Introduction
Bats (Chiroptera) are the second largest order of mammals, with > 1,116 species (Simmons, Reference Simmons, Wilson and Reeder2005). Among them c. 183 belong to the family Pteropodidae and are essentially vegetarian, eating fruit, pollen, nectar and leaves, and the remainder have more varied diets that include frugivory, although most are insectivores. But within the Old World, including India, only pteropodids eat fruit, consuming a wide variety of commercial and non-commercial species (Marshall, Reference Marshall1985). They play a pivotal role as pollinators and seed dispersers of many plants and in tropical forest succession (Start & Marshall, Reference Start, Marshall, Burley and Styles1976; Fleming & Estrada, Reference Fleming and Sosa1993; Fleming & Sosa, Reference Fleming and Estrada1994; Banack, Reference Banack1998; Muscarella & Fleming, Reference Muscarella and Fleming2007). At least 300 plant species of nearly 200 genera rely mainly on Old World fruit bats for their propagation (Marshall, Reference Marshall1983, Reference Marshall1985; Fujita & Tuttle, Reference Fujita and Tuttle1991). Furthermore, these plants produce c. 500 economically valuable products including fruits, dyes, tannins, timber, medicines, fibres and fuelwood (Fujita & Tuttle, Reference Fujita and Tuttle1991). The importance of bats for the future availability of these products is substantial and has been seriously underestimated.
Bats as pollinators and seed-dispersers
Fruit bats are mobile foragers (Fleming & Sosa, Reference Fleming and Sosa1994), moving genetic material in the form of pollen between isolated fragments of vegetation and depositing seeds over large areas (Young et al., Reference Young, Boyle and Brown1996; Law & Lean, Reference Law and Lean1999). They are the sole or prime pollinators of several nectariferous plants that flower only at night (e.g. kapok Ceiba, petai Parkia, durian Durio, Oroxylum, stinking passion Passiflora and banana Musa), many of which are economically important. For example, Ceiba pentandra yields a commercially valuable fibre, which is used for stuffing cushions and mattresses (Fujita & Tuttle, Reference Fujita and Tuttle1991). Durio and Parkia fruits are commercially important foods in some parts of South-East Asia (Fujita, Reference Fujita1988).
Zoochory (seed dispersal by animals) is particularly widespread among pioneer plants and nearly half of the most abundant species are bat-dispersed (Charles-Dominique, Reference Charles-Dominique, Estrada and Fleming1986). Fruit bats disperse seeds in two ways. Fruits may be carried away from the parent tree and the seeds subsequently dropped under a feeding roost. Alternatively, smaller seeds are ingested along with the fruit pulp and pass through the gut to be voided in the faeces, often away from parent trees. The success of self-regeneration for many tropical trees improves if their propagules are moved away from the parents (Janzen, Reference Janzen and Golley1983). Smaller pteropodid bats can fly up to 35 km nightly in search of food, and the larger ones (Pteropus spp.) can fly for longer distances (Nelson, Reference Nelson1965). Because they defecate or drop large numbers of seeds in flight, these bats are able to move seeds over longer distances and wider areas than any other rainforest animals. In addition, it has been suggested that passage through the bat gut improves the levels of seed germination (Izhaki et al., Reference Izhaki, Korine and Arad1995).
Plants that have relatively large-seeded fruits are consumed by fewer dispersers, and depend on fewer species of mammals (Corlett, Reference Corlett1998). The carriage of such fruits is considered to be the primary dispersal role of Pteropus (Richards, Reference Richards1995). These pteropodid bats are thus important vehicles of plant dispersal and are able to bridge the gaps between widely separated forest fragments (Corlett, Reference Corlett1998). Several of the world's most important domesticated food staples, including bananas, plantain, breadfruit and mangoes, continue to rely on flying foxes for their propagation in the wild (Fujita & Tuttle, Reference Fujita and Tuttle1991). However, bananas are spread vegetatively and mangoes are planted by humans in rural villages in India. Wide-ranging seed dispersal encourages genetic exchange between fragments of forests or isolated populations of certain species and decreases the chances of inbreeding (Loveless & Hamrick, Reference Loveless and Hamrick1984). Through pollination of bat-dependent flowers and dispersal of seeds into forest gaps and clearings, tropical bats play an essential role in forest ecology (Cox et al., Reference Cox, Elmqvist, Pierson and Rainey1991; Fujita & Tuttle, Reference Fujita and Tuttle1991). Thus, they play an important role in secondary succession as well as in maintaining the compositional heterogeneity of tropical forests (Wang & Smith, Reference Wang and Smith2002).
Fruit bats of India
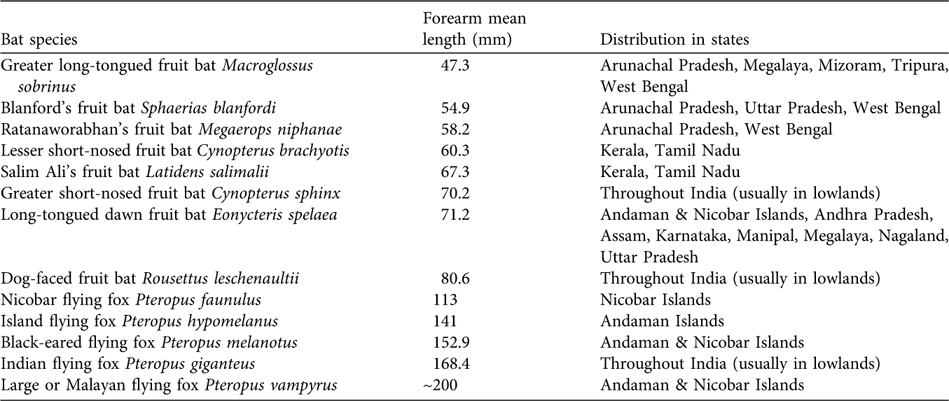
Thirteen of the 120 bat species in India are pteropodids (Table 1; Bates & Harrison, Reference Bates and Harrison1997). Pteropus giganteus, Rousettus leschenaultii and Cynopterus sphinx are distributed throughout most of India, generally in lowland areas. The remaining 10 species are restricted mainly to forested and island areas (Bates & Harrison, Reference Bates and Harrison1997). For example, the lesser short-nosed fruit bat Cynopterus brachyotis (Balasingh et al., Reference Balasingh, Ronald, Thiruchenthil and Suthakar1999) and Salim Ali's fruit bat Latidens salimalii (Singaravelan & Marimuthu, Reference Singaravelan and Marimuthu2003) are confined to the south, especially to the Western Ghats of Tamil Nadu (and possibly also in Kerala). The island flying fox Pteropus hypomelanus lives only on the Andaman islands, the Nicobar flying fox Pteropus faunulus is endemic to the Nicobar islands, and the large or Malayan flying fox Pteropus vampyrus and black-eared flying fox Pteropus melanotus are present on both island archipelagos (Aul, Reference Aul2006). Similarly Ratanaworabhan's fruit bat Megaerops niphanae and the greater long-tongued fruit bat Macroglossus sobrinus are restricted to north-east India.
Table 1 Name, size and distribution of the 13 species of fruit bats in various states of India (Bates & Harrison, Reference Bates and Harrison1997).
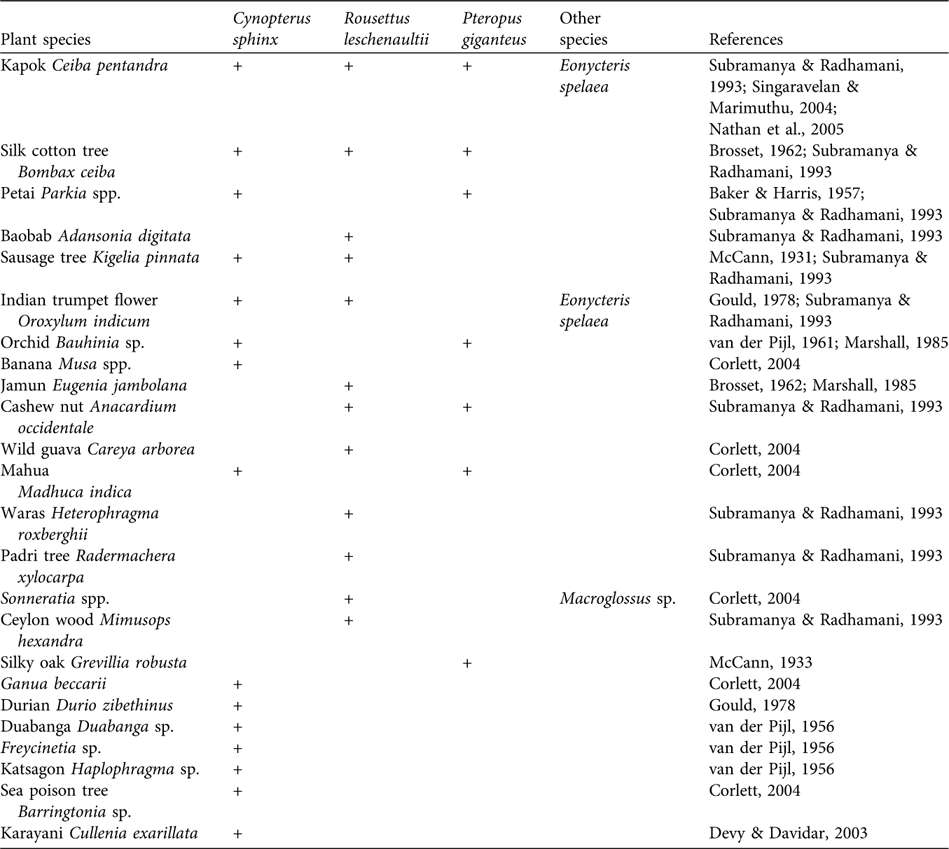
Detailed studies on the foraging activity of the three ubiquitous bats P. giganteus, R. leschenaultii and C. sphinx reveal their role as pollinators of several plant species (Table 2). There is no evidence of individuals of the remaining 10 species, including Pteropus species on the Nicobar and Andaman islands, either being caught in lowland areas or causing damage to commercial fruit orchards. This suggests that these species rely on wild fruits that are available in their forest and island habitats. For example, remnants of wild fruits (the bead tree Eleocarpus oblongus, the Ceylon plum Prunus ceylanicus and the Indian laurel fig Ficus macrocarpa) were collected at the night roosts of L. salimalii (Singaravelan & Marimuthu, Reference Singaravelan and Marimuthu2003). Eonycteris spelaea feeds mainly on nectar and pollen and pollinates flowers (Bumrungsri et al., Reference Bumrungsri, Harbit, Benzie, Carmouche, Sridith and Racey2008) that yield commercial fruits (Bates & Harrison, Reference Bates and Harrison1997).
Table 2 List of plants that are pollinated by the three ubiquitous fruit bats C. sphinx, R. leschenaultii and P. giganteus, and pollination of these plants by other bat species.
Fruit bats and the Indian Wildlife (Protection) Act
Despite their beneficial role, fruit bats have long been hunted as a source of protein and for medicinal use and persecuted as fruit-eating pests. The Indian Wildlife (Protection) Act, 1972, categorizes fruit bats as vermin (which can be captured or killed) under Schedule V. However, killing of bats with such a low reproductive rate (often only 1–2 young per year) results in a reduction in their numbers with consequent effects on pollination and seed dispersal. A breakdown of plant–pollinator and plant–seed-disperser relationships and subsequent loss of genetic diversity (heterozygosity and allelic diversity) could be one of the most threatening consequences of forest fragmentation (Bawa, Reference Bawa1990; Young et al., Reference Young, Boyle and Brown1996). Although categorizing plant-visiting bats as vermin is no longer acceptable, when the Act was formulated (1972) there was a lack of adequate scientific evidence about the ecological roles of fruit bats, with ecological research, particularly on plant-visiting bats, in its infancy. However, three amendments have been made to the Act. Although the efforts of bat conservationists, particularly the Chiroptera Conservation and Information Network of South Asia (CCINSA), to shift India's fruit bats to Schedule I (i.e. to be protected) met with little early success, the Wildlife (Protection) Amended Act, 2002 placed the endemic fruit bat L. salimalii as well as Wroughton's free-tailed bat Otomops wroughtoni in Schedule I. This fell short of removing all fruit bat species from the list of vermin, which is the aim of CCINSA (Molur et al., Reference Molur, Marimuthu, Srinivasulu, Mistry, Hutson and Bates2002) and other organizations, including Bat Conservation International (Mistry, Reference Mistry2003) and IUCN's Species Survival Commission (Mickleburgh et al., Reference Mickleburgh, Hutson and Racey1992, Reference Mickleburgh, Hutson and Racey2002).
Mitigating bat damage to commercial fruits
Bat researchers have documented the incidence of bats visiting orchards. Large-scale commercial growing of fruits has led to conflicts between fruit growers and bats in Australia (Loebel & Sanewski, Reference Loebel and Sanewski1987; Tidemann et al., Reference Tidemann, Kelson and Jamieson1997), Israel (Moran & Keidar, Reference Moran and Keidar1993), South Africa (Jacobsen & DuPlessis, Reference Jacobsen and DuPlessis1976), Malaysia and Indonesia (Fujita, Reference Fujita1988) and India (Vergheese, Reference Vergheese1998; Srinivasulu & Srinivasulu, Reference Srinivasulu and Srinivasulu2001). The most serious conflicts may occur where the supply of native fruits has been reduced through habitat loss (Tidemann & Nelson, Reference Tidemann and Nelson1987).
Although fruit bats are considered to be pests in commercial orchards, a recent study (Singaravelan, Reference Singaravelan2002) argues that the menace of bats is mainly restricted to those guava and mango orchards where harvesting is delayed and that c. 60% of fruits that were damaged by bats were ripe or overripe. The latter are not marketable even if the bats did not damage them. Fruit growers usually sell the ripe fruits to local markets where they often fetch half the price of less ripe fruit, which is sold further afield.
Recently C. sphinx has been blamed for damage to grapes (Vergheese, Reference Vergheese1998; Srinivasulu & Srinivasulu, Reference Srinivasulu and Srinivasulu2001, Reference Srinivasulu and Srinivasulu2002). However, the bats inflicted only 4% of the observed damage and birds (purple sunbird Nectaria asiatica, red-vented bulbul Pycnonotus cafer, white-eye or spectacle bird Zosterops palpebrosa) ate or damaged c. 30% of the grape crops (Singaravelan, Reference Singaravelan2002).
To reduce the damage caused by fruit bats in orchards, several non-destructive control measures have been suggested (Hall & Richards, Reference Hall and Richards1987; Vergheese, Reference Vergheese1998). There are two feasible and effective measures that yield considerable success: crop protection at the orchard and farm management. The former includes the use of scare guns, light grids over orchards, chemical repellents and netting. The latter involves removal of early ripening fruits and the establishment of suitable sacrificial crops. However, crop protection at orchards is suggested for large-scale commercial fruit growers where Pteropus damage is substantial, as in Australia. Bat attacks on those orchards that incorporate harvesting were minimal (Singaravelan, Reference Singaravelan2002). Thus, changes in farm management practice may be the most feasible and successful means of reducing bat damage to commercial fruit.
Although early picking of fruit and early removal of overripe fruit may be effective, planting alternative forage trees with a high sugar content in the proximity of the orchards could alleviate the problem of bat-attacks. The Singapore cherry Muntingia calabura produces a fruit on which several species of bats feed (Bonaccorso & Gush, Reference Bonaccorso and Gush1987). For example, C. sphinx gathers in swarms of 10–30 individuals while feeding on Muntingia fruits. The number of nightly visits of C. sphinx to M. calabura was much higher than to any other commercial fruits (Singaravelan & Marimuthu, Reference Singaravelan and Marimuthu2006). Thus, it appears that M. calabura serves as an effective alternative forage source when planted adjacent to orchards (Singaravelan & Marimuthu, Reference Singaravelan and Marimuthu2006).
Is it still justifiable to categorize plant-visiting bats as vermin?
Although there has been much debate about fruit bat damage to commercial orchards, the beneficial role of such bats and the ecological services they provide has not been fully appreciated. Publications in peer-reviewed journals, which detail the ecological roles of those bats (Cox et al., Reference Cox, Elmqvist, Pierson and Rainey1991; Fujita & Tuttle, Reference Fujita and Tuttle1991; Fleming & Sosa, Reference Fleming and Sosa1994) may not yet have reached policy makers and conservation planners. In spite of the damage to orchards by the three ubiquitous species (P. giganteus, R. leschenaultii and C. sphinx), their beneficial roles cannot be ignored. Unlike other small mammals, fruit bats usually give birth to a single young (occasionally two, such as Eonycteris), either once (e.g. P. giganteus) or twice (e.g. R. leschenaultii and C. sphinx) per year. If the fruit bats of India are not protected their populations will be drastically reduced because of this low reproductive rate. Such a situation may have a cascading effect on ecosystems, with potentially serious ecological consequences and economic disadvantages (Fujita & Tuttle, Reference Fujita and Tuttle1991; Elmqvist et al., Reference Elmqvist, Cox, Rainey and Pierson1992).
With respect to the other 10 species of pteropodids, in a long-term study with a total of 1,858 mist net hours from April 2000 to August 2003 we never captured individuals of L. salimalii, C. brachyotis and E. spelaea in lowland areas (Singaravelan, Reference Singaravelan2002; Singaravelan & Marimuthu, Reference Singaravelan and Marimuthu2006, Reference Singaravelan, Raja and Marimuthu2007), and there is no report of the other seven species visiting lowland areas or complaints of damage to orchards. We thus rule out the possibility that these 10 species visit commercial orchards.
We recommend that the remaining 12 species of fruit bats should all be removed from the list of vermin in the Indian Wildlife (Protection) Act and placed in Schedule I along with L. salimalii. Detailed long-term studies on the distribution, breeding and foraging behaviour of all 10 species of pteropodids that live in the forests of India should be a priority.
Acknowledgements
The MoEF, Government of India, supported the work through a grant to GM. NS acknowledges the support of a scholarship from Bat Conservation International. We are grateful to Richard Jenkins for comments on the draft manuscript and two anonymous referees for their critical comments.
Biographical sketches
Natarajan Singaravelan has studied behavioural and conservation ecology of plant-visiting bats. He is a member of the Chiroptera Conservation Information Network of South Asia (CCINSA) and is popularizing the conservation needs of bats in general and the vital role they play in restoration ecology. Ganapathy Marimuthu has worked on bats throughout his career. He is a member of the Bat Specialist Group of IUCN's Species Survival Commission and was founding Chairman of CCINSA. Paul Racey works on the ecology and conservation biology of bats in temperate and tropical latitudes, particularly Madagascar. He is Co-Chair of IUCN's Bat Specialist Group and is Vice Chairman of Fauna & Flora International.






