No CrossRef data available.
Article contents
The crystal structure of trisodium hexachlororhodate (Na3RhCl6)
Published online by Cambridge University Press: 14 February 2018
Abstract
Commercially available trisodium hexachlororhodate (Na3RhCl6) was dehydrated and characterized by laboratory X-ray powder diffraction. The crystal structure is isostructural to the Na3CrCl6 structure type with space group P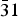 $\bar 31$c. Unit-cell parameters are a = 6.8116(1) Å, c = 11.9196(2) Å, V = 478.95(2) Å3, and Z = 2.
$\bar 31$c. Unit-cell parameters are a = 6.8116(1) Å, c = 11.9196(2) Å, V = 478.95(2) Å3, and Z = 2.
Keywords
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2018




