Introduction
Invasive feral swine Sus scrofa cause extensive damage to natural systems and affect many species of native wildlife and vegetation, both directly and indirectly (Seward et al., Reference Seward, VerCauteren, Witmer and Engeman2004). In addition, feral swine have been implicated as a major threat to sensitive habitats and ecosystems (Engeman et al., Reference Engeman, Stevens, Allen, Dunlap, Daniel, Teague and Constantin2007). The negative impact of exotic species on native species and ecosystems is exceeded only by human-caused habitat destruction (Wilcove et al., Reference Wilcove, Rothstein, Dubow, Phillips and Losos1998; Parker et al., Reference Parker, Simberloff, Lonsdale, Goodell, Wonham and Kareiva1999). In the USA exotic species have played a role in the listing of 42% of the species protected by the Endangered Species Act (Stein & Flack, Reference Stein and Flack1996). Worldwide, invasive species are a leading contributor to extinctions of birds, fish and mammals (Clavero & García-Berthou, Reference Clavero and García-Berthou2005). Unlike many invasive species that may have primarily direct effects on other species through predation or competition, feral swine can have both direct effects as predators and indirect effects by drastically altering habitats (Zengel & Conner, Reference Zengel and Conner2008; Barrios-García & Ballari, Reference Barrios-García and Ballari2012; Rossell et al., Reference Rossell, Clarke, Schultz, Schwartzman and Patch2016). Non-native predators tend to be a greater threat than native predators to prey populations (Salo et al., Reference Salo, Korpimäki, Banks, Nordström and Dickman2007). Feral swine have been implicated as a threat to several threatened wildlife species in the USA, including the lesser prairie-chicken Tympanuchus pallidicinctus, shorebirds and marine turtles (USDA, 2002; Engeman et al., Reference Engeman, Duffiney, Braem, Olsen, Constantin and Small2010, Reference Engeman, Addison and Griffin2016), but direct and indirect impacts on small, fossorial species, including amphibians, have not been as well documented. Land managers tasked with the recovery of endangered wildlife require an increased understanding of the existence and nature of conflicts between feral swine and threatened amphibians.
Reticulated flatwoods salamanders Ambystoma bishopi and frosted flatwoods salamanders A. cingulatum have been identified, along with three other amphibian species in Florida that are either federally or state listed, as being threatened by the activities of feral hogs (USDA, 2002). Although direct predation of salamanders by feral swine has been reported (Howe et al., Reference Howe, Singer and Ackerman1981), it has been suggested that habitat disturbance from extensive rooting has the most serious impact on amphibians with specialized habitat requirements (Means & Travis, Reference Means and Travis2007). Feral hogs create short-term disturbance of vegetation and soil, removing emergent plants and exposing bare soil (Arrington et al., Reference Arrington, Toth and Koebel1999; Doupé et al., Reference Doupé, Schaffer, Knott and Dicky2009; Rossell et al., Reference Rossell, Clarke, Schultz, Schwartzman and Patch2016). They can also affect the long-term vegetation structure, as certain plants appear to respond positively and others, such as seedlings of the longleaf pine Pinus palustris, which as canopy trees provide an important source of fuel to maintain the fire-dominated ecology of this habitat, may be consumed preferentially by hogs (Simberloff, Reference Simberloff1993; Arrington et al., Reference Arrington, Toth and Koebel1999; Means, Reference Means, Jose, Jokela and Miller2006; Boughton & Boughton, Reference Boughton and Boughton2014). These impacts are particularly challenging for flatwoods salamanders because these species rely on hydric and mesic longleaf pine–wiregrass Aristida stricta savannah habitat for both the aquatic and terrestrial phases of their life cycles (Palis, Reference Palis1997).
The reticulated flatwoods salamander was listed as federally endangered in 2009 (USFWS, 2009) and is categorized as Vulnerable on the IUCN Red List (Palis & Hammerson, Reference Palis and Hammerson2008). The decline of the species has been rapid, and fewer than 30 breeding wetlands across the species’ range are known to have been occupied in recent years (USFWS, 2009; Semlitsch et al., Reference Semlitsch, Walls, Barichivich and O'Donnell2017). Eglin Air Force Base in Florida is one of few remaining public lands where this species occurs, and it is the only remaining property within the entire range of the species to support two populations, each occurring in wetland complexes having > 2 occupied wetlands (Gorman et al., Reference Gorman, Haas and Bishop2009).
The flatwoods salamander depends on complex herbaceous vegetation for all aspects of its life history. The ecotone between deeper wetland areas and uplands provides a diverse habitat structure, and this type of shallow, well-vegetated littoral zone is known to be important to many species of pond-breeding amphibians (Porej & Hetherington, Reference Porej and Hetherington2005; Shulse et al., Reference Shulse, Semlitsch, Trauth and Gardner2012). Flatwoods salamanders lay their eggs in dense herbaceous ground cover in the ecotone (Gorman et al., Reference Gorman, Powell, Jones and Haas2014), and developing larvae feed and seek cover there as well (Sekerak et al., Reference Sekerak, Tanner and Palis1996; Gorman et al., Reference Gorman, Haas and Bishop2009). Frequently metamorphs and adults are observed climbing and perching above the ground in wiregrass near breeding wetlands (Jones et al., Reference Jones, Hill, Gorman and Haas2012). This dense understorey herbaceous vegetation is maintained by the presence of an open pine canopy, which provides fine fuels and a short (1–3 years in uplands, 3–5 years in wetlands) fire return interval, although in much of the salamander's range fire suppression has facilitated the encroachment of midstorey shrubs that shade and change this habitat (Bishop & Haas, Reference Bishop and Haas2005; Gorman et al., Reference Gorman, Haas and Himes2013).
In the face of many complex challenges, habitat management for reticulated flatwoods salamanders at Eglin has increased significantly since 2010 and includes mechanical removal of midstorey shrubs in wetland basins (e.g. Gorman et al., Reference Gorman, Haas and Himes2013) and use of growing-season prescribed fire. However, with continuing challenges associated with frequent drought years, smoke management risks that inherently accompany prescribed fire, and slow natural dispersal by the salamanders into recently restored habitat, the species faces a long, difficult road to recovery.
Feral swine are relatively ubiquitous at Eglin, where their damage to wetlands has been documented for many years (Engeman et al., Reference Engeman, Stevens, Allen, Dunlap, Daniel, Teague and Constantin2007). Nevertheless, for > 10 years only occasional sets of hog tracks had been observed in the vicinity of monitored flatwoods salamander breeding wetlands, and damage to the sites had never been documented prior to 2013. However, while surveying for larval flatwoods salamanders during the 2013–2014 autumn–winter breeding season we observed a significant increase in hog sign, including extensive damage from rooting in known breeding wetlands of the flatwoods salamander. The addition of this new threat to an already challenging recovery poses a potentially serious problem, the severity of which needs to be assessed.
Effects of invasive species on rare species may be more pronounced in a location such as Florida, where rare species may be particularly vulnerable because extensive development has depleted many of the native habitats on which they depend (Engeman et al., Reference Engeman, Constantin, Gruver, Rossi, Columbus and Kuznetsov2009). Invasive species often present novel control situations for managers, requiring the acquisition of biological knowledge and the development and testing of control technologies and strategies (e.g. Engeman & Vice, Reference Engeman and Vice2001). Our objective was to assess the amount of hog sign and damage in these wetlands and to take corrective management actions to curb additional impacts from this invasive mammal. We report on our monitoring procedure as well as the management practices to document the benefits of regular monitoring in facilitating a rapid response.
Study area
Our study area comprised longleaf pine–wiregrass flatwoods with scattered ephemeral wetlands of various sizes on Eglin Air Force Base, Okaloosa County, Florida. Within this area we selected 28 wetlands, of which 17 were historical breeding sites for reticulated flatwoods salamanders. Eleven of the 17 historical sites were occupied by salamander larvae in 2013–2014. The selected wetlands typically had an overstorey consisting of a combination of slash pine Pinus elliottii, pond cypress Taxodium ascendens and black gum Nyssa sylvatica, a midstorey consisting of some combination of myrtle dahoon Ilex cassine var. myrtifolia and titi Cyrilla racemosa as well as the aforementioned overstorey species, and an understorey of wiregrass, Curtiss’ sandgrass Calamovilfa curtissii, longleaf threeawn Aristida palustris, pipeworts Eriocaulon spp., shortbristle horned beaksedge Rhynchospora corniculata, and a rich diversity of graminoids, forbs and small shrubs.
Methods
Damage assessment
Following our initial observations of hog damage in several wetlands during the salamander breeding season in winter 2013–2014, we surveyed 28 wetlands for hog sign and damage during 2 April–20 August 2014. These included all 11 wetlands that were known to be occupied by flatwoods salamander larvae during the 2013–2014 breeding season, as well as six that have been occupied historically and 11 potential breeding sites, six of which are near historically or recently occupied sites, and five of which are in an area that is undergoing restoration to support future recovery efforts for flatwoods salamanders.
Sites were surveyed by walking along the approximate perimeter of the wetland within the ecotone (wetland edge, heavily used by breeding salamanders), and along perpendicular transects into the approximate centre of the wetland basin, at intervals of 10–30 m, depending on visibility. Additional surveys for hog sign were conducted along adjacent roads and surrounding uplands within 50 m of the wetland edges. At each site we recorded the type(s) of hog sign observed (tracks, scat, rooting damage), location of damage (wetland basin, ecotone, uplands < 50 m of outer edge of ecotone), approximate area damaged or disturbed by rooting, and percentage of overall wetland disturbed by rooting. We also assessed whether damage was focused on herbaceous vegetation, woody vegetation, or a near-equal combination of the two. Observers had several years of experience identifying hog damage in both upland and wetland habitats, as well as training from U.S. Department of Agriculture Wildlife Services personnel.
Mitigation activities
Our initial reporting of hog damage led natural resources personnel at Eglin to install hog exclusion fencing in two areas that enclosed five of the 11 flatwoods salamander breeding sites that were occupied in 2013–2014, as well as some surrounding uplands. In total, 2,295 m of fencing was required, at a cost of USD 19.70 per m, including the cost of site preparation, equipment, labour and materials. Materials included 1.8 m metal T-posts, 4 gauge 0.86 × 4.88 m (34″ × 16′) hog fence panels (Behlen Manufacturing Co., Columbus, USA), and wire clips. The fence panels had a mesh width of 20 cm, and a mesh height ranging from 5 cm at the bottom to 15 cm at the top. We surveyed these fenced breeding wetlands for hog presence a minimum of three times following the completion of the installation on 16 December 2014, to ensure that no hogs had been enclosed within the fence perimeters. Fifteen unfenced sites, including the remaining six breeding sites that were occupied in 2014 and initially had been surveyed for hog presence, were resurveyed during the same period following 16 December 2014. Moreover, following the initial reports of damage, U.S. Department of Agriculture Wildlife Services personnel based at Eglin immediately (April 2014) began implementing hog trapping across all affected areas. Swine captured in pen traps were euthanized. The swine trapping process required identifying the most favourable locations to carry out control activities, placing baits (soured corn), constructing pen traps around bait piles that were visited consistently by swine, allowing the swine to acclimatize to the presence of the traps, and then setting the traps for capture. The traps were custom-designed and collapsible for portability, durable, and large enough to capture groups of feral swine, including the largest individuals (Engeman et al., Reference Engeman, Duffiney, Braem, Olsen, Constantin and Small2010).
Statistical analyses
The prevalence of damage caused by feral hogs was compared among the three categories of wetlands, based on use by flatwoods salamanders (potential breeding sites, currently occupied breeding sites, and historical breeding sites), using Fisher's exact test. For breeding sites with damage, the percentage of damage within the ecotone (where most of the suitable vegetation associated with breeding occurs in our sites) was compared to that observed in the associated wetland as a whole using repeated measures ANOVA. This would indicate whether the salamanders’ potential breeding locations were preferred rooting habitat of feral swine.
Results
Hog presence was recorded in 19 of the 28 (68%) wetlands surveyed, and damage caused by hogs was recorded in 15 of those 19 (78% of wetlands with hog presence, or 54% of total sites). Critically, six of the 15 damaged sites had ≥ 20% of the wetland ecotone damaged by feral hog activity, and two of those six were occupied by flatwoods salamanders at that time (Plate 1). In all 15 sites where hog damage was recorded, the damage was focused exclusively on herbaceous vegetation.

Plate 1 (a) Intact flatwoods wetland ecotone, and (b) flatwoods wetland ecotone with recent rooting damage to herbaceous vegetation caused by feral swine Sus scrofa, on Eglin Air Force Base, Florida, USA.
The prevalence of hog presence among the historical breeding wetlands was 65% (11 of 17 sites), with 73% (8 of 11) of those having damage (47% of the 17 historical breeding sites). Of paramount concern, hogs were present and damaged 55% (6 of 11 sites) of the breeding wetlands that were occupied by reticulated flatwoods salamanders in 2013–2014. Hog sign was present but there was no observed damage in 9% (1 of 11) of current known breeding sites, and no sign or damage was observed in four other wetlands (36%) in this category. Thus, although a little more than half of the currently occupied breeding sites were damaged, 86% of sites where hog presence was recorded were damaged (Fig. 1).
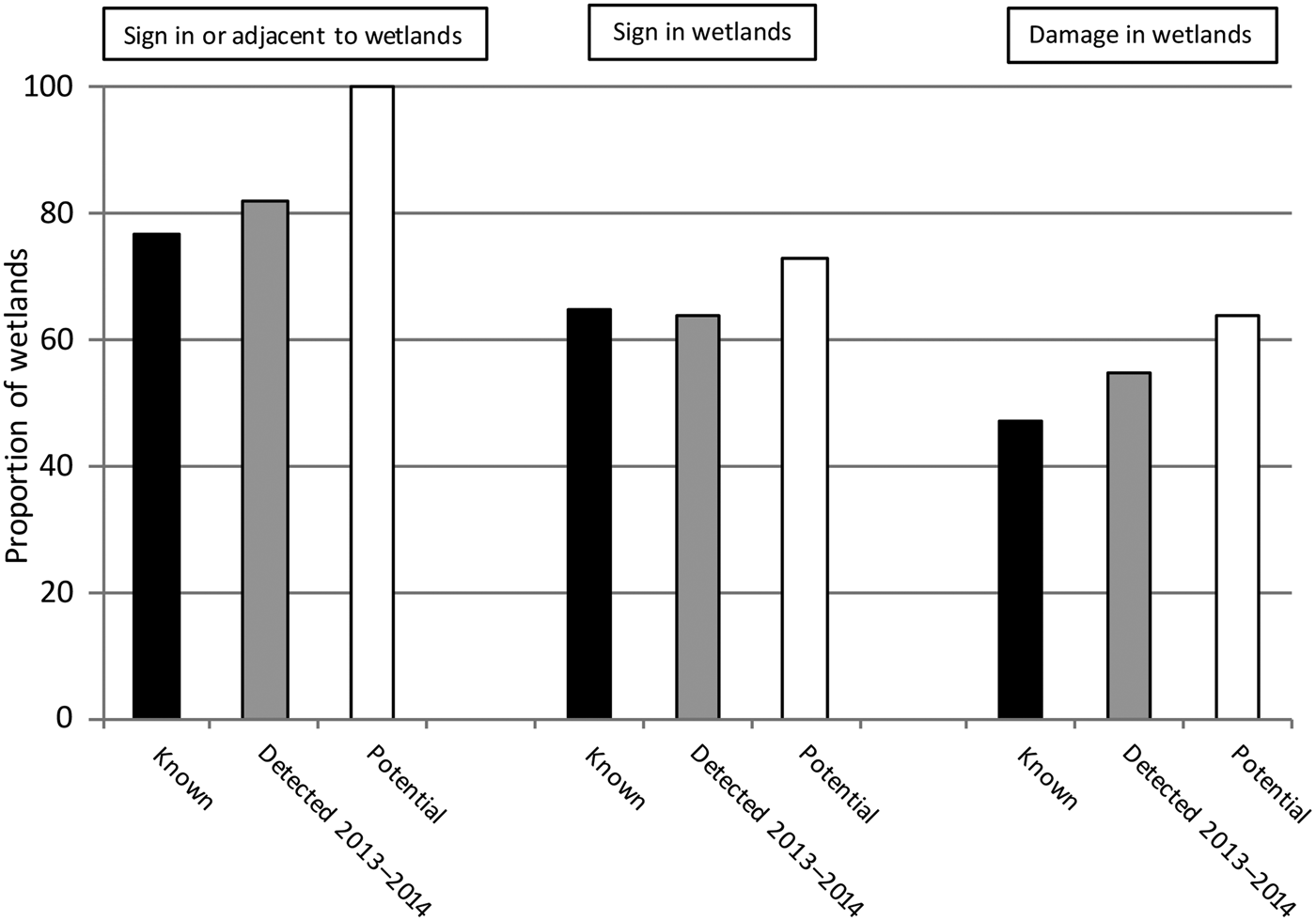
Fig. 1 Proportion of wetlands (n = 28; grouped according to presence of hog Sus scrofa sign and damage) on Eglin Air Force Base, Florida, USA, used by reticulated flatwoods salamanders Ambystoma bishopi, based on surveys conducted during 2 April–20 August 2014. Known (n = 17) represents all sites where the occurrence of A. bishopi was ever recorded, including sites detected during 2013–2014; Detected 2013–2014 (n = 11) represents sites occupied during the 2013–2014 breeding season; and Potential (n = 11) represents sites where the species was not detected but which have potential breeding habitat.
No differences in prevalence of damage were detected (Fisher's exact test, P = 0.55) among potential breeding sites (64%), current breeding sites (55%), and historical breeding sites (not including sites occupied in 2013–2014; 33%). Of concern for salamander breeding success, the mean percentage of area damaged by rooting within wetland ecotones (14 ± SE 12.98%) was higher (F 1,14 = 5.50, P = 0.034) than for the associated wetlands as a whole (6.3 ± SE 3.99%), indicating that areas most suitable for salamander breeding may also be attractive to rooting swine.
During follow-up surveys (December 2014–May 2015) for hog presence within fenced areas, no hog sign was detected inside the perimeter of any of the fenced areas. Hog sign and damage were detected both in the general vicinity and immediately along the outside of the fenced areas, indicating that hogs were still present, although they were being excluded from these areas. Furthermore, of the 15 unfenced sites that were resurveyed during this same time period, of which eight had recent hog damage during initial surveys (53%), six (40%) had recent hog rooting damage (December 2014–May 2015) and were the only sites with any observed hog sign.
Trapping efforts began in early April 2014 across the three areas where all of our monitoring sites were located, and a total of 50 feral hogs had been trapped by 15 May 2015 in two major areas of concern (20 in one and 30 in the other). In one of these areas, trapping efforts were made both inside and outside the fence perimeter but hogs were trapped only outside the fenced area, and to date there has been no observed evidence of hogs within the fenced areas. No hogs have yet been trapped in the third area, largely because of frequent tampering of the traps by people.
Discussion
Our assessments indicate that feral swine are damaging habitat that is critical to egg laying (Gorman et al., Reference Gorman, Powell, Jones and Haas2014), larval development (Gorman et al., Reference Gorman, Haas and Bishop2009), and above-ground activity of metamorph and adult flatwoods salamanders (Jones et al., Reference Jones, Hill, Gorman and Haas2012). Our findings show that whether a site is currently used for breeding, known to have been used for breeding previously, or could potentially be used for breeding, if hogs are present there is a high likelihood they will damage the site. These habitats are characterized by dense coverage of herbaceous vegetation, including Aristida spp., Eriocaulon spp. and Xyris spp. among others (Gorman et al., Reference Gorman, Haas and Himes2013), and we found that it was exactly these complex herbaceous plant communities that were the focus of hog rooting in these valuable wetlands. There are few remaining known breeding sites for the federally endangered reticulated flatwoods salamander, and the damage caused by feral swine in Eglin Air Force Base flatwoods ponds was effectively reversing wetland restoration efforts aimed at increasing the coverage of complex herbaceous vegetation (e.g. Gorman et al., Reference Gorman, Haas and Himes2013). Given the importance of the breeding sites at Eglin for the species, persistent damage to these sites by feral swine could jeopardize the long-term survival of the species and increase the cost of future recovery actions.
We knew from an ongoing study that, for several weeks leading up to these observations, dozens of recently metamorphosed reticulated flatwoods salamanders had been moving out of the breeding pond basin into areas damaged by hogs. We have also observed metamorphs sheltering under clumps of wiregrass near the surface while still in the vicinity of the breeding site (Jones et al., Reference Jones, Hill, Gorman and Haas2012). Threats to metamorphs sheltering in this way were indicated on 27 May 2014, when we observed the partial remains of an apparently swine-predated eastern glass lizard Ophisaurus ventralis in a freshly rooted patch of vegetation in the wiregrass-dominated ecotone of one of the few remaining breeding wetlands occupied by reticulated flatwoods salamanders (Jones et al., Reference Jones, Rincon, Gorman, Haas and Engeman2015). If swine can depredate a glass lizard while rooting, it seems likely that they could also depredate a flatwoods salamander. For many ephemeral pond-breeding species successful reproduction occurs sporadically, with most metamorphosis concentrated in time. In many breeding wetlands used by flatwoods salamanders, including the one where this observation occurred, successful recruitment into the adult population occurs in < 50% of years (Gorman & Haas, Reference Gorman and Haas2014; Chandler et al., Reference Chandler, Rypel, Jiao, Haas and Gorman2016). Given the concentration of new cohorts of amphibians in a restricted space and time, there is potential for feral hogs to consume entire cohorts, posing a serious threat to the continued existence of the species.
Since we recognized and reported the increase in hog activity in and around the breeding wetlands of flatwoods salamanders, focused trapping efforts have been implemented in these areas. These efforts are ongoing and have reduced hog activity from 53 to 40% of resurveyed sites. Other measures to control hogs may need to be considered in these vulnerable habitats of endangered species, as there is evidence that people continue to release hogs into these sensitive areas. At the five occupied breeding wetlands where fencing was installed in an attempt to exclude hogs, this appears to have been successful. The hog fencing has required some repairs following damage by a falling tree or by adult black bears Ursus americanus climbing over it but most checks yielded no observations of damage. The potential breeding area where human interference rendered trapping efforts ineffective has now been closed to motorized vehicles. Whereas in the Brazilian Pantanal, for example, hunting of feral hogs provides an important source of protein to local residents, reduces the harvest pressure on native wildlife, and limits growth of the hog population (Desbiez et al., Reference Desbiez, Keuroghlian, Piovezan and Bodmer2011), in the USA and Australia hunting of feral swine seems to be self-reinforcing, with hunters (often illegally) transporting and releasing hogs to new areas for sport-hunting (Spencer & Hampton, Reference Spencer and Hampton2005; Barrios-García & Ballari, Reference Barrios-García and Ballari2012; Bevins et al., Reference Bevins, Pedersen, Lutman, Gidlewski and Deliberto2014). The rapid expansion of the range of feral swine in the USA is believed to be primarily a result of people transplanting them to increase recreational hunting opportunities (USDA, 2015), and thus spreading their negative impacts through disease and damage to the environment, agriculture, wildlife (especially rare species), domestic animals, and other human interests (Seward et al., Reference Seward, VerCauteren, Witmer and Engeman2004; USDA, 2015). At Eglin Air Force Base recreational hunting had been taking place for many years and was demonstrated to have some impact on reducing hog numbers and their damage to seepage slopes, but the beneficial impacts from operational hog trapping were much greater (Engeman et al., Reference Engeman, Stevens, Allen, Dunlap, Daniel, Teague and Constantin2007).
There have been reports of feral hogs consuming amphibians at other locations, and their ability to consume large numbers of amphibians during mass migrations to or from breeding sites is a concern, especially for species limited to only a few remaining breeding sites (Schley & Roper, Reference Schley and Roper2003; Jolley et al., Reference Jolley, Ditchkoff, Sparklin, Hanson, Mitchell and Grand2010; Wishart et al., Reference Wishart, Lapidge, Braysher, Sarre and Hone2015). Feral hogs may also degrade the quality of breeding habitats by disturbing vegetation, spreading invasive plants, altering invertebrate communities (which are both important prey and predators of salamanders), reducing dissolved oxygen, and altering the microtopography (e.g. Kaller & Kelso, Reference Kaller and Kelso2006; Engeman et al., Reference Engeman, Stevens, Allen, Dunlap, Daniel, Teague and Constantin2007; Bankovich et al., Reference Bankovich, Boughton, Boughton, Avery and Wisely2016). Such destructive effects have been observed elsewhere, including at breeding sites of the Houston toad Bufo houstonensis, another federally endangered amphibian (Brown et al., Reference Brown, Jones, Bell and Forstner2012). Our results support the conclusions of others that fencing (supported by trapping) can mitigate the negative effects of feral hogs on wetland-breeding amphibians and reptiles (Doupé et al., Reference Doupé, Schaffer, Knott and Dicky2009; Brown et al., Reference Brown, Jones, Bell and Forstner2012). Although there may be negative consequences of using hog fencing around wetlands, such as excluding alligators and large turtles, other control measures cannot fully protect threatened amphibians and sensitive habitats. Trapping at Eglin has been demonstrated to substantially reduce hog numbers (Engeman et al., Reference Engeman, Stevens, Allen, Dunlap, Daniel, Teague and Constantin2007). However, eradication of hogs from Eglin is not feasible because of a lack of complete access to an extensive area of challenging terrain, boundaries with rivers and terrain that cannot be maintained to exclude hogs, and illegal translocation of hogs to the area by local people for recreational hunting purposes. For an extremely restricted habitat of a rare species such as the reticulated flatwoods salamander, a single hog can have a significant negative impact. Thus, fencing the breeding sites serves as a localized eradication of hogs from around the breeding sites. During the study period, fencing reduced damage by 100%.
Our ability to identify the disturbance quickly through monitoring efforts, in concert with the presence of trained wildlife control professionals, facilitated a rapid response to conserve the reticulated flatwoods salamander. We encourage others who manage land that supports populations of rare or threatened species to institute pre-emptive practices. A first step is to make sure that personnel on the ground know how to identify likely invasive species and their signs, and to liaise with damage management professionals for advice on how to survey and respond. Regular site visits should then be implemented to monitor sensitive areas, using survey techniques such as those described here or by Zengel & Conner (Reference Zengel and Conner2008). We recommend that land managers work with invasive species control specialists to implement techniques that will reduce or prevent damage to sensitive habitats and species.
Acknowledgements
We thank the Natural Resources Branch (Jackson Guard) of Eglin Air Force Base and the Department of Fisheries and Wildlife Sciences at Virginia Tech for their financial and logistical support. We specifically thank the leadership and supporting staff at Jackson Guard for their rapid response, Sandy Pizzolato for coordinating fence installation, and the U.S. Fish and Wildlife Service Panama City Ecological Services team for permitting support.
Author contributions
KJ and TG developed the survey methodology, and KJ and BR conducted the damage surveys. RE conducted statistical analyses. KJ guided and oversaw fence installation. JA provided training in hog sign identification, conducted swine trapping, and recorded trapping success. KJ, TG, RE and CH wrote the article, and all authors contributed to editing the article.
Biographical sketches
Kelly Jones coordinates field operations for Virginia Tech's work on threatened species at Eglin Air Force Base. His interests include natural history, habitat management and conservation of rare species and ecosystems. Thomas Gorman manages aquatic resources and conducts research on wildlife–habitat relationships, habitat management, and approaches to conserving amphibians and reptiles. Brandon Rincon is a wildlife biologist whose interests include ecology and ecosystem management, especially within the longleaf pine system. John Allen has been the lead technician in the implementation of the Eglin feral hog management programme since it began in 2003. He has also conducted beaver control, threatened and endangered species protection, and bird/animal aircraft strike hazard-related control work. Carola Haas conducts research on wildlife populations and behaviour in managed habitats. Richard Engeman carries out research to develop wildlife indexing and ecological sampling methods, primarily focusing on applications for management of invasive species and protection of threatened species.






