Introduction
Antimicrobial resistance (AMR) is a natural, evolutionary process hastened by human activity. It occurs when microbes, including disease-causing bacteria, evolve to evade the antimicrobial drugs we rely on to treat them, such as antibiotics. This biological process gives rise to the creation of new, mutated microbes that cause drug-resistant infections. As a result, existing antimicrobial drugs—which are among the most effective treatments in modern medicine—become ineffective. In other words, AMR transforms previously curable infections into untreatable and often deadly diseases.
AMR can occur whenever antimicrobials are used, and the same antimicrobials are still widely used across many sectors of activity, including in human health, animal health, and agricultural settings. Microbes, including resistant ones, spread easily across humans, animals, and the environment, and travel around the world through humanity’s global circuits of movement. Global governance has not sufficiently risen to meet this challenge. Almost a century’s worth of human antimicrobial use in the absence of adequate mitigation and adaptation strategies has now accelerated AMR to the point of a global crisis: over 1.27 million people died from AMR in 2019 (Murray et al. Reference Murray, Ikuta, Sharara, Swetschinski, Aguilar, Gray and Han2022).
Like many of today’s greatest challenges, mitigating the threats posed by AMR requires new ideas and practices for governance (Denyer Willis and Chandler Reference Denyer Willis and Chandler2019). The inability of prevailing policy practices to sufficiently address the emergence and reemergence of mutated zoonotic and resistant disease variants, as well as the uneven global realities that they create, suggests a problem with the way that global health challenges are conceived. Thus, it is argued, a paradigm shift is needed to reconceptualize the objects, subjects, and methods of global health governance before even attempting to craft global health institutions that are more effective at achieving their stated goals. Otherwise, old conceptions in global health governance will continue to yield miscalculations and failures; reproduce class, race, and gender inequities; and deepen unjust and lingering colonial power relations. This is a conclusion increasingly recognized after several governance failures during the COVID-19 pandemic (Assefa et al. Reference Assefa, Gilks, van de Pas, Reid, Gete and Van Damme2021; Büyüm et al. Reference Büyüm, Kenney, Koris, Mkumba and Raveendran2020; Fukuda-Parr, Buss, and Ely Yamin Reference Fukuda-Parr, Buss and Yamin2021; Wenham, Eccleston-Turner, and Voss Reference Wenham, Eccleston-Turner and Voss2022), which can also be applied to the governance of AMR.
The challenge is that AMR is not just another health problem to be solved. Nor is it just another technocratic challenge in need of scientific innovation to maintain existing human ways of life (Hinchliffe, Butcher, and Rahman Reference Hinchliffe, Butcher and Rahman2018). Rather, it is an enduring global phenomenon caused by the innate ability of microbes to adapt and develop resistance to human intervention (Wallinga, Rayner, and Lang Reference Wallinga, Rayner and Lang2015). From this perspective, AMR represents a socioecological problem that demands robust institutions capable of managing the array of human behaviors with the potential to shape the human–microbial nexus (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020; Léger et al. Reference Léger, Lambraki, Graells, Cousins, Patrik, Harbarth and Carson2021). To maximize the likelihood of achieving sustainable antimicrobial use for all, strategies to improve AMR governance should, therefore, aim to minimize the ways that human activities conflict with the natural tendencies of microbial life as these two interlinked worlds continue to evolve into the future.
Guided by this alternative conceptualization of AMR, new insights can be generated by considering how well global institutions align with the ecological characteristics of the problem, including the geographical scope, temporal scales, and natural tendencies of complex microbial ecologies. This concept of “ecological fit” emerges from the understanding that ecological systems have various unique qualities, and the institutions that govern them should be tailored to accommodate those unique qualities—much like a key that is designed to fit a specific lock (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015). Put differently, it shifts the question from asking whether AMR institutions can achieve their stated objectives (i.e., “are they fit for purpose?”) to instead ask whether they are suited to the problem that they govern (i.e., “do they fit the challenge of AMR?”). Gaining insight into how well global health institutions fit the biophysical features of the problems they are meant to govern could significantly improve the likelihood of creating more effective and “fit-for-purpose” global health institutions.
This article adopts a socioecological perspective to examine the ecological fit between the social institutions governing AMR and the ecological nature of the problem. It begins by reviewing emerging ecological scholarship to frame AMR as an enduring, intersectoral, and widespread problem inextricably linked to other ecological processes. Next, drawing on research conducted in a complementary study (Weldon, Yaseen, and Hoffman Reference Weldon, Yaseen and Hoffman2022), it adopts the concept of the regime complex for AMR governance, which is defined as the array of global institutions converging around the problem, to frame the current social systems that govern human behavior around the challenge. By systematically comparing the logics of AMR governance with the ecological features of the problem, this article identifies 18 spatial, temporal, threshold, and cascading misfits. While not a comprehensive list, the results of this investigation illustrate how the concept of ecological fit can unlock new perspectives on recurring global health governance challenges. Finally, because the concept of ecological fit has been used extensively in biodiversity and ecosystems governance research, this article proposes five institutional design principles for AMR governance arising from lessons learned in those settings.
Part 1: Adopting a Socioecological Perspective to Investigate AMR
The socioecological perspective emphasizes the dynamic and complex relationships among people and their environments, both social and physical (Folke Reference Folke2016). It can unlock new ideas for conceptualizing problems and identifying their dynamic causes, while generating solutions to address today’s complex and interlinked global challenges, including AMR.
The perspective begins by observing that individuals exist within human social systems that are embedded in natural environments and ecosystems. Following this observation, it aims to contend with the interdependencies across these spheres (Wallinga, Rayner, and Lang Reference Wallinga, Rayner and Lang2015). In contrast to this large scope, however, prior research has historically privileged one set of systems and relationships at the expense of the other, focusing on only the social or the ecological. For example, Arild Underdal (Reference Underdal, Miles, Andresen, Carlin, Skjærseth, Underdal and Wettestad2001) outlines a theory that suggests that problem structure and institutional problem-solving capacity are two independent variables that determine regime effectiveness. But these factors, while important, only pertain to human institutions and their normative understandings of the problem at hand. The socioecological perspective, on the other hand, grapples with the relationship between human social systems, including their various institutional and normative aspects, and the biophysical characteristics of ecosystems (Folke Reference Folke2016).
By emphasizing the interlinkages between human social and natural ecological systems, the socioecological approach provides a greater awareness of the shared material, planetary, and biospheric reality in which human institutions operate, and which human activity partly constitutes, compared to other human-centric political science and public health approaches. This advantage allows for investigations across AMR’s multiple interdependent facets, encompassing both human and biological dynamics of the problem.
Prior research that emphasizes the ecological nature of AMR has identified at least four important socioecological characteristics (Green Reference Green2020; Ventola Reference Ventola2015; Wallinga, Rayner, and Lang Reference Wallinga, Rayner and Lang2015). Specifically, AMR is simultaneously (1) a widespread intersectoral problem; (2) a global and local—or “glocal”—problem; (3) a long-enduring process that, because it is caused by the innate evolutionary ability of microbes, is unlikely to ever truly be “solved”; and, finally, (4) a problem that is interlinked with other complex problems, such as the emergence of zoonotic disease, climate change, and biodiversity loss—all of which also have the potential to accelerate AMR. Table 1 expands on each of these characteristics in turn.
Table 1 Ecological Characteristics of Antimicrobial Resistance

A starting point for considering how these socioecological interdependencies play out in global politics is Robert Cox’s (Reference Cox1981) conception of the “three domains” present within any world order. These are the global political economy, the interstate system, and the global ecosystem. For Cox, “these three components are both autonomous in having their inherent dynamics, and, at the same time, interdependent with each other. Contradictions are generated within each of the three spheres, and contradictions arise in the interrelationships among the three” (1981, 161). When put in these terms, the socioecological approach essentially focuses on the contradictions that arise in the relationship between prevailing systems of human behavior (i.e., the global political economy and the interstate system) and the biosphere (i.e., the global ecosystem). In doing so, it endogenizes that which would appear to be exogenous to approaches that only look at human activity.
As Cox’s ideas aptly suggest, the relationship among human and microbial domains is historically specific and dialectical—determined in part by the prevailing forms of social organization, technological capacities, and material realities of the time (R. Cox Reference Cox1981). The configuration of these domains, moreover, is not fixed, but rather changes over the long run through various historical processes (e.g., changing material capabilities, ideas, and social forces, though the relative importance of which is a major source of debate). In a time of overlapping planetary crises, the critical need to understand how the current configuration of these domains emerged, and the ways that it may be changing, must be balanced against the need to address and mitigate the urgent problems that exist within it (Hoffman, Bakshi, and Rogers Van Katwyk Reference Hoffman, Bakshi and Van Katwyk2019). But conversely, those who adopt problem-solving approaches to confront these urgent problems must be aware of how assumptions about the fixity of the current configuration can embody ideologies associated with conserving it. Indeed, while Cox critiques problem-solving approaches that are unquestioning of the changing orders in which they operate, he notes that critical problem-solving perspectives can emerge “with a normative choice in favor of a social and political order different from the prevailing order” (R. Cox Reference Cox1981, 130).
Cox’s ideas are useful because they emphasize how patterns of human activity cannot be separated from, nor should they be considered autonomous from, the global ecosystem. This kind of thinking can foster the conception that culture is somehow separable from nature and lead to fallacies associated with thinking about “nature as something to be controlled by culture” (Agathangelou Reference Agathangelou2016; Latour Reference Latour and Porter2004). Instead, a better way to conceptualize the relationship is to see human activity as occurring within specific material ecosystems. These ecosystems, together with human activity, constitute one interdependent and complex planetary system (Gill and Benatar Reference Gill and Benatar2019; Rockström et al. Reference Rockström, Steffen, Noone, Persson, Stuart III Chapin, Lambin and Lenton2009a; Reference Rockström, Steffen, Noone, Åsa Persson, Lambin and Lenton2009b). In this system, there is a growing acceptance that human activity is the single biggest driver of change—in what is now defined as “the Anthropocene”—but, importantly, the full effects and outcomes of those changes are not entirely under human control (Malhi Reference Malhi2017; Rockström et al. Reference Rockström, Steffen, Noone, Persson, Stuart III Chapin, Lambin and Lenton2009a).
When deployed to investigate AMR, the socioecological perspective directs attention to the way that microbes form part of the natural ecological basis that orders human social relations. Since the discovery of effective antimicrobials in the early twentieth century, societies have formed systems that critically depend upon them to function (Chandler Reference Chandler2019). Antimicrobials have attained such an important role in modern societies that they operate like invisible infrastructure, enabling more productive food, labor, and healthcare systems. But this dependency, characterized by deeply engrained structures and practices that incentivize antimicrobial use for quick fixes for productivity, abundance, and profits, has generated a wide range of human activities that affect microbial adaptive behaviors (Denyer Willis and Chandler Reference Denyer Willis and Chandler2019). Specifically, these human activities precipitate biological change and accelerate microbial evolution toward drug resistance. AMR, in turn, undermines our social orders that rely upon antimicrobials to provide medical care and avoid more costly investments in sanitation and hygiene.
Put another way, AMR is a problem of interacting worlds. The discovery of antimicrobials enabled a shift in the relationship between human societies and invisible microbial ecologies (Green Reference Green2020). New human social orders were built around the ability to artificially affect microbes, making them more conducive for specific kinds of human activity (e.g., antimicrobials are most often used to reduce the likelihood and consequence of infection in animal farming operations and increase overall agricultural productivity [Van Boeckel et al. Reference Van Boeckel, Brower, Gilbert, Grenfell, Levin, Robinson, Teillant and Laxminarayan2015]). The increasing failure of antimicrobials, though, now reveals the extent to which our social orders are precariously based upon effective antimicrobial drugs as ecology-transforming tools. This precariousness, moreover, manifests as social problems arising from drug resistance, including higher morbidity and mortality, reduced productivity, and declining agricultural yields from drug-resistant diseases (World Bank 2017). And with increasing calls to address the social structures and incentives that shape human behavior around antimicrobial use (Chandler Reference Chandler2019; Denyer Willis and Chandler Reference Denyer Willis and Chandler2019; Weldon, Rogers Van Katwyk, et al. Reference Weldon, Liddell, Van Katwyk, Hoffman, Minssen, Outterson, Stephanie Palmer and Viñuales2022), there is an emerging normative project to achieve a new human–microbe relationship that reduces the rate at which human activity induces microbial evolution. In explicit terms, it requires a political order that pursues a more harmonious configuration among human governance institutions and microbial life to maximize the sustainability of effective antimicrobials for current and future generations.
Growing recognition of the significant findings generated by more holistic perspectives is fostering academic projects that aim to grapple with the ecological nature of today’s interlinked health challenges, including AMR. There have even been journals established to consider these projects (e.g., see Lancet Planetary Health). Yet, there remains a need to apply this perspective to investigate the relationship between these challenges and the social institutions that govern them.
Part 2: Existing Governance Systems for AMR
As described in depth in a complementary study, AMR is currently governed by a global regime complex (Weldon, Yaseen, and Hoffman Reference Weldon, Yaseen and Hoffman2022). Regime complexes have emerged as a defining feature of global governance in the twenty-first century (Alter and Raustiala Reference Alter and Raustiala2018). They are defined as arrays of international regimes that converge around the same issue in global politics. As such, they represent the informal, formal, and legal architectures that structure existing human institutions in the current conjuncture. Although these architectures are evolving, their present, historically contingent form represents the socioecological niche into which resistant pathogens are currently emerging (Hruschka and Henrich Reference Hruschka and Henrich2013). Thus, investigating the regime complex for AMR governance in its current form can illuminate the various ways that the existing landscape of human institutions enables and accelerates microbial evolution and spread.
Indeed, the regime complex for AMR governance provides a systematic framework to understand the social order by which AMR governance takes place. But to fully understand the regime complex for AMR governance and its relevance to this article’s socioecological perspective, it is first helpful to discuss the concept of an international regime and the evolution in regime theory that led to the emergence of the concept of regime complexes.
International regimes are often defined, using Stephen Krasner’s seminal definition, as
“sets of implicit or explicit principles, norms, rules, and procedures around which actors’ preferences converge in a given area of international relations. Principles are beliefs of fact, causation, and rectitude. Norms are standards of behavior defined in terms of rights and obligations. Rules are specific prescriptions or proscriptions for action. Decision-making procedures are prevailing practices for making and implementing collective choice. (Reference Krasner1982, 186)
Since Krasner introduced the idea of an international regime in 1982, scholars have used the concept to analyze the global governance of specific issues and domains, including money, trade, the environment, health, and nuclear technology (Gottemoeller Reference Gottemoeller2015; Lakoff Reference Lakoff2017; Ruggie Reference Ruggie1982; Young Reference Young2011). There are, however, some important limitations that arise when employing the concept of an international regime to understand the dynamics of global governance systems, which many scholars began to identify in the late 1990s and early 2000s (Aggarwal Reference Aggarwal and Aggarwal1998; Oberthür Reference Oberthür2002; Rosendal Reference Rosendal2001; Stokke Reference Stokke and Young1997; Young Reference Young1996). Specifically, there was a recognition of at least three major limitations that prevent regime analysis from accurately capturing how global governance takes place. First, global issues are usually interdependent as the boundaries of one issue flow fluidly into others (Keohane and Nye Reference Keohane and Nye2000; Raustiala and Victor Reference Raustiala and Victor2004). Second, issues are rarely if ever solely governed by a discrete regime. There may be one regime that features more prominently in certain discourses, but in the same way that problems blur into one another, so too do the social systems that emerge to govern them. Third, the international system evolves in a path-dependent way, meaning that new regimes are always created and are shaped in a system already densely populated by other regimes. They cannot and do not start tabula rasa, but instead must connect, relate to, and interact with other regimes, which often causes overlap (Alter and Raustiala Reference Alter and Raustiala2018).
Following these observations, Kal Raustiala and David Victor argued in 2004 that rather than being governed by discrete individual regimes, global issues are usually governed by regime complexes, which are defined as arrays of three or more international regimes with overlapping membership and conflicting principles, norms, rules, and procedures (Morin and Orsini Reference Morin and Orsini2013; Morin et al. Reference Morin, Louafi, Orsini and Oubenal2017; Orsini, Morin, and Young Reference Orsini, Morin and Young2013). And unlike investigating a single international regime, regime complex research necessarily starts by recognizing the densely populated, sometimes nebular, and path-dependent trajectory of the global political system, where regimes do not emerge, exist, or operate in isolation (Keohane and Victor Reference Keohane and Victor2011).
Thus, the idea of regime complexes aligns nicely with the socioecological perspective. Both share a commitment to see the interdependence of complex issues and note that these issues give rise to functionally interdependent social systems. Based on this understanding of regime complexes, it is possible to map the existing regime complex for AMR governance, including the various principles, norms, rules, and procedures across the elemental international regimes that comprise it.
The Anatomy of the Regime Complex for AMR Governance
The far-reaching nature of AMR makes it simultaneously a human health, animal health, agricultural, environmental, developmental, and trade issue, with no single global institution poised to comprehensively address it (Rogers Van Katwyk, Weldon, et al. Reference Rogers Van Katwyk, Giubilini, Kirchhelle, Weldon, Harrison, McLean, Savulescu and Hoffman2020; Weldon, Yaseen, and Hoffman Reference Weldon, Yaseen and Hoffman2022). Instead, there are at least seven elemental international regimes coalescing in what can be identified as a textbook example of a global regime complex. These are (1) the human health security regime, (2) the humanitarian biomedicine regime, (3) the animal health regime, (4) the agriculture regime, (5) the trade regime, (6) the development regime, and (7) the environment regime (table 2).
Table 2 The Regime Complex for AMR GovernanceFootnote 3
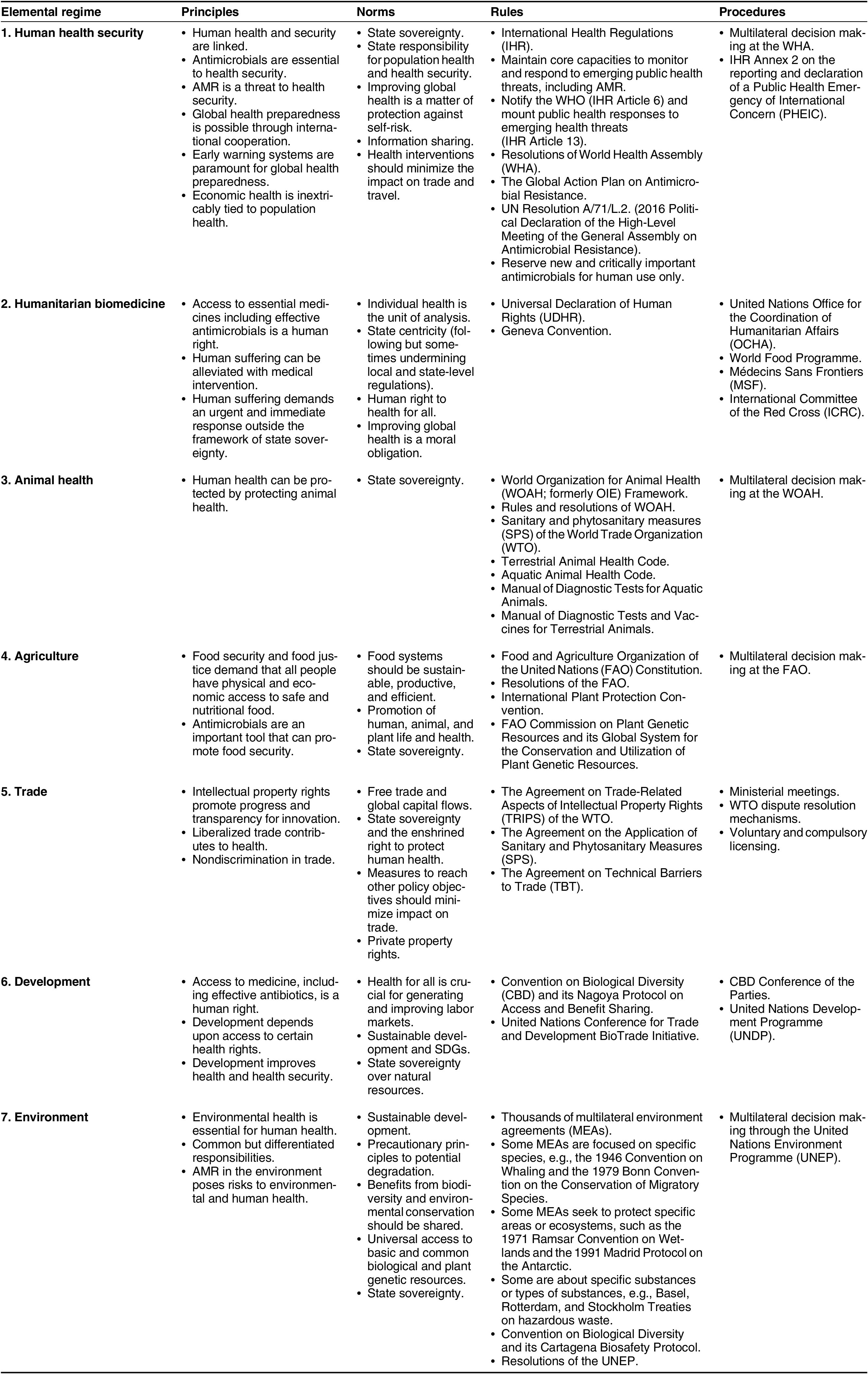
Some of these elemental regimes have evolved with highly institutionalized agreements containing fully spelled-out expectations and behaviors. For example, the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs) under the auspices of the World Trade Organization (WTO) espouses a set of principles, norms, rules, and procedures that formally structures part of the international trade regime. The International Health Regulations (IHR) under the auspices of the World Health Organization (WHO), which are the legally binding rules that govern how states respond to infectious disease outbreaks, are another example of these kinds of formal rules.
Elemental regimes may also contain formally established agencies devoted to the implementation of desired goals and plans. For example, the Food and Agriculture Organization (FAO), which is the United Nations’ agency for ending hunger and promoting nutrition, forms part of the global agricultural regime. Alternatively, behavior in an international regime may be guided by a looser arrangement of principles, norms, rules, and procedures. Such is the case for the humanitarian biomedicine regime, where many principles, norms, rules, and procedures guide actor behavior, but do not emanate from specific formalized agreements. Rather, these structures have been shaped historically through informally codified behaviors and sporadic international agreements.
There are several instances of overlapping and sometimes conflicting principles, norms, rules, and procedures across the regime complex for AMR. These overlaps and conflicts, moreover, have been found to have both positive and negative consequences. For example, synergistic overlaps include the shared commitment of human health security and trade regimes to minimize the impact of policy interventions on trade (Hoffman, Weldon, and Habibi Reference Hoffman, Weldon and Habibi2022).
However, other aspects of the trade regime have previously obstructed the achievement of global health goals (Barlow et al. Reference Barlow, McKee, Basu and Stuckler2017), especially the principles, norms, rules, and procedures around intellectual property, investment, and financing for new medicines. For instance, some studies have found that regulations adopted and implemented through the international trade regime have previously prevented the adoption of health regulations (Mercurio Reference Mercurio and Rensmann2017). Moreover, the trade regime places a significant emphasis on the importance of intellectual property rights as a means to enhance health innovation. In addition to this emphasis, specific regulations outlined in TRIPs are designed to support this principle. However, it has been observed that these measures have historically presented an impediment to the fundamental principle of providing access to medicine, which is advocated by both development and humanitarian biomedicine regimes as an essential human right (Motari et al. Reference Motari, Nikiema, Ossy, Kniazkov, Loua, Sougou and Tumusiime2021). Indeed, the belief that intellectual property rights improve innovation has proven especially ineffective in stimulating research and development for new antimicrobials, which is a market with unique challenges (Kesselheim and Outterson Reference Kesselheim and Outterson2011). Yet, attempts to address these obstacles and move beyond traditional market systems for antimicrobials has previously conflicted with the economic interests of powerful actors, such as pharmaceutical firms, their shareholders, and the states in which they operate (Lopert and Gleeson Reference Lopert and Gleeson2013).
Amid growing calls to reevaluate the priorities of global health governance, there remains a need to determine whose interests and which principles, norms, rules, and procedures prevail when elemental regimes interact with conflicting perspectives and aims. Similarly, where there are calls to identify and transform the suite of human behaviors that threaten the sustainability of antimicrobial therapy, there remains a need to identify and transform these governance institutions to better align with the ecological nature of AMR.
Part 3: Assessing the Ecological Fit of AMR Governance Systems
With an understanding of both the ecological characteristics of AMR (table 1) and the social systems that currently govern it (table 2), it is now possible to consider the ecological fit across these domains. The concept pertains to how well social institutions fit with the problem at hand (Young Reference Young2002). Specifically, it is defined as “the congruence or compatibility between ecosystems and institutional arrangements created to manage human activities affecting these systems” (Young Reference Young2002, 20).
The concept of ecological fit has been used extensively to explain governance challenges in biodiversity and ecosystems management (Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008), where research has shown how institutions are likely to fail if they do not fit the problems that they are trying to govern (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015). Some research in global health has identified a similar theme of misfits between the dynamics of emerging health threats and existing institutions of global health governance. For example, Steven Hoffman and Sarah Silverberg (Reference Hoffman and Silverberg2018) note several political and technical barriers that prevent the IHR from responding to infectious disease outbreaks promptly, signaling a temporal incongruity between the rules of global health governance and the rate of infectious disease spread. Yet, none have systematically analyzed the full range of misfits or their consequences.
Operationalizing the Concept of Ecological Fit
We follow a relatively simple process to operationalize the concept of ecological fit for application to AMR governance. Specifically, we adopt what Graham Epstein and colleagues (Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015, 36) describe as a straightforward process of “characterizing the attributes of the ecological problem and then comparing these to the attributes of governing institutions.” Much has been written about theoretical and practical challenges associated with measuring fit (M. Cox Reference Cox2012; Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015; Vatn and Vedeld Reference Vatn and Vedeld2012), which has produced many different approaches and methods. For instance, any assessment of the fit between a problem and the institutions that govern it at least partially depends on a socially constructed and historically specific understanding of the problem and its corresponding institutions. Someone with a different understanding of these matters could diagnose other important aspects of fit and misfit. Exactly what “fit” looks like, moreover, will also change as human institutions, microbial ecologies, and their relationship evolve. Furthermore, while the metaphor of a key and lock can be helpful, it is important to not necessarily see the “lock” and “key” as separate; as noted above, a more appropriate socioecological framing would see social systems as embedded in the global biosphere. These challenges notwithstanding, the benefit of adopting this simple approach is that it provides a replicable method, while demonstrating the power of the concept and its potential when applied to the global health governance of AMR.
Prior research in biodiversity governance, ecosystems management, and systems dynamics theory suggests that four different kinds of misfits can occur between institutions and the problems they seek to address (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015; Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008; Rockström et al. Reference Rockström, Steffen, Noone, Persson, Stuart III Chapin, Lambin and Lenton2009a). These are (1) spatial, (2) temporal, (3) threshold, and (4) cascading misfits (table 3). Through a theory-driven analysis, we use qualitative analytical reasoning guided by principles of similarity-matching to systematically evaluate the fit between the ecological characteristics described in part one with the governance attributes described in part two (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015). Each governance attribute across the regime complex was considered in relation to the overarching ecological characteristics of AMR. In doing so, the analysis identifies, categorizes, and elucidates 18 spatial, temporal, threshold, and cascading misfits in global AMR governance (table 3).
Table 3 Summary of Ecological Misfits in AMR Governance

1. Spatial Misfits
Spatial misfits occur when the jurisdiction of a governance arrangement is too small or too large to cover or affect the ecosystem that it seeks to govern (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015; Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008). For AMR, there is a spatial misfit between the globalizing way that microbes transcend borders and the statist norms and principles that underpin the regime complex for AMR governance (Hoffman, Weldon, and Habibi Reference Hoffman, Weldon and Habibi2022; Weldon and Hoffman Reference Weldon and Hoffman2021; Wenham, Eccleston-Turner, and Voss Reference Wenham, Eccleston-Turner and Voss2022). This spatial misfit reflects what Clare Wenham, Mark Eccleston-Turner, and Maike Voss (Reference Wenham, Eccleston-Turner and Voss2022) identify as a contradiction between the globalist need for health governance and the anarchical system of sovereign states in which global health governance necessarily takes place.
Another important spatial misfit in AMR is the uneven distribution of global access to effective antimicrobials, which is caused by failures in the global political-economic system to distribute these lifesaving products equitably and efficiently. Enduring legacies of colonialism, weak healthcare systems, lack of infrastructure, and inabilities to pay all confound this challenge, which essentially means that places where antimicrobials are needed the most face an extremely unjust triple burden marked by high infectious disease, poor access to antimicrobials, and high mortality and morbidity from drug-resistant infections (Murray et al. Reference Murray, Ikuta, Sharara, Swetschinski, Aguilar, Gray and Han2022). Somewhat paradoxically, this lack of access to antimicrobials is a key driver of resistance such that global efforts to mitigate AMR must simultaneously expand access in these locations while reducing use in others.
Finally, there is a related spatial misfit between the sites where the socioecological interaction happens (i.e., where the antibiotics are consumed by individuals around the world) and the sites where governance happens through the regime complex (e.g., in multinational corporation board rooms, UN agency meeting rooms in Geneva and New York, etc.).Footnote 1
2. Temporal Misfits
Temporal misfits occur when institutional dynamics are too fast or too slow compared with their relevant ecological dynamics. They can occur when institutions are ignorant of, ignore, or miscalculate the timespan of an ecological process, or when responses take too short or too long a time to have a positive effect (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015; Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008). Temporal misfits in global AMR governance are caused by the dynamics of the regime complex writ large, the IHR, and the process of innovation for new drugs in the economic system enshrined by the global trade regime. For the regime complex writ large, it is evident that as global AMR mortality rates rise, it is because the global institutions designed to mitigate the threat of AMR cannot match the pace at which microbes evolve, travel, and infect populations.
Another two temporal challenges arise in the event-focused nature of the current IHR.Footnote 2 First, the primary focus of the IHR, as outlined in its preamble, is to improve the international response to emergency events. Arising from this aim, articles 12–17 of the IHR outline a set of rules and responsibilities for responding to disease outbreaks, but which are ill-suited to the enduring process of AMR (Kamradt-Scott Reference Kamradt-Scott2011). Specifically, these articles project a dichotomous understanding of time, implying that the world is either in crisis mode or noncrisis mode. The enduring process of AMR, however, underscores the need for action and mechanisms to address the underlying causes and drivers of resistance to prevent emergencies from happening in the first place (Staupe-Delgado and Rubin Reference Staupe-Delgado and Rubin2023).
Second, as noted above, another temporal misfit is the slow response time that it generally takes for the WHO to declare public health emergencies of international concerns (PHEICs). This procedure, which is outlined in articles 6–15 and Annex 2 of the IHR, is used by the WHO to enact emergency global response protocols to emerging disease threats (Hoffman and Silverberg Reference Hoffman and Silverberg2018). However, several technical and political barriers often prevent a timely declaration of a PHEIC, which hinders an effective global response.
The innovation pipeline for new antimicrobial drugs faces several market challenges, highlighting additional socioecological tensions that arise from human economic practices. This lack of innovation is an outcome shaped by principles, norms, rules, and procedures in the trade regime, demonstrating that the traditional system of conferring intellectual property rights to support innovation has largely failed for antimicrobials (Caceres et al. Reference Caceres, Singh, Minssen, Van Katwyk and Hoffman2022). This system has not been able to provide new antimicrobials at a rate commensurate with increasing human-induced resistance—meaning that existing antimicrobials are becoming less effective faster than new ones are coming out.
More specifically, the antimicrobial market presents numerous barriers that make the traditional intellectual property rights approach ineffective, such as high research and development costs and societal expectations for low-cost and affordable medicines. Furthermore, the usefulness of antimicrobials is often time limited due to inevitable microbial resistance, while the need to limit use and conserve their effectiveness further reduces the antimicrobial market (Kesselheim and Outterson Reference Kesselheim and Outterson2011).
In response, it has become increasingly necessary to explore models that delink the cost of research and development from the sales volume of antimicrobials. Strategies like market entry rewards, subscription models (“Netflix models”), or full patent buyouts offer potential solutions to the failure of the market in developing new antimicrobials (Anderson et al. Reference Anderson, Panteli, Van Kessel, Ljungqvist, Colombo and Mossialos2023; Singer, Kirchhelle, and Roberts Reference Singer, Kirchhelle and Roberts2020).
As demonstrated by ongoing failures to distribute COVID-19 vaccines, another challenge is the allocation of medical resources via market mechanisms, as new technologies are not distributed equally or on equal time horizons once discovered. Rather, these scarce technologies continue to be distributed along global income, racial, and colonial lines, with high-income countries enjoying the bulk of their benefits (Bajaj, Maki, and Stanford Reference Bajaj, Maki and Stanford2022).
In other words, the inability to replenish the depleting pool of antimicrobial effectiveness and optimally distribute new countermeasures represent temporal misfits between the social dynamics of drug development and distribution and the socioecological factors that accelerate microbial evolutionary processes. Moreover, they signal yet another temporal challenge created by the uneven temporal scales that manifest when microbes spread around the world.
3. Threshold Misfits
Threshold misfits happen when institutions cannot prevent, fail to recognize, or cause abrupt shifts in biophysical systems. They can occur when institutions fail to provide adequate response contingencies, initiate action, or provide buffers against mechanisms that lead to irreversible shifts in ecosystems (Folke Reference Folke2016; Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008). For AMR, the historical failure of global policy to sufficiently address the issue is itself a threshold problem (Living with Resistance Project Reference Resistance Project2018). Specifically, the evolution of societies dependent on antimicrobials for improving productivity and as quick fixes means that institutions have hitherto failed to recognize and prevent irreversible shifts in the ecology of microbes, thereby accelerating AMR to the point of a global crisis (Chandler Reference Chandler2019; Kirchhelle et al. Reference Kirchhelle, Atkinson, Broom, Chuengsatiansup, Ferreira, Fortané and Frost2020; Rogers Van Katwyk, Giubilini, et al. Reference Rogers Van Katwyk, Weldon, Giubilini, Kirchhelle, Harrison, McLean, Savulescu and Hoffman2020).
Furthermore, AMR is such a complex challenge, meaning several potential threshold problems can arise if policy interventions focus on some aspects of the problem at the expense of others (Weldon, Liddell, et al. Reference Weldon, Liddell, Van Katwyk, Hoffman, Minssen, Outterson, Stephanie Palmer and Viñuales2022). One way these misfits can occur is with the overreliance on popular models such as “the global common-pool resource” model, which draws attention to the conservation of and innovation for the common pool of antimicrobial effectiveness. The risk is that narrowly applied models can obscure important aspects of the issue. For example, the global common-pool resource model, which assumes global nonexcludability, suggests that people cannot be prevented from accessing antimicrobials. This assumption, however, is not borne out in our current reality. As noted above, many are indeed excluded from enjoying the benefits of antimicrobial therapies. Consequently, models like “the global common-pool resource challenge” can overlook concerns for access, and interventions drawn from that model may further restrict access or ignore it. Conversely, however, focusing on access without conservation and innovation would accelerate the depletion of antimicrobial effectiveness and increase the likelihood of resistance while squandering precious new antimicrobial stocks. These related threshold effects underscore the interdependencies across the functionally complex challenge of AMR (Hoffman and Outterson Reference Hoffman and Outterson2015).
4. Cascading Misfits
Finally, cascading misfits happen when institutions trigger or are unable to buffer the “effects between or among biophysical and/or social and economic systems” (Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008, 153). Cascading misfits for AMR draw attention to problematic gaps in global governance around multisectoral and cross-issue planning and action. In particular, the global AMR regime complex contains no crosscutting contingency plans that unite the elemental regimes. This gap means that even if a resistant pathogen from AMR triggered the declaration of PHEIC under the IHR, this human health-specific mechanism has no bearing on how the other elemental regimes in the regime complex operate. Moreover, there is an information gap and related governance gap for mechanisms that can connect the causes and consequences of AMR with those of today’s other greatest challenges, including climate change, biodiversity loss, and zoonotic spillover.
More specifically, biodiversity, climate change, and zoonotic pandemics all have the potential to accelerate AMR either directly or indirectly (Gilchrist et al. Reference Gilchrist, Greko, Wallinga, Beran, Riley and Thorne2007; Singer et al. Reference Singer, Shaw, Rhodes and Hart2016; Strathdee, Davies, and Marcelin Reference Strathdee, Davies and Marcelin2020; Van Boeckel et al. Reference Van Boeckel, Brower, Gilbert, Grenfell, Levin, Robinson, Teillant and Laxminarayan2015). AMR, in turn, exacerbates these challenges by diminishing the ability to respond with medical countermeasures while heightening the risk of deadly infection. While the regime complex for AMR includes institutions that also focus on these functionally interdependent problems, they are not equipped with the kind of intersectoral arrangements to address the complex linkages among them.
Part 4: Improving the Ecological Fit of AMR Governance Systems
This analysis illuminates the magnitude of the challenge in making progress toward mitigating and adapting to the risks of AMR. Presently, the institutions governing AMR—embodied by a decentralized regime complex—have primarily evolved with an emphasis on developing the capacity needed to address AMR as a medical problem. However, these problem-solving efforts have largely failed to achieve the interrelated goals of sustaining antimicrobial effectiveness and promoting global health for all. They have also led to the creation of social systems fundamentally misaligned with the ecological characteristics of the problem that they are meant to govern.
The 18 identified misfits reveal deeply rooted structural challenges with the current system of global health governance, as well as the prevailing approach to designing the institutions that constitute it. The persistent inability of existing social systems to adequately address the ecological nature of AMR suggests the need for a paradigm shift in AMR governance, where AMR is approached as a socioecological problem rather than a medical one. This conceptual shift could guide extensive and profound transformations to the many social, political, and economic practices that have the potential to alter microbial ecologies through antimicrobial use. Drawing on our analysis of ecological misfits, this final, more normative section considers such a paradigm shift for global AMR governance. We end by proposing five institutional design principles to navigate the delicate balance between enacting immediate action to mitigate AMR and transitioning to a future of sustainable antimicrobial governance.
A Paradigm Shift for AMR Governance
The inevitability of microbial resistance underscores the inherent unsustainability of current antimicrobial therapies, as well as the need for new ideas to change social practices (Denyer Willis and Chandler Reference Denyer Willis and Chandler2019; Ventola Reference Ventola2015; Weldon, Rogers Van Katwyk, et al. Reference Weldon, Yaseen and Hoffman2022). It also calls into question the “war on superbugs” analogy often deployed to raise awareness of the challenge (Wallinga, Rayner, and Lang Reference Wallinga, Rayner and Lang2015). On one hand, the current rate of antimicrobial innovation needs acceleration, which can be accomplished through mechanisms that address various market failures in the pharmaceutical industry (e.g., via novel market or entirely nonmarket mechanisms). However, a “weapons” approach, fixated on innovation, fails to address the broader context in which antimicrobials are used and distributed. It would mean that the current pace of innovation, which is already inadequate, would need to be radically if not impossibly accelerated to keep pace with the rising demand for new therapies. Instead of engaging in an unwinnable arms race—where microbial evolution typically outstrips our ability to develop and distribute new therapies—shifting our approach to designing social systems that can optimize antimicrobial use, minimize AMR, and maximize the time-limited effectiveness of antimicrobial drugs offers better chances of achieving sustainability. Without such paradigmatic changes, our response to AMR will remain a reactive one, always struggling to outpace microbial evolution rather than sustainably managing it.
Rather than solely focusing on innovating new technologies to solve problems and maintain the status quo of human social systems, a paradigm shift could change the way we approach AMR, leading to new systems that reconfigure human–microbial relations for future sustainability (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020). With this shift, the object of governance extends beyond merely the social response to infectious disease threats. It also encompasses the coevolution of human societies with and within microbial ecologies—a concept referred to as “coevolutionary governance” (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020). Coevolutionary governance builds on principles of adaptive comanagement by recognizing, anticipating, and analyzing “interdependent eco-evolutionary dynamics [to] guide human societies toward identified goals” (485). This concept acknowledges that the evolution of human culture is dialectically connected with various forces of microbial evolution, underscoring the need for adaptable institutions capable of sustainably guiding these interactions.
Transitioning to a Future of Antimicrobial Sustainability
Transitioning from the current configuration of human–microbial relations calls for practical changes and transformations to align existing institutional approaches with socioecological understandings of the problem. Guided by a paradigm shift, this transformation would include the development of more sustainable forms of organization, while simultaneously ensuring that the benefits of antimicrobials are accessible to all. Such a transformation will entail a comprehensive reassessment of existing labor, care, agriculture, and land practices, where antimicrobials are currently employed infrastructurally as expedient solutions to support abundance, profits, and productivity (Denyer Willis and Chandler Reference Denyer Willis and Chandler2019). This endeavor will, among other things, require substantial investments in hygiene and sanitation, addressing global poverty, and more broadly transforming the conditions under which people live, work, and seek safety (Rogers Van Katwyk et al. Reference Rogers Van Katwyk, Balasegaram, Boriello, Farrar, Giubilini, Harrison and Kieny2019). Otherwise, global efforts would be akin to treating the symptoms without addressing the underlying illness.
This transformation, moreover, will involve a tough examination of the ways in which the principle of state sovereignty manifests as an obstacle to unified, global strategies for ecological crises in the Anthropocene (Biermann Reference Biermann2012). In global governance, for example, the principle of sovereignty contradicts microbial ecological realities and the world’s resulting shared vulnerability to infectious disease. This vulnerability is facilitated, in part, by the myriad pathways through which humans, animals, and microbes travel around the world and put all countries at risk. Despite these challenges, immediate solutions will have to be pioneered and implemented by states within this very system (Wenham, Eccleston-Turner, and Voss Reference Wenham, Eccleston-Turner and Voss2022). Therefore, while institutional transformations guided by new conceptions of AMR seem warranted, the current problem of AMR remains a problem both of and for the existing system of sovereign states.
The deep-rooted ideas shaping behaviors that drive AMR suggest that a paradigm shift may be difficult to implement, especially given the ingrained principles and norms of global governance. Certain stakeholders even harbor vested interests in resisting change. For example, pharmaceutical companies continue to oppose regulatory changes that jeopardize their profit margins, while governments resist measures they perceive as encroaching upon their sovereignty. But while initiating and implementing a paradigm shift in AMR governance will be challenging, it is not impossible. For example, the contradictions in today’s prevailing principles and norms analyzed in this article indicate ruptures through which new principles and norms may emerge to inform future action.
Balancing Long-Term Transformation with the Urgent Need for Immediate Action
In the interim, adjustments can be made to the existing system to alleviate the dire human suffering caused by the urgent problem of AMR (Hoffman, Bakshi, and Rogers Van Katwyk Reference Hoffman, Bakshi and Van Katwyk2019). However, it is crucial that these adjustments are designed to support rather than detract from larger transformative efforts. Indeed, this approach does not mean forgoing the above-argued shift in approaches to AMR governance. Rather, informed by an understanding of the regime complex’s ecological misfits, specific adjustments can simultaneously (1) respond to the problem of AMR within the current configuration of human–microbial relations, (2) mitigate the deeper drivers of AMR in the first place, and (3) transform the existing configuration for long-term sustainability.
Socioecological studies on institutional design, particularly in biodiversity and ecosystems management, provide useful starting points for responding to AMR as an enduring ecological challenge—balancing immediate action with long-term transformation (Folke Reference Folke2016; Galaz et al. Reference Galaz, Olsson, Hahn, Folke, Svedin, Young, King and Schroeder2008). Five interrelated design principles emerging from decades of empirical investigations on governing socioecological challenges stand out as especially important for improving the fit of AMR governance systems. These principles recognize the inherent complexities and rapidly evolving nature of AMR, and advocate for responsive, informed, and multifaceted strategies. Collectively, these principles represent an alternative normative foundation to the prevailing paradigm informing global AMR action, which we propose could inform future deliberations on designing institutions capable of adapting global AMR governance for coevolution.
Five Principles for Governing AMR as a Socioecological Challenge
1. Acknowledge There Is No Panacea or Silver Bullet for AMR
First, when addressing complex and multifaceted socioecological challenges, including AMR, it is crucial to recognize that there is no one-size-fits-all solution or panacea. Indeed, one of the few generalizable findings from socioecological investigations on institutional design is that universal, simple, and silver-bullet solutions do not exist (Ostrom, Janssen, and Anderies Reference Ostrom, Janssen and Anderies2007). Socioecological problems manifest differently across different geographies, ecosystems, and cultures. Each region and community may face unique challenges based on specific socioeconomic, environmental, and political conditions (Epstein et al. Reference Epstein, Pittman, Alexander, Berdej, Dyck, Kreitmair, Rathwell, Villamayor-Tomas, Vogt and Armitage2015). This diversity necessitates localized, tailored solutions. Attempting to apply a uniform approach across all these different contexts is likely to result in ineffectiveness or even unintended harmful consequences.
Similarly, the issue of AMR is not monolithic; rather, the challenge varies depending on location, ecosystem, and social context. AMR is an ecologically complex and diverse problem, with different populations facing unique social, economic, and environmental contexts (e.g., some populations overuse antimicrobials while others lack access). Therefore, institutions will need to adapt to fit local circumstances, where each social and microbial ecosystem presents its own set of challenges and considerations (de Campos-Rudinsky Reference de Campos-Rudinsky2023). For instance, the strategies suitable for a hospital in a high-income country will be different from those needed for a farm in a lower-middle-income country. Tailoring local AMR policies for the specific needs of an area, and considering factors like local population density, healthcare access, prevalent pathogens, and labor, care, and land practices can help to address many of the spatial misfits in AMR governance. However, this will require substantial action to overcome the political and economic challenges that currently hinder the optimal global distribution of vital resources, such as effective antimicrobial treatments.
2. Design Agile and Adaptable Institutions with Iterative Approaches and Rapid Cycles of Learning
The second principle revolves around building governance systems that remain adaptable over time. The complexity of ecological challenges, often characterized by high degrees of dynamism, uncertainty, and the potential for abrupt shifts, as well as evolving human preferences, values, and interests, means that what works today may not work tomorrow (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020). Solutions must therefore be adaptable and capable of evolving along with the changing ecologies that they aim to govern (Ostrom et al. Reference Ostrom, Burger, Field, Norgaard and Policansky1999). In this context, durable institutions are not static ones that will stand the test of time, but rather ones that are agile and flexible enough to respond and adapt to inevitable changes.
Agile institutions, designed with iterative approaches to rapid learning, would generate practices more commensurate with the temporal dynamics of microbial evolution and spread. Given many existing uncertainties and a rapidly evolving science and social science evidence base, these integrated processes will inevitably involve trial and error in policy making. In recognizing this challenge, built-in and regular reviews and adjustments can foster institutional cultures of social learning-by-doing, enabling continuous adaptation based on experience and feedback.
The dynamic nature of AMR requires flexible, evolving strategies rather than static ones (Léger et al. Reference Léger, Lambraki, Graells, Cousins, Patrik, Harbarth and Carson2021). Resistant strains can emerge rapidly, and existing treatments can quickly become ineffective. In response, our governance structures must be adaptable and ready to react to changes swiftly. This principle means that governance structures need to be flexible over time. Developing more fitting institutions for AMR will require normalizing policy experiments, embracing failures, and making revisions. In practice, this principle could be accomplished in various settings, from local to global, by incorporating policy feedback systems where the effectiveness of AMR strategies is evaluated and refined in iterative cycles. The process in the 2015 Paris Agreement, where national commitments to climate action are regularly assessed and ratcheted-up in ambition, provides a useful example (Weldon, Rogers Van Katwyk, et al. Reference Weldon, Liddell, Van Katwyk, Hoffman, Minssen, Outterson, Stephanie Palmer and Viñuales2022). Similar mechanisms for AMR governance could help to address temporal misfits by quickly implementing new guidelines in response to emergent resistant strains, as well as threshold misfits by reassessing and recalibrating interventions when microbial resistance thresholds are crossed.
3. Diversify Practices and Generate a Living and Comprehensive Evidence Base for the Many Facets of AMR
A wide range of diversified practices is essential for promoting location-specific and adaptable policy experiments to address ecological challenges across time and space (Dietz, Ostrom, and Stern Reference Dietz, Ostrom and Stern2003). Similar to how biodiversity improves the likelihood of ecosystem resilience, diversity in social systems can improve resilience to political and social shifts and shocks, create and sustain systemwide adaptability and flexibility, and unlock multiple pathways to success (Plummer and Armitage Reference Plummer and Armitage2007). In tandem with the need for locally adapted policies, agility, and rapid learning, the endeavor to systematically diversify practices can be purposively designed to cogenerate a robust evidence base on what techniques can best support effectiveness in relation to social values (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020; Léger et al. Reference Léger, Lambraki, Graells, Cousins, Patrik, Harbarth and Carson2021). This evidence base, derived from a diverse range of practices for the many facets of AMR, could inform adaptation and adjustments, isolating conditions for success where they exist.
The myriad determinants of antimicrobial use—itself a testament to the extent to which antimicrobials are relied upon in various aspects of daily life—mean that diverse practices are needed at varying levels of society. At the biomedical level, diversity is accomplished by exploring alternative therapies to antimicrobials. In agriculture, traditional and alternative practices could be explored to promote yields and sustainability while lessening the reliance on antimicrobials. At the social level, diverse practices could explore means for promoting health and welfare beyond pharmaceutical interventions to more holistic approaches to prevention. At the cultural level, alternative perceptions of food security and more sustainable diets could promote nutrition beyond industrially produced meat and animal products. In the market, a range of public–private partnerships or entirely public models could diversify responses to the economic challenges associated with antimicrobial innovation. And at the governance level, diversity can be accomplished by implementing a range of strategies to regulate and monitor antimicrobial use, foster collaboration across sectors, promote international cooperation, and empower decision making that includes a broad spectrum of stakeholders. By integrating diversity in practices across all levels, it is possible to formulate a comprehensive and effective response to the complex challenge of AMR. Throughout these domains, moreover, a living evidence base can be cogenerated with experiments, informing revisions and adaptations in accordance with new knowledge and evidence as it emerges.
4. Create Links across Locations and Scales
Diversification can help to build resilience and reduce the risk of AMR, but it also requires coordination across different sectors and levels of governance. Evidence from socioecological management of shared resource systems has found that adaptive governance is most effective when smaller efforts are connected to larger networks, especially when challenges are complex and global (Ostrom Reference Ostrom1990). Promoting connectivity across various scales—from local to global—ensures that bottom-up initiatives at the community to national levels do not operate in isolation, but rather are woven into a broader, global strategy. It is therefore crucial to create links between different actors, sectors, and scales when managing rapidly evolving and transnational phenomena, such as AMR.
The need for coordination across scales and geographies underscores the importance of bridging organizations—entities that can pioneer the much-needed linkages across diverse sectors, geographies, and scales (Olsson et al. Reference Olsson, Folke, Galaz, Hahn and Schultz2007). Bridging organizations perform crucial functions of governance, such as facilitating the sharing of knowledge, resources, accountability, and best practices.
For AMR, cross-scale and cross-sectoral approaches are indispensable given the problem’s transboundary and multifaceted nature. Local solutions and grassroots initiatives are vital, but their true potential can only be harnessed when they are integrated into a larger, global framework that recognizes and addresses AMR as a shared, worldwide problem. Bridging organizations can facilitate this integration, connecting localized efforts with global strategies and ensuring that innovations and solutions are supported, visible, and can be adapted to different contexts. For example, a successful approach to AMR in healthcare in one community could be scaled up and incorporated into national and even global health policies, ensuring that lessons learned at one level inform strategies at others. Bridging organizations could enable the scalability of successful local approaches to AMR, fostering the transfer of lessons from the community level to national and global health policies. Equally important is their role in creating connections across sectors—healthcare, agriculture, and environmental management, among others—fostering a multisectoral response to AMR. In strengthening vital linkages across locations and scales, we can cultivate a more holistic and adaptable governance system capable of managing the complexities of AMR.
5. Promote Participation among Stakeholders
Finally, the effectiveness of these measures depends on the involvement of stakeholders (Chaffin, Gosnell, and Cosens Reference Chaffin, Gosnell and Cosens2014). This principle involves mechanisms for participation and incorporating diverse perspectives into decision-making processes. It also necessitates creating rules and policies that are flexible, adaptable, and reflect the needs and circumstances of different stakeholders. Examples from biodiversity and climate governance underscore that institutions designed to improve ecological fit may inadvertently create social misfits (Moss Reference Moss, Breit, Engels, Moss and Troja2003). This can produce tensions among social values and competing conceptions of objectivity, universality, and desirability across different contexts and over time—conflicts already apparent in the vast regime complex for AMR governance. In addition to questions about improving ecological fit, there are also important questions that remain about how best to sustain social cohesion, uphold democratic principles, and other social values during uncertain and transformative times.
In cultivating participation, the new “AMR Multi-Stakeholder Partnership Platform” hosted by the WHO, FAO, WOAH, and UNEP offers an opportunity to bring together diverse actors across different levels and sectors (WHO 2022), making AMR governance not only more ecologically fitting but also socially acceptable and feasible. In recognizing that the challenge of AMR cannot be tackled by any one sector, organization, or level of governance alone, the platform promises to involve stakeholders from the health, agricultural, environmental, and other sectors, as well as representatives of various levels of governance (from local, to national, to global) and various societal groups (e.g., patients, healthcare professionals, farmers, etc.).
The effectiveness of this global platform for AMR could be gauged by its ability to perform several functions. These include acting as a hub for knowledge exchange and learning, and sharing information, best practices, and lessons learned. The platform could foster policy coordination and harmonization across different sectors and levels of governance, ensuring a more coordinated and effective response to AMR. Additionally, it may also play a role in raising awareness about AMR, advocating for policy changes, enhancing accountability, and mobilizing resources for AMR prevention and control.
Despite ongoing challenges and acknowledged limitations, these lessons from biodiversity and ecosystems governance offer valuable insights for improving AMR governance. Specifically, these strategies provide a road map for research to inform practice; emphasize the need for sustainability, adaptability, and resilience; recognize the importance of democratic principles; and underscore the importance of striking a balance between addressing immediate needs and pursuing long-term goals. While global biodiversity governance has experienced lackluster results in some areas due to insufficient funding, political will, and enforcement, these principles have catalyzed action, providing a basis to evaluate progress. In AMR governance, on the other hand, governance efforts are missing these necessary steps, including metrics, benchmarks, and evidence to inform current and future action. By learning from both successes and shortcomings in these areas, we can work toward more effective and inclusive governance structures that inform the development of context-specific approaches to AMR governance, consider the complex dynamics of microbial evolution and human activity, and address the challenges of AMR in a comprehensive and sustainable manner.
Conclusion
When we ask whether an institution is “fit for purpose,” we are essentially asking if it can achieve its stated objectives. This raises several important political questions about what and whose purposes and objectives global governance is serving. But the question also directs our attention in a particular way by preempting the idea that institutions solve problems. That is, by asking whether institutions can address certain issues, the question assumes that human institutions are self-contained entities that fix problems out in the world, making the site of the intervention external to the institution. What this article posits, however, is that the central question for enduring global health challenges like AMR is not “how do we ‘solve’ this problem,” but rather, “how do we craft social systems that are better aligned with ecological and microbial systems in a shared planetary environment?” Put differently, we should be asking how our institutions can better fit the process of AMR to (1) create systems that enable harmonious coevolution with microbes within planetary systems, (2) minimize the socioecological contradictions that accelerate AMR, and (3) maximize the effectiveness of antimicrobial treatments for infectious disease (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020). This transition internalizes the issue, bringing the site of intervention from outside somewhere in the world to instead thinking about designing institutions that are contained within and aligned with material planetary realities.
The path to a more fitting relationship with microbes depends on improving our understanding of the intricacies of the human–microbial nexus, where many agree that the human–microbial relationship is overall net positive and symbiotic (Jørgensen et al. Reference Jørgensen, Folke, Patrik, Malmros, Troell and Zorzet2020). Yet, this article revealed 18 ways that human activity is at odds with microbial processes, accelerating their evolution from symbiotic to pathogenic. It turned attention to the challenges surrounding the principles, norms, rules, and procedures by which AMR is globally governed and drew a series of five principles for designing institutions for AMR, suggesting that it is possible to identify opportunities to transform the prevailing principles, norms, rules, and procedures by which AMR is currently governed. Additionally, by adopting a core International Relations (IR) concept to examine the global social systems emerging around AMR, this article sought to help bridge IR’s relative silence about the salience and intensity of today’s many pressing ecological challenges, as pointed out by IR scholars (Agathangelou Reference Agathangelou2021; Burke et al. Reference Burke, Fishel, Mitchell, Dalby and Levine2016).
The process of AMR is inevitable and enduring, but its manifestation as a social problem is contingent on the prevailing interplay between human activities and microbial ecologies. As this interplay evolves, the development of new institutions that guide human cultural evolution in tandem with the inherent evolutionary tendencies of microbial life could help to minimize human-induced disease mutations caused by AMR. As we continue to look for ways to mobilize and sustain appropriate global action for AMR, finding political and policy strategies that better fit the problem could help to achieve sustainable antimicrobial use for all. Indeed, the optimal relationship between human societies and microbial ecologies may necessitate forms of social organization that transcend those currently in existence.
Acknowledgments
The authors thank Anna Agathangelou, James Orbinski, Clare Wenham, and Sydney Wilson for their constructive feedback on previous versions of this article. This research was conducted as part of Isaac Weldon’s PhD dissertation at York University, Toronto, Canada.





