an individual level for estimating the intake of folate with the Belgian Health Council guidelines( 23 ) as reference values, because 33 % of the women would lose the possibility of receiving a required intervention (daily folate intake <200 μg). The specificity and sensitivity of the other nutrients were more acceptable.
Reproducibility study
The correlation between repeated administrations was good (r=0·62–0·83) with a maximal misclassification of 7 % for betaine (κ w=0·44–0·66).
Lower mean intakes of all of the nutrients (7–12 % lower) were observed in the second questionnaire administration. Villamor et al.( Reference Villamor, Rifas-Shiman and Gillman 33 ) found similar results when they assessed maternal intakes of folate, vitamin B12, choline, betaine and methionine during the first and second trimesters of pregnancy. For betaine and choline a lower mean intake was found during the second trimester of pregnancy, an increase in mean intake was found for methionine, and no changes were found in mean intake for folate. Seasonal variation cannot explain this difference because both FFQ were administered during spring. A possible explanation is that boredom was higher and motivation was lower during the second administration.
In the present study, the intra-class correlation coefficients between the first and the second FFQ ranged from 0·62 for betaine to 0·83 for folate. These good correlations between the repeated administrations indicate that the random response error, sometimes due to lack of interest or motivation of the respondent, is rather small. In reproducibility studies, the correlation coefficients generally range from 0·5 to 0·7. Bidulescu et al.( Reference Bidulescu, Chambless and Siega-Riz 34 ) reported correlations of 0·49 (folate) and 0·48 (choline) in men and women aged 45–64 years using a version of the Willett sixty-one-item FFQ. A study on intake of methyl-group donors during the first and second trimesters of pregnancy using a 166-item FFQ showed correlations of 0·32, 0·55, 0·51 and 0·43 for folate, choline, betaine and methionine, respectively( Reference Villamor, Rifas-Shiman and Gillman 33 ). These correlations are lower than the correlations reported in the present study: 0·83 (folate), 0·80 (choline), 0·62 (betaine) and 0·78 (methionine). This lower correlation for folate (0·55) was also found by Longnecker et al.( Reference Longnecker, Lissner and Holden 26 ). On the other hand, a high correlation for folate (0·72) was also found by Pufulete et al.( Reference Pufulete, Emery and Nelson 28 ) who similarly assessed the intake of folate (in women) with a short newly developed FFQ.
The cross-classification analysis and κ w values indicated a moderate to good agreement between the repeated administrations, with a maximal misclassification of 7 % for betaine.
Strengths and limitations
To our knowledge, the present study is the first validation study that assesses the intake of methyl-group donors with a newly developed FFQ designed for these specific nutrients. This FFQ will be used in an observational study, providing us with a convenient and reliable instrument to rank individuals according to their intake of methyl-group donors( Reference Cade, Thompson and Burley 11 ). A key step in the validation process is the selection of a reference method for the tool to be validated against. Since there is no gold standard, it is important that the errors of both methods are as independent as possible. The EDR was chosen as reference method in the present study. Unlike the FFQ method, the EDR does not depend on memory, is moreover open-ended and involves direct estimation of portion size( Reference Cade, Thompson and Burley 11 ). The EDR also takes into account the within-person variability in food intake, which is necessary because in women there is a strong day-of-the-week effect( Reference Beaton, Milner and Corey 20 ). Therefore intra-class correlations were corrected for attenuation, which improved the correlations for all nutrients. EDR (instead of weighed diet records) were used because they have the same order of accuracy when ranking individuals and the respondent burden is lower( Reference Chinnock 35 ). Food diaries with seven consecutive recording days were used to deal with day-to-day variation and to cover all days of the week equally. Seven consecutive dietary records might decrease the subject compliance and therefore decrease the accuracy of the dietary records collected at the end of the 7 d period. Therefore, we compared the energy intake calculated during the first three record days with that calculated during the last three record days. There was no significant difference (P=0·121) between energy intakes from the first 3 d and last 3 d. Structured diaries, as well as an example for filling in the EDR, guided the women to report all consumption, even for easily forgotten snacks such as soft drinks and candy. In the literature we found a wide range in sample sizes to assess validity and reproducibility, with a median of 110 participants. A sample size of at least fifty, and preferably 100 or more participants, is desirable( Reference Cade, Thompson and Burley 11 ). Because the performance of a dietary assessment instrument depends on the characteristics of the study population and considering the target population in which the FFQ will be used, women of reproductive age (18–35 years) were recruited, leaving us with a large sample size and homogeneous group of women.
A limitation of this method is that it requires high motivation of the participants (many recording days), lack of which may lead to under-reporting of intake and inadequate food description( Reference Willett 13 ). In the present study, 61 % of the participants returned two FFQ and an EDR. This is a good response rate knowing how intensive it is to fill in the 7 d EDR. It was higher than the 15 % response rate seen in other validation studies( Reference Bingham, Gill and Welch 36 ). Women who filled in an EDR were promised nutritional advice in return, leading to some selection bias of volunteers who are more concerned about health and diet. But forcing non-motivated women to participate in the study might influence the quality of the data as well( Reference De Henauw, Brants and Becker 37 ). A limitation for the reproducibility study could be a possible memory effect during completion of the second FFQ as women could possibly remember what they filled in 6 weeks ago. Furthermore, because of dietary changes, for example food cravings and aversions, nausea, vomiting( Reference Bayley, Dye and Jones 38 , Reference Niebyl 39 ), during pregnancy and even possible dietary restrictions (e.g. restrictions to avoid toxoplasmosis), it might be more difficult to complete such FFQ during pregnancy. However, because of logistical reasons it was not feasible to perform this validation study in a group of pregnant women.
One methyl-group donor, folic acid, derived from the diet is missing from our FFQ. In Belgium many food items, for example breakfast cereals, are enriched with folic acid. At this time point there is no list available of food items enriched with folic acid in Belgium, and folic acid is not included in the Belgian food composition database. Food folate (without folic acid) was used to calculate the daily folate intake, since the fortification of products is different in other countries. Therefore the intake of folic acid could not be calculated. A last limitation is the use of food composition databases from different countries. Differences between databases include description of foods, calculation of nutrient content, recipe calculation, etc.( Reference Hakala, Knuts and Vuorinen 40 ).
Conclusion
The results found in the present validation and reproducibility study indicate that the FFQ is a reliable instrument with acceptable validity for ranking according to methyl-group donor intakes (except for a poor agreement for choline (κ w=0·10) and a fair ranking ability for betaine (r=0·32)) in Flemish women of reproductive age.
Acknowledgments
Acknowledgements: The authors acknowledge the women who volunteered to take part in the study. Financial support: Funding for the present study was provided by a PhD grant (grant number 11B1812N) from The Research Foundation-Flanders (FWO) and the Flemish Institute of Technological Research (VITO). FWO and VITO had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: S.P. and I.D. were responsible for the field work and data entry. I.H. helped with the statistical analysis of the results. All authors helped in the evaluation of the results and the writing of the manuscript. All authors have read and have approved the manuscripts before submission. Ethics of human subject participation: The study was approved by the UZ Leuven-Committee for Medical Ethics (reference number: ML7975).
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980014003140











