Introduction
Osteoarthritis (OA) is a very common problem, which has a major impact on the quality of life of older people. Around 8.5 million people in the United Kingdom are affected by joint pain which could be attributed to OA (Arthritis Care Resarch, 2004). It is expected, by the year 2020, OA will be the fourth leading cause of disability (Woolf and Pfleger, Reference Woolf and Pfleger2003). It commonly affects joints in the hands, hips and knees, often resulting in reduced function and mobility (Felson et al., Reference Felson, Lawrence, Dieppe, Hirsch, Helmick, Jordan, Kington, Lane, Nevitt, Zhang, Sowers, McAlindon, Spector, Poole, Yanovski, Ateshian, Sharma, Buckwalter, Brandt and Fries2000). The World Health Organization (2003) reports 80% of people with OA have some level of movement limitation and 25% are restricted in their daily life activities. These physical restrictions can have an impact on individuals’ quality of life and mental health.
Pharmacological treatments, such as non-steroidal anti-inflammatory drugs (NSAIDs), which produce some symptom relief, are commonly used for patients with OA. The high incidence of adverse events from NSAIDs is well documented (Gotzsche, Reference Gotzsche2004). NSAIDs can have serious, sometimes fatal side effects including gastrointestinal bleeding, renal failure, heart failure and cardiovascular risk (Smith, Reference Smith1989). Few of the very elderly can safely be prescribed NSAIDs because of the high incidence of comorbidities. There are also concerns about the use of opioid analgesics in the elderly; one case control study found they had more serious adverse events than NSAIDs (Solomon et al., Reference Solomon, Rassen, Glynn, Lee, Levin and Schneeweiss2010). Even with Paracetamol (acetaminophen), the risk may outweigh the benefits for those with comorbidities (McAlindon et al., Reference McAlindon, Bannuru, Sullivan, Arden, Berenbaum, Bierma-Zeinstra, Hawker, Henrotin, Hunter, Kawaguchi, Kwoh, Lohmander, Rannou, Roos and Underwood2014).
People living with OA have more comorbidities than their peers without OA (Kadam et al., Reference Kadam, Jordan and Croft2004; Stang et al., Reference Stang, Brandenburg, Lane, Merikangas, Von Korff and Kessler2006; Gabriel et al., Reference Gabriel, Crowson and O’Fallon1999a). Within arthritic conditions, comorbidities are predictors of poor physical function (Kadam and Croft, Reference Kadam and Croft2007) and mortality (Gabriel et al., Reference Gabriel, Crowson and O’Fallon1999b). Common comorbidities in OA include obesity (Rosemann et al., Reference Rosemann, Grol, Herman, Wensing and Szecsenyi2008), cardiovascular disease (Marks and Allegrante, Reference Marks and Allegrante2002; Gabriel et al., Reference Gabriel, Crowson and O’Fallon1999a), diabetes (Caporali et al., Reference Caporali, Cimmino, Sarzi-Puttini, Scarpa, Parazzini, Zaninelli, Ciocci and Montecucco2005) and psychological conditions such as depression and anxiety (Katon et al., Reference Katon, Lin and Kroenke2007). The National Institute for Health and Care Excellence (NICE) have made treating comorbidities in OA a research priority and have recommended exercise as one of the core treatments for people with OA, irrespective of age, comorbidity, pain severity and disability (NICE, 2008; 2014). Beneficial effects of exercise in patients with OA include improvements in pain, disability and physical performance (Ettinger et al., Reference Ettinger, Burns, Messier, Applegate, Rejeski, Morgan, Shumaker, Berry, O’Toole, Monu and Craven1997; van Baar et al., Reference van Baar, Dekker, Oostendorp, Bijl, Voorn, Lemmens and Bijlsma1998; Deyle et al., Reference Deyle, Henderson, Matekel, Ryder, Garber and Allison2000; Fransen et al., Reference Fransen, McConnell, Hernandez-Molina and Reichenbach2009; Fransen and McConnell, Reference Fransen and McConnell2009). Physical activity in general has a wide range of health benefits including preventative and therapeutic effects on cardiovascular disease, stroke, hypertension, type 2 diabetes, osteoporosis, obesity, colon cancer, breast cancer, anxiety and depression (Department of Health, 2004; Haskell et al., Reference Haskell, Lee, Pate, Powell, Blair, Franklin, Macera, Heath, Thompson and Bauman2007; Blake et al., Reference Blake, Mo, Malik and Thomas2009). The major problem with attempting to introduce or increase levels of physical activity is patient compliance.
Behaviour change interventions are well researched and commonly used in health care interventions (Michie and Abraham, Reference Michie and Abraham2004). Strategies to promote and maintain behaviour change can help to improve self-efficacy and subsequently improve overall health and well-being (Bandura, Reference Bandura1997). The most effective physical activity interventions in the elderly incorporate behavioural or cognitive strategies, together with education (Ettinger et al., Reference Ettinger, Burns, Messier, Applegate, Rejeski, Morgan, Shumaker, Berry, O’Toole, Monu and Craven1997; Warsi et al., Reference Warsi, Lavalley, Wang, Avorn and Solomon2003). They incorporate elements of goal setting, self-monitoring, feedback, support and relapse prevention into a physical activity programme. This is useful because the effects sizes of studies adopting self-management tends to be small (May, Reference May2010; McKnight et al., Reference McKnight, Kasle, Going, Villanueva, Cornett, Farr, Wright, Streeter and Zautra2010), therefore combined interventions maybe more effective. The beneficial effects of exercise in this population, even if small in magnitude, will result in large relative improvements in health status (Hansen et al., Reference Hansen, Daykin and Lamb2010).
The aim of this feasibility study was to develop and test an intervention of group exercise with integrated self-management to help older adults living with OA manage their comorbidities. We addressed the following objectives:
(1) from the literature, to identify and describe effective components of group exercise, self-management and behaviour change for older adults with OA;
(2) to integrate these components into a combined intervention;
(3) to evaluate the intervention by conducting a feasibility study and process evaluation.
The ultimate aim of the feasibility study is to design an intervention to be tested for clinical and cost effectiveness in a randomised controlled trial.
Design
This study involves a multi-component feasibility study designed to address the aims described in the previous section. It consists of the following:
∙ scoping literature reviews to inform the development of the intervention package;
∙ integration and testing of the intervention within a pilot study;
∙ process evaluation involving participant interviews and observation of the intervention in action.
Ethics
Ethical approval for the pilot study and qualitative component was granted by The Black Country Research Ethics Committee (10/H1202/57).
Methods
Literature review
The aim of the literature review was to inform the development of the intervention, which would then be tested in the pilot study. We broadly reviewed the literature on effective components of exercise, behaviour change and self-management. There is an abundance of recent and relevant systematic reviews related to this topic, particularly in the area of exercise prescription. Therefore, we focussed on analysing these systematic reviews in order to develop the intervention for the pilot study.
Behaviour change and self-management
A scoping search of the key databases include Medline, Embase, Amed CINAHL, PsycINFO and CENTRAL was performed. We searched for keywords relating to OA, behavioural interventions, behavioural therapy and self-management. The identified papers and reviews were used to identify key components of self-management interventions for use in our pilot study.
In summary, research applying concepts of self-management in patients with arthritis have shown improvements in pain, fatigue, health distress, well-being, functioning and self-efficacy (Ersek et al., Reference Ersek, Turner, Mccurry, Gibbons and Kraybill2003; Heuts et al., Reference Heuts, De Bie, Drietelaar, Aretz, Hopman-Rock, Bastiaenen, Metsemakers, Van Weel and Van Schayck2005; Devos-Comby et al., Reference Devos-Comby, Cronan and Roesch2006; Osborne et al., Reference Osborne, Wilson, Lorig and Mccoll2007; Coleman et al., Reference Coleman, Briffa, Carroll, Inderjeeth, Cook and Mcquade2012). NICE recommend patients should have access to information, advice and education as well as support for self-management (NICE, 2008; 2014). A strong body of literature focuses on the Arthritis Self-Management Programmes (ASMP) that provides long-term follow-up evidence (Astin, Reference Astin2004; Barlow et al., Reference Barlow, Turner, Swaby, Gilchrist, Wright and Doherty2009). Such programmes include many core topic areas for self-management. In a trial of ASMP six weekly, 2 h sessions were delivered to patients with arthritis. The sessions were interactive with short presentations introducing topics followed by group discussion, problem solving and role play. Topics included information about arthritis, overview of self-management principles, exercise, cognitive symptom management, dealing with depression, nutrition, communication and goal setting. The trial showed improvement in self-efficacy, health behaviours and health status (Barlow et al., Reference Barlow, Turner, Swaby, Gilchrist, Wright and Doherty2009). Monitoring progress through diaries or behaviour change records is also important in self-management interventions.
Exercise
In order to gather evidence regarding an appropriate exercise regime for older adults, a search of the Cochrane library was performed for systematic reviews related to exercise and OA, and exercise and older adults. A similar search was performed for guidelines from the NICE, the British Association of Sport and Exercise Sciences and the American College of Sports and Exercise Medicine (ACSM).
Evidence for exercise in OA: the majority of evidence for OA and exercise is related to knee OA and few studies have considered the hip or other joints (NICE, 2008). However, the evidence that does exist suggests that exercise has at least short-term benefit in terms of reduced pain and improved physical function. Although the magnitude of the reported effect is small, it is comparable to estimates reported for NSAIDs (Fransen and McConnell, Reference Fransen and McConnell2008).
Type of exercise: the ACSM recommend that exercise for older adults should include aerobic training, muscle strengthening and flexibility exercises. They also suggest balance training for individuals at risk of falling. These are broadly in line with the NICE guidelines for exercise in OA (NICE, 2008; 2014).
A systematic review of resistance training in older adults concluded that it was an effective way for improving physical functioning, including improving strength, and for reducing pain in people with OA (Liu and Latham, Reference Liu and Latham2009).
Ashworth et al. reported conflicting findings regarding whether home-based or centre-based exercise programmes were better in terms of outcome in older adults. The few studies included involved different clinical conditions, which may account for the variability in results. Aquatic exercise programmes appear to have some short-term benefit with regard to pain but no impact on function. However, no long-term effects on pain or function have been documented in people with knee or hip OA (Bartels et al., Reference Bartels, Lund, Hagen, Dagfinrud, Christensen and Danneskiold-Samsøe2007). Stronger evidence exists for land-based exercise for people with knee OA, at least in the short term, with reported benefits of reduced knee pain and improved function (Fransen and McConnell, Reference Fransen and McConnell2008).
Dosage: dosage encompasses concepts such as intensity, duration, frequency and loading. ACSM guidelines for exercise in older adults suggest that a total of 30–60 min/day (in bouts of at least 10 min each) to achieve a total of 150–300 min/week of physical activity of at least moderate intensity or, at least 20–30+ min/day to total 75–150 min/week of vigorous intensity activity (or an equivalent combination of moderate/vigorous activity) should be engaged in to improve aerobic capacity (Chodzko-Zajko et al., Reference Chodzko-Zajko, Proctor, Fiatarone Singh, Minson, Nigg, Salem and Skinner2009). Based on the results of one study, Brosseau et al. concluded that both high and low intensity aerobic exercise are suitable for people with OA of the knee for functional status, gait, pain and aerobic capacity.
With regard to resistance training, the ACSM recommend at least two sessions/week of between 8 and 12 repetitions/exercise targeting the major muscle groups at an intensity of 5–8 on a rating of perceived exertion scale. Higher intensity training has been reported as having a larger effect on strength than low to moderate intensity training (Liu and Latham, Reference Liu and Latham2009) as well as beneficial effects on bone mineral density (Chodzko-Zajko et al., Reference Chodzko-Zajko, Proctor, Fiatarone Singh, Minson, Nigg, Salem and Skinner2009).
Pilot study
Design
A pilot study that included the testing of a package of outcome measures. A process evaluation looked at the implementation of the pilot study, using qualitative methods (interviews and observations).
Participants
Participants were eligible for inclusion if they were aged ⩾75 years with a diagnosis of peripheral OA. Confirmation of diagnosis in our clinical assessment was based on the NICE guidance (persistent joint pain worse with use; age ⩾45 years; morning stiffness<30 min) (NICE, 2008; 2014).
All participants had to have the ability to provide informed consent. Our exclusion criteria were:
∙ severe psychiatric/personality disorder;
∙ terminal/critical illness;
∙ fracture/surgery in the last six months;
∙ on a surgical waiting list;
∙ ⩾10 on the 15-item Geriatric Depression Scale indicative of depression (Sheikh and Yesavage, Reference Sheikh and Yesavage1986);
∙ contraindication for exercise;
∙ non-English speaking.
Recruitment
Participants were recruited from a single GP practice in Warwickshire. Their database was searched in February 2012 for patients 75 years or over with a previous recorded diagnosis of OA, and excluded palliative care using the appropriate Read codes. Patients meeting the search criteria were sent an invitation pack containing an invitation letter, patient information sheet, screening questionnaire, reply slip and prepaid envelope. Those that returned the relevant documentation were contacted by the research physiotherapist. The first 10 people eligible and interested were invited to attend an initial assessment where written informed consent was obtained.
Assessment
We did not specify a primary outcome measure. Instead, we selected a battery of outcome measures used in previous trials in similar populations to evaluate for feasibility. Baseline assessments took place in July 2012 at the GP practice. At this appointment, they had the opportunity to meet both the research physiotherapist and psychologist. The research physiotherapist evaluated general health and OA and then asked them to complete a battery of physical tests. The tests evaluated muscle strength/endurance, aerobic endurance, flexibility and body composition as recommended for older people by the British Association of Sport and Exercise Sciences (Saxton, Reference Saxton2007).
∙ Sit-to-stand test: number of repetitions of sit-to-stand in 30 s.
∙ Arm curl test: number of bicep curls in 30 s using a weight (1.8 kg females; 3.6 kg males).
∙ Grip strength: maximal grip strength was measured using a dynamometer (MIE Digital Grip Analyser). The maximum from three trials was used for the dominant hand (Helliwell et al., Reference Helliwell, Howe and Wright1987).
∙ Step test: number of repetitions of stepping on the spot in 2 min.
∙ Flexibility: sit-and-reach test for lower back and hamstring flexibility; back scratch test for shoulder/upper limb flexibility.
∙ Body mass index.
Information about previous orthopaedic surgery (eg, arthroplasty) and walking aids was also recorded. Subsequently, participants completed a baseline questionnaire that incorporated the following:
∙ Chronic Pain Grade (Von Korff et al., Reference Von Korff, Ormel, Keefe and Dworkin1992), measures overall pain and pain related disability over the past six months. We used an amended version looking at the preceding one month.
∙ 15-item Geriatric Depression Scale (Sheikh and Yesavage, Reference Sheikh and Yesavage1986) measures depressive mood.
∙ Arthritis Impact Measurement Scale 2 (Lorish et al., Reference Lorish, Abraham, Austin, Bradley and Alarcon1991) measures the overall impact of arthritis including physical activity and function.
∙ The EuroQol EQ-5D, is a five-item questionnaire measuring health utility (EuroQol, 1990).
∙ Troublesomeness grid, measures levels of troublesomeness of pain in different parts of the body over the previous month (Parsons et al., Reference Parsons, Carnes, Pincus, Foster, Breen, Vogel and Underwood2006).
∙ Satisfaction with health, a five-point Likert scale from very dissatisfied to very satisfied.
∙ Expectations for the future, a five-point Likert scale from much worse to much better.
We collected demographic data, pain duration and presence of comorbidities as well as the selected outcomes measures at baseline. Follow-up data was collected at three months after the baseline data was collected. Adherence with the programme was assessed from programme attendance records.
Analysis
As this was a small feasibility study, no formal sample size was calculated. A total of 10 participants were included because this was a suitable and safe number for a group intervention requiring supervision in a gym environment.
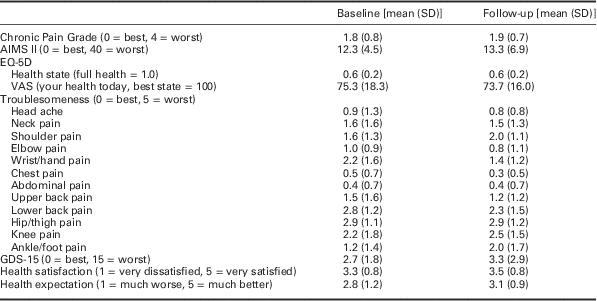
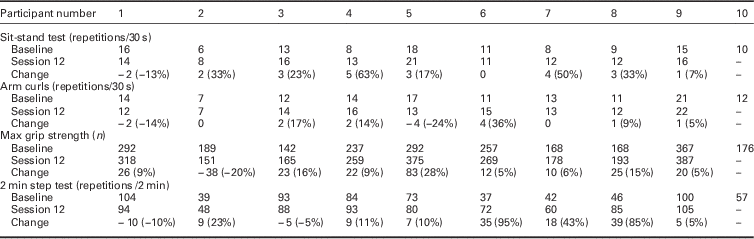
Owing to the small sample, descriptive data only at baseline and follow-up were reported. These are presented in tables as appropriate (Table 1 and Table 2). No inferential statistics were carried out.
Table 1 Summary of outcome data collected at baseline and three month follow-up

GSD=Geriatric Depression Scale.
Table 2 Change score for physical tests performed at baseline and during the final session

Missing data (–), negative change scores indicate a decline in ability.
Intervention
Our intervention was designed in line with the Medical Research Council framework for complex interventions (Craig et al., Reference Craig, Dieppe, Macintyre, Michie, Nazareth and Petticrew2008). The intervention was delivered by a Health Psychologist and a Physiotherapist, with additional support from two further physiotherapists during the exercise session.
Behaviour change and self-management: based on the literature, we included sessions on OA and other comorbidities, understanding self-management and the process of behaviour change, SMART (Specific, Measurable, Attainable, Realistic and Timely) goal setting, introduction to exercise and exercise diaries, managing pain – pacing activities, dealing with emotions, unhelpful thoughts, relaxation and visualisation, managing setbacks and communication with health professionals.
Exercise component: based on the broad parameters regarding type and intensity of exercise from the literature, an exercise programme was developed combining muscle strengthening, general aerobic fitness and balance. The specific exercises to be performed were selected in accordance with the facilities available at the chosen venue. The exercise sessions were designed by the research physiotherapist and monitored by a team of three physiotherapists and the psychologist. Staffing levels and inclusion or not of existing gym staff were other aspects of the intervention to be considered as part of the pilot study.
Each participant was provided with an exercise booklet with instructions and photos on how to perform each exercise as well as providing a space for recording the weight and number of sets/repetitions or time. Load/intensity was individualised for each participant for each exercise using a modified Borg scale (Borg, Reference Borg1982). This was also used to gauge their ability to progress over the six-week period. The emphasis was on the participants working as hard as they could within pre-determined limits of self-reported exertion using the Borg scale. By using this scale, the load used by each participant varied but the intensity level was roughly equivalent across the group. Therefore, those with lesser ability or greater levels of deconditioning were catered for.
The programme was set up as a circuit within an existing public gym facility. Initially, participants completed one circuit but, as they became accustomed to the programme, they increased this to two or three depending on ability. In certain cases, specific exercises had to be modified or substituted due to pre-existing conditions (eg, hip arthroplasty). Participants were also encouraged to be as independent as possible in completing the programme and to increase their general level of physical activity outside of the programme sessions.
Each session was for two-and-a-half hours, twice a week for six weeks and followed a similar structured format:
∙ outline – the agenda for the session and any questions/concerns addressed;
∙ delivery of planned session, which was usually 1 h on a self-management topic followed by a warm up and an hour in the gym;
∙ break – each session comprised a 20–30 min break before the exercise component;
∙ homework – this is an important part of a behavioural approach, therefore behavioural monitoring in the form of physical activity diaries were kept by each participant and reviewed in the sessions.
Process evaluation
The main evaluation within this feasibility study was a process evaluation. Within the process evaluation we employed qualitative methodology exploring the implementation of the intervention. The aim of the process evaluation was to inform the future development of the intervention and trial procedures for use in a randomised control trial.
Observation and interviews were used. The observations were carried out by a researcher independent of the study development team (DE) and involved observing delivery of the group sessions over the period of the project (some in the early weeks some in the middle and finally at the end). The aim of the observations was to observe the interactions between the facilitators and the participants and between the participants. Field notes were made at each visit. We did structured telephone interviews with all participants one month after the intervention ended. Interviews with participants aimed to capture their views on:
∙ attendance/acceptability of the programme;
∙ study processes, including assessment and consent procedures;
∙ outcome measures;
∙ session content and length;
∙ delivery in a community based group environment.
Data from field notes and interview transcripts were subjected to thematic content analysis.
Quotations are used to exemplify themes, a code made up of a participant’s gender (M or F) and their age is used as an identifier (eg, F, 88).
Results
Pilot study
A total of 10 participants took part in this intervention (Figure 1). There were four males and six females aged between 75 and 92 years (mean 82, SD 6.2).

Figure 1 Consort chart. OA=Osteoarthritis
We delivered two sessions/week for six weeks resulting in 12 sessions in total with an average attendance of 89%. Eight of the participants reported some form of comorbidity such as obesity, cardiovascular disease, diabetes, osteoporosis, angina, high cholesterol and high blood pressure. Of those that reported such comorbidities at baseline, all reported some form of benefit at three month follow-up.
Mean and standard deviation data for baseline and follow-up for the various outcome measures have been presented in Table 1. At three month follow-up we asked patients if they were engaging in any exercise and we found eight had continued to do some form of regular exercise. Five participants continued with the exercise programme used in the intervention.
At three months we also collected treatment benefit and satisfaction data as well as global change measures. Seven participants had a positive change in their OA since starting the study, nine reported either moderate or substantial benefit from the advice or exercise received as part of this study, six were either satisfied or very satisfied with the programme and seven had better or much better overall health since attending the programme.
The change scores for the physical tests show a general trend towards improvement at the end of the six week programme (Table 2).
Process evaluation
Observation
DE attended 5 of the 12 sessions. These observations revealed that interactions within and between the group were very good. It was noted that the group were very positive and indeed all very willing and receptive. Initial shyness was soon replaced with participants who all took an active role within the groups. Facilitators generally stuck to the timetable and during breaks or when one of the others was facilitating mixed with the group. Discussions were generally lively and participants were not afraid to ask questions. Facilitators did not ‘lecture’ rather they encouraged discussion. Friendships quickly developed and it seems were enduring. It is noted that this group are all very motivated and this has helped them get the most out of the intervention. The setting within the sports centre was very good. Most of the participants had never set foot in the place before and most had never been to a gym. However, with the support of the physiotherapist they all were able to participant in the gym sessions that were at the end of each session. Indeed, it was clear while observing that they loved it; lots of smiles and laughter. As the weeks progressed the participants became more confident in their own abilities and indeed some became quite competitive. In addition, as the weeks progressed members of the public and gym staff often talked to our participants commenting on how well they were doing for example. The group cohesion at the end was excellent and indeed one of the participants took it on himself to organise a continuation of the gym sessions after the study finished. Universally they were all very sad when it ended as all were feeling the benefit.
Interviews
All 10 participants agreed to be interviewed post-intervention. The results below map onto the interview questions where relevant observations were added as supportive data.
Attendance and acceptability: when asked about attending the programme and its acceptability all participants reported they were very pleased. Even those who were a little sceptical at the start found it an enjoyable and useful experience (Figure 2).

Figure 2 Comments about attending the programme and its acceptability
Recruitment, consent and outcome measures: all participants recall recruitment taking place at their GP’s surgery, whereas some of the finer details around assessment and consent varied. Some experienced problems with completing the outcome measures and felt that older people could be put off by long questionnaire packs. Others felt the process was straightforward and the instructions were clear. It was suggested that time could be made during the sessions to complete them so that some help was available.
Session content and length: asked about the content and length of the sessions it was clear that all were happy with what they received and in most cases wanted more. Most were surprised how much they enjoyed the gym and overall liked the way the group was facilitated (Figure 3).

Figure 3 Comments about content and length
The venue: most were very happy with the venue. It was a new experience for many who had never been to a gym before.
Post intervention: discussions in the early weeks of the study between the participants resulted in one of the participants organising the group and making arrangements for the gym sessions to continue after the study finished. Half of the participants took this up and continued to attend. It was noted that the gym staff were very helpful but a number did note that without the physiotherapist and a set programme of work they found it hard. One participant noted that future programmes should include the gym staff more in both the talk sessions and the work in the gym as this helps to build a good relationship and understanding. Several were not attending the follow-up due to health problems (Figure 4).

Figure 4 Comments about post-intervention attendances at the gym
Discussion
We have shown it is possible to combine a self-management and exercise intervention for adults ⩾75 years with OA living in the community. The programme was delivered as intended and for the total six week duration. The overall outcomes indicate that this population is able to engage in strength training, adapt to equipment used in a gym environment and experience positive outcomes on their self-reported comorbidities.
We developed an intervention based on research evidence and clinical expertise of the research team. The study is only a small scale feasibility study run in a very specific population in Warwickshire. Despite this, the intervention was very well received as indicated in the independent process evaluation. It is difficult to compare the outcomes of this feasibility study with other published research in this area as we have not attempted to analyse the results in any way. However, the positive changes seen in physical strength and ability, self-report of benefit, satisfaction and overall health provides some indication that this combined intervention may have the potential for clinical benefit in this population. It is also encouraging that at follow-up, 80% of participants were continuing to engage in exercise and 50% of these were continuing with the exercise intervention delivered in the programme. This is a good indicator that the combined intervention has helped to engage this population in behaviour change. A proportion of these participants also reported some beneficial changes in their comorbidities and OA, both again reinforcing the beneficial effects of exercise.
The process evaluation supports the notion of the intervention’s effectiveness with participants reporting physical and psychological benefits. They not only enjoyed the self-management sessions but they developed into a group who were very comfortable to be with each other even when the groups ended. Continuing with some form of exercise post-intervention was an important part of the study. However, the inclusion of gym staff in some of the taught sessions as well as the gym sessions would be advantageous. This would allow the participants to become familiar and comfortable with the staff who would support them post-intervention, as well as increasing ‘buy-in’ of the staff to the study. The length of sessions and the mix seems to have been satisfactory. A number did suggest they wanted more time in the gym, which might be something to consider for a larger study.
The process evaluation highlights areas for future consideration, especially around the consent and assessment process. All participants gave consent for this study but many were unable to recall this. An increasing number of older adults have some degree of cognitive impairment and researchers need to be aware of this and behave appropriately. In addition, half of the participants found completing the outcome measures difficult. Again, future researchers need to minimise the burden on our older participants and maybe offering alternative ways of completing measures, perhaps with the support of a researcher.
The benefits of physical activity are well documented for both OA and associated comorbidities. More importantly, combined exercise and self-management is likely to be a safer option among this elderly population, who often suffer from other comorbid conditions, compared to the long-term use of NSAIDs.
This small feasibility study has shown that it is possible to deliver a programme like this to older adults with OA. We have demonstrated that older people will use a gym with support and enjoy group activities, which help them manage their condition. We must not forget that the sample is self-selected and those responding to our invitation may naturally have been more motivated, which in turn may have helped with compliance and outcomes. However, the problem of self-selection is common in most research. Although this feasibility has been well received by patients it is important to be able to demonstrate the clinical and cost effectiveness of such an intervention on a larger scale.
The intervention was well received and has encouraged 80% of participants to continue exercising after the programme. The small but positive changes seen in comorbidities, benefit of the intervention, satisfaction and general health are promising. Randomised controlled trial evidence of effectiveness is needed before such interventions can be recommended.
Acknowledgements
The authors thank their lay representative for his input in the early study design phase.
This project benefitted from facilities funded through Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands (AWM) and the Wolfson Foundation.
Financial Support
This work was supported by The Bupa Foundation (grant number TBF-PPW09-036). The Bupa Foundation had no involvement in the research.
Conflicts of Interest
Authors had financial support in the form of a research grant for the submitted work from the Bupa Foundation.
M.U. received grants from NIHR Research for Patient Benefit Programme and NIHR Senior Investigator award during the conduct of the study. Outside the submitted work M.U. also received support from NICE, BMJ learning and Osteoarthritis Research Society International; he is the director and shareholder of Clinvivo Ltd.
SP has been a co-applicant on NIHR and ARC-UK funded research. She is a director and shareholder of Health Psychology Services Ltd.