Introduction
Aspirin (acetylsalicylic acid, ASA) and omega-3 (n3) polyunsaturated fatty acids (PUFA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are well-established substances for preventing atherosclerotic cardiovascular disease (CVD) events and colorectal adenoma(Reference Arnett, Blumenthal and Albert1–Reference Sezai, Unosawa and Taoka3). Research has demonstrated the efficacy of ASA alone and EPA and DHA alone in preventing worsened health outcomes and in improving quality of life(Reference Arnett, Blumenthal and Albert1–Reference Sezai, Unosawa and Taoka3). ASA alone has long been studied in the primary prevention of atherosclerotic disease in high-risk patients aged 40–70 years(Reference Arnett, Blumenthal and Albert1). Unfortunately, bleeding associated with high doses of ASA (100 mg/d or higher) can limit its use in patients with CVD or with colorectal adenomas who are at a high risk to bleeding(Reference Arnett, Blumenthal and Albert1). EPA and DHA ingestion have also been researched in lowering triacylglycerol levels in patients with dyslipidaemia(Reference Arnett, Blumenthal and Albert1,Reference Sezai, Unosawa and Taoka3) . The use of ASA and omega-3 supplementation may be a probable alternative in patients who require higher doses of ASA in CVD and colorectal adenoma(Reference Lev, Solodky and Harel4).
According to the World Health Organization (WHO), CVD was the leading cause of death globally in 2016, and an estimated 17·9 million lives are lost each year(5). Also, according to NHANES 2013–2016 data, the prevalence of CVD in its multiple forms including coronary artery disease, vascular disease, heart failure, stroke and hypertension was estimated to account for 48 % of the US population aged 20 years or older. The majority of CVD cases are due to hypertension. Excluding hypertension, the prevalence is only 9 %(6). Risk factors for CVD include poor diet, hypertension, obesity, dyslipidaemia, diabetes, tobacco smoking, alcohol and inactivity(Reference Roth, Johnson and Abate7). The American Heart Association (AHA) cited that high dietary trans fatty acid and low dietary n3 PUFA have been attributed to increased cardiovascular mortality(Reference Danaei, Ding and Mozaffarian8). The risk for CVD has shown to be associated with individuals who are unemployed with a rapid increase in disability adjusted life year burden from age 40 years to a total of 20 % of all disability adjusted life year burden by the age of 60 years(Reference Roth, Johnson and Abate7,Reference Blackwell and Villarroel9) . Across sexes, CVD had twice the burden on males when compared with females in ischaemic heart disease, cardiomyopathy and myocarditis. Across races, African Americans and American Indians shared the highest total CVD burden within the United States. However, this is most likely attributed to disparities in healthcare access(Reference Roth, Johnson and Abate7).
The overall cost burden associated with CVD within the United States in 2016 was $555 billion ($318 billion in direct cost and $237 billion in indirect costs) and is estimated to increase to $1·1 trillion by the year 2035. As such, low-cost prevention methods such as ASA and n3 PUFA supplementation can be critical in reducing overall economic burden in patients with CVD(10).
According to the American Cancer Society, colorectal cancer is the third most common diagnosed cancer with lifetime risks of 1 in 23 for men and 1 in 25 for women(Reference Siegel, Miller and Goding Sauer11). In 2020 alone, there will be an estimated 147 950 new cases with 53 200 colorectal cancer-related deaths. Although mortality associated with colorectal cancer has been declining due to better screening for adenomas and polyps, mortality associated with colorectal cancer for individuals aged 55 or younger has been increasing at 1 % each year for the past 9 years(Reference Siegel, Miller and Goding Sauer11). Risk factors associated with colorectal cancer include being overweight or obese, diabetes mellitus, inactivity, diets rich in red meat, smoking, high alcohol consumption, history of colorectal polyps, history of inflammatory bowel disease or genetic disposition. Across sexes, the incidence rate for colorectal cancer is 31 % higher in males, and evidence supports this due to shorter life expectancy compared with females(Reference Siegel, Miller and Goding Sauer11). This disparity between sexes is found in individuals aged 45 years or less but increases to 40–50 % higher in males for those aged 55–74 years old. This is speculated to be a result of cumulative exposure to risk factors and probably sex hormones. Across races, African Americans and Jews of Eastern European descent have a disproportionally higher colorectal cancer incidence and mortality across all racial groups within the United States. The increased risk for developing adenomas and being unable to achieve timely and high-quality colonoscopy could also contribute to increased mortality(Reference Siegel, Miller and Goding Sauer11). As a result of the increased risk, preventative measures from well-established medications such as ASA and higher blood levels of EPA and DHA are studied to improve the quality of life for patients with colorectal adenomas and cancer.
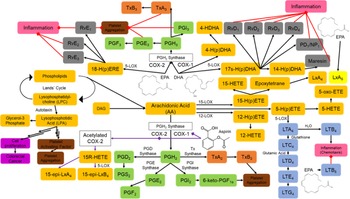
Currently, ASA is used alone or in combination with anticoagulants to prevent or treat acute coronary syndromes(Reference Levine, Bates and Bittl12). ASA is also used for primary and secondary prevention of atherosclerotic CVD and ischaemic stroke from doses of 75 mg/d to 325 mg/d(Reference Levine, Bates and Bittl12). ASA is an irreversible inhibitor of cyclooxygenase 1 (COX-1) at low doses and at high doses also exhibits non-selective inhibitory effects on COX-2 through acetylation on the production of eicosanoids, such as prostaglandins, thromboxane and leukotrienes(Reference Serhan13). Low-dose ASA (81 mg) has been associated with cardioprotective effects by lowering the production of inflammatory lipid mediators such as prostaglandin E2 (PGE2) and prothrombic factors such as thromboxane A2 (TxA2)(Reference Serhan13). ASA can also induce the biosynthesis of potent specialised pro-resolving lipid mediators (SPM) known as ASA-induced SPM, such as lipoxins (Lx), D-series resolvins (RvD), E-series resolvins (RvE), protectin (PD) and maresin (MaR)(Reference Chiang, Bermudez and Ridker14,Reference Sorokin, Yang and Remaley15) where EPA, DHA and arachidonic acid are substrate for these SPM. Through these mediators, as shown in Fig. 1, ASA works to reduce thrombosis, inhibit activation of inflammation and stimulate resolution of inflammation(Reference Franzese, Bliden and Gesheff16). The further inhibition of neutrophil recruitment is also beneficial in preventing inflammation-type reactions as seen in atherosclerotic plaque development. Although ASA has been associated with increased bleeding risk, lower doses of ASA (81 mg) through the mechanisms described herein have been beneficial in preventing thrombosis and CVD.
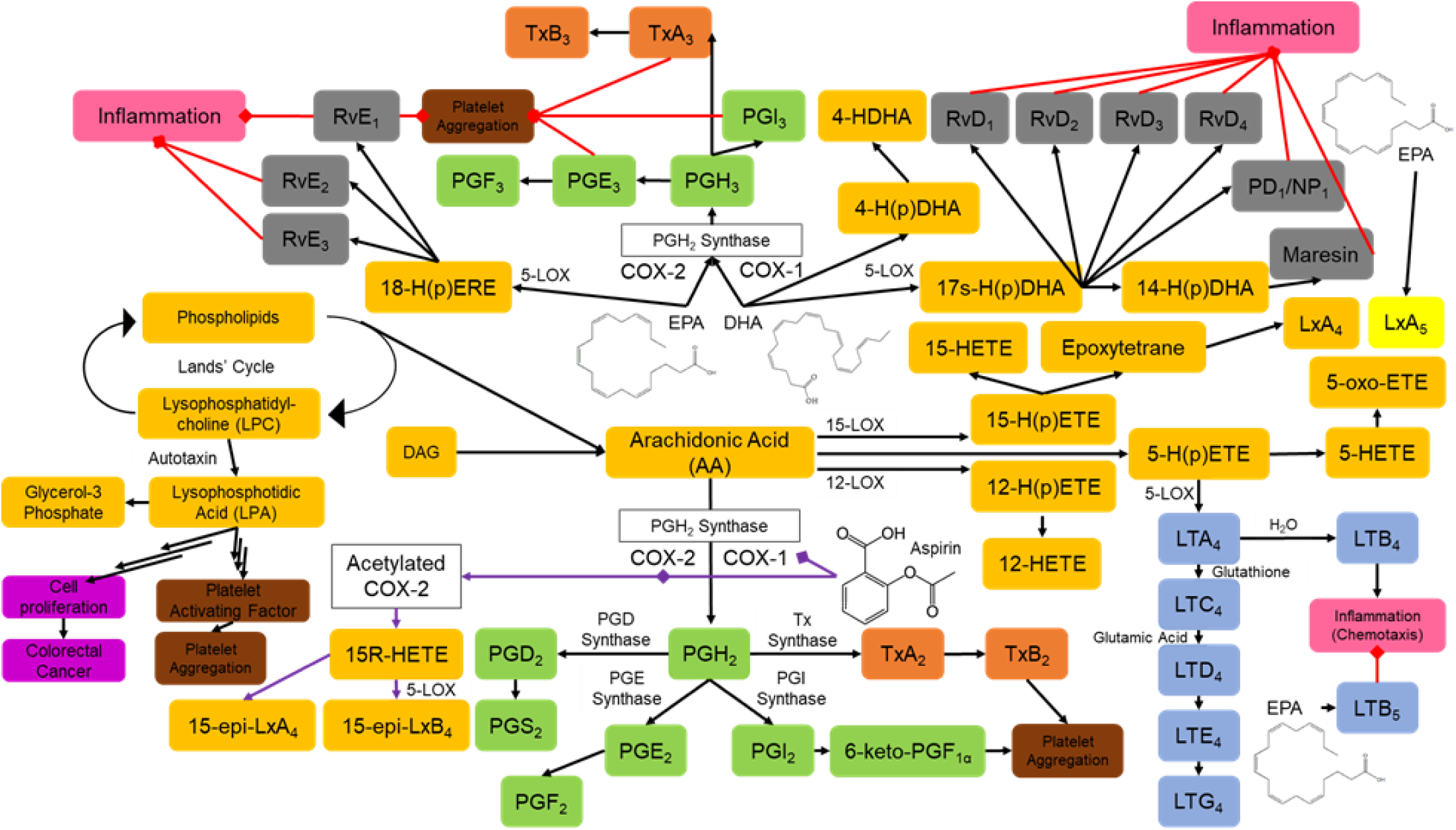
Fig. 1. Synthesis of endogenous lipid mediators, eicosanoids, specialised pro-resolving mediators (SPM) and their effects. Aspirin is a COX-1 inhibitor at low doses and a non-selective COX-1 and COX-2 inhibitor though acetylation at higher doses. EPA and DHA lead to decreased synthesis of PGH2 and arachidonic acid derived eicosanoids in favour of increased production of PGH3 and subsequently TxA3 and PGE3 associated with the inhibition of platelet function. Lipid mediator intermediates (yellow); prostaglandins (green); leukotrienes (blue); thromboxane intermediates (orange); SPMs (grey); responses leading to inflammation (pink); responses to previous mediators leading to platelet aggregation (brown); responses leading to cell proliferation (purple); direct effects and subsequent mediators induced by aspirin (purple arrows); inhibitory signals (red arrows). COX, cyclooxygenase; LT, leukotrienes; PG, prostaglandin; Rv, resolvins; Tx, thromboxane; 5-LOX, 5-lipoxygenase; 5-HPETE, 5-hydroperoxyeicosatetraenoic acid.
Similar to ASA, marine-derived n3 PUFA have also been shown to be beneficial in CVD. Ingestion of these n3 PUFA increased EPA by 400 % and DHA by 50 % in erythrocyte membranes and significantly in plasma(Reference Block, Duff and Lawrence17–Reference Siniarski, Haberka and Mostowik20). This suggests that EPA and DHA supplementation leads to displacement of pro-inflammatory fatty acids, in favour of anti-inflammatory n3 PUFA(Reference Sorokin, Yang and Remaley15). By displacing arachidonic acid, EPA and DHA decrease the synthesis of TxA2 and PGE2, leading to inhibited platelet aggregation and thrombosis. Increased concentrations of EPA and DHA allow for the incorporation by prostaglandin synthase and thromboxane synthase into PGH3 and TxA3, which are less potent than their endogenous counterparts in platelet aggregation and inflammation activation(Reference Franzese, Bliden and Gesheff16). Ingestion of n3 PUFA can decrease the synthesis of fatty acids and increase their oxidation, which can lower plasma triacylglycerol. Together, the mechanisms of n3 PUFA in regulating eicosanoids and inflammation show promise in preventing CVD.
The FDA has approved four different n3 PUFA containing products for dyslipidaemia presented in Table 1 (Reference Amarin Pharma21–Reference Trygg Pharma24). Although all the brands contain n3 PUFA, the amount and the ratio of EPA and DHA differs. Thus, these brands are not interchangeable due to the effect on the synthesis of SPM from EPA and DHA and their functions in CVD.
Table 1. FDA-approved omega-3 PUFA containing products for dyslipidaemia, EPA and DHA content per 1 g capsule and approved adult daily dosage in dyslipidaemia
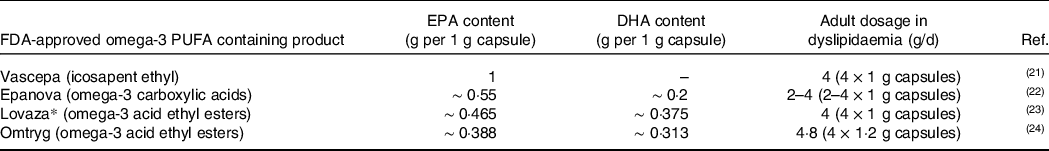
FDA, Food and Drug Administration; PUFA, polyunsaturated fatty acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
*The brand name Omacor was switched to Lovaza to avoid medication errors. Some of the included studies refer to Omacor.
The biosynthesis of SPM, which activate inflammation termination, are derived from EPA and DHA; thus, supplementation with them can increase the production of SPM(Reference Chiang, Bermudez and Ridker14,Reference Sorokin, Yang and Remaley15) . The ingestion of EPA and DHA increases oxygenated products of DHA, 14- and 17-hydroxydocosahexaenoic acids (HDHA), the precursors to MaR and PD responsible for wound healing and neuroprotection. RvD are synthesised from DHA, which are responsible for preventing cellular infiltration and cytokine and chemokine production. RvE have a similar role to RvD but are synthesised from the oxygenated products of EPA, 18-hydroxyeicosapentaenoic acid (HEPE) with a similar function as RvD(Reference Serhan13,Reference Kalish, Le and Fitzgerald25) . SPM directly instigate the termination of inflammation, which is critical in CVD prevention.
Previous research in murine models has indicated that high-fat diets and hyperlipidaemia can disrupt the homeostasis of SPM, leading to disrupted inflammation resolution(Reference Merched, Serhan and Chan26). In patients with peripheral arterial disease, EPA and DHA supplementation caused a 5–9 % increase in the Omega-3 Index (ratio of EPA and DHA in erythrocytes to percent of fatty acids). Their ingestion positively correlated with plasma lipid mediator production and decreased thrombosis and the risk of peripheral vascular disease(Reference Ramirez, Gasper and Khetani19,Reference Schaller, Zahner and Gasper27) . However, in patients with high-risk CVD and risk factors such as diabetes mellitus, EPA and DHA did not improve endothelial function(Reference Siniarski, Haberka and Mostowik20). In a similar study that looked at platelet function, coagulation, fibrin clot properties and markers of inflammation or metabolic status in diabetes mellitus patients with atherosclerosis, there were no significant changes with n3 PUFA treatment(Reference Poreba, Mostowik and Siniarski18). These studies suggest that n3 PUFA treatment alone in diabetic patients may not be enough to help prevent CVD events in high-risk individuals.
The biosynthesis of lipid mediators includes arachidonic acid, lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA), which are known to mediate various biological activities and play an important role in cellular signal transduction. LPC can be further diverted into LPA synthesis with the removal of choline, whose downstream mediators act as a platelet-activating factor and stimulate cell growth(Reference Hishikawa, Shindou and Kobayashi28,Reference Shimizu, Ohto and Kita29) . LPC and LPA contain one PUFA (arachidonic acid, EPA, or DHA), and knowledge regarding the effects of each of these is limited. In addition, PGE2 has been demonstrated to increase the risk of cancer by stimulating cell proliferation, inhibiting apoptosis and promoting angiogenesis, which can increase the aggressiveness and invasiveness of cancerous cells(Reference Wang and Dubois30). Previous research indicated that n3 PUFA alone has limited benefits in preventing and reducing the size of colorectal adenomas(Reference West, Clark and Phillips31).
Both ASA use alone and n3 PUFA use alone have efficacy in preventing platelet activation and inflammation in CVD and colorectal cancer(Reference Guillot, Caillet and Laville32). The overlapping mechanism of ASA and n3 PUFA in regulating platelet activity and inflammation suggests potential synergistic effects (Fig. 1)(Reference Franzese, Bliden and Gesheff16). In patients who are ASA resistant and require higher doses of ASA for cardioprotective effects, combinational use of ASA with n3 PUFA can be beneficial in achieving similar antithrombotic effects comparable to increased ASA dose (325 mg) without increased risk for bleeding(Reference Lev, Solodky and Harel4). Additionally, higher doses of EPA and DHA (over 1 g) can cause peroxidation, which can lead to platelet agonistic effects causing more CVD. The anti-inflammatory properties of ASA may negate the effects of EPA and DHA that further suggest combination use(Reference Guillot, Caillet and Laville32). The potential synergistic effects of ASA and n3 PUFA can be helpful in patients who do not see efficacy from ASA use alone or n3 PUFA use alone.
Previous reviews have not explored the combined use of ASA and n3 PUFA use in combination with regard to CVD and colorectal cancer outcomes. Although reviews regarding ASA use and n3 PUFA use alone in CVD and colorectal cancer exist, recent data on combinational use with the provided synergistic effects suggest further exploration. Thus, the goal for this review is to gather, highlight and summarise the established literature on combinational ASA and n3 PUFA use in the prevention and subsequent treatment of CVD and colorectal cancer.
Methodology
Using PubMed, Google Scholar and Cochrane Library, relevant articles published within the last 10 years (January 2010 to December 2020) reporting use of ASA and EPA and DHA in CVD and colorectal cancer were identified in this narrative review. To analyse the dose of ASA in CVD and colorectal cancer prevention and treatment, relevant articles published within the last 30 years (January 1990 to December 2020) were used. Included articles were limited to important meta-analyses, clinical trials and randomised controlled trials. Previous review articles were also included to help rationalise and reinforce conclusions made by the authors. Articles that included the use of food supplemented EPA and DHA were excluded in this narrative review because of the lack of compliance data documenting adherence to the intake of those supplements. Using keyword searching, terms such as ‘aspirin’, ‘fish oil’, ‘eicosapentaenoic acid’, ‘docosahexaenoic acid’, ‘NSAIDs’, ‘thrombosis’, ‘hypertension’, ‘stroke’, ‘myocardial infarction’, ‘cardiovascular disease’, ‘colorectal cancer’, ‘colorectal adenomas’ and ‘colorectal polyps’ were used.
ASA and n3 PUFA in CVD
Using the aforementioned parameters, eleven articles were selected that demonstrated combinational ASA and EPA and DHA supplemental use in CVD. Below we present a proof-of-concept study done in a murine model and then focus on ASA and EPA and DHA supplementation in healthy adults and at-risk adults to explore the indication of ASA and EPA and DHA use in different CVD-related outcomes.
As a proof of concept, published by Sorokin and colleagues using a murine model (n = 10)(Reference Sorokin, Yang and Vaisman33) that was later confirmed in humans by Block and colleagues(Reference Block, Abdolahi and Tu34), the combination of ASA (equivalent to 100 mg/d in humans) and n3 PUFA (equivalent to 4 g/d in humans) for 13 weeks demonstrated a significant reduction in omega-6 (n6) PUFA such as arachidonic acid and increased n3 PUFA such as EPA by 55-fold and DHA by 6-fold when compared with baseline diet. The increased concentrations of EPA and DHA were more prominent than in n3 PUFA use alone. Additionally, combination treatment showed a greater reduction in atherosclerotic lesions in the aorta compared with ASA or n3 PUFA use alone. Genes associated with cholesterol transport, efflux, catabolism and metabolism were all down-regulated(Reference Sorokin, Yang and Vaisman33). The combinational use demonstrated a more potent reduction in inflammatory markers such as MCP-1 as well as arachidonic-acid-derived SPM and eicosanoids. EPA-derived HEPE (5-, 12-, 15- and 18-HEPE) and DHA-derived HDHA (4-,7-, 14- and 17-HDHA) were elevated in n3-PUFA-supplemented mice regardless of ASA administration, demonstrating a need for EPA and DHA intake in order to generate these compounds(Reference Sorokin, Yang and Vaisman33). Although murine data can be difficult to extrapolate into humans, these results still suggest a promising outlook for combinational treatment in humans. Block et al. demonstrated in humans that the concentrations of EPA and DHA mediate the ASA (81 mg/d) effects on LPA and platelet function after 7 d. The mediated effect is not linear but rather reflects a ‘V-shaped’ relationship as shown in Fig. 2 (Reference Block, Abdolahi and Tu34). The ‘V-shaped’ relationship showed that ASA was more effective at lowering LPA concentrations when the combined EPA and DHA were at a concentration of approximately log(4·5). Thus, baseline EPA and DHA concentration in the body determined levels of n3 PUFA that would benefit ASA’s inhibition of platelet activation and aggregation(Reference Block, Abdolahi and Tu34). In the same way, patients who are ASA resistant lack a particular plasma concentration of EPA and DHA needed for ASA effect on platelet inhibition. The results found by Block et al. were similar to those of Sorkin et al., which demonstrated that EPA and DHA concentrations in the body are pertinent to ASA’s role in CVD.

Fig. 2. Effects of baseline concentration of EPA and DHA on LPA concentration lowering, associated with cardiovascular disease prevention in patients receiving 7 d of aspirin (81 mg). The x-axis represents the log-transformed EPA+DHA (originally measured in nM) concentration, and the y-axis represents the log-transformed LPA concentrations (originally measured in nM). The LOWESS (a regression analysis tool) curve represents the effects of chronic aspirin ingestion on the production of different species of LPA (16:0, 16:1, 18:0, 18:1, 18:2n6, 18:3, 20:4n6, 20:5n3, 22:4n6, 22:5n3 and 22:6n3). The lowest point on the ‘V-shaped’ curve shows the optimal concentration of EPA and DHA corresponding to optimal aspirin efficacy. Reprinted from Prostaglandins Leukot. Essent. Fat. Acids, 96, Block, R. C. et al., The effects of aspirin on platelet function and lysophosphatidic acids depend on plasma concentrations of EPA and DHA, 17–24 Copyright 2015, with permission from Elsevier. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LPA, lysophosphatidic acid; LOWESS, locally weighted scatterplot smoothing.
The study of ASA (81 mg/d) and EPA and DHA (4 g/d, Lovaza) use in healthy individuals (n = 25) for one day was first reported by Block and colleagues(Reference Block, Kakinami and Jonovich35), and Table 2A summarises all of the relevant studies of combinational ASA and n3 PUFA use in CVD prevention. Combination use in the Block et al. study showed a beneficial effect on inhibiting platelet function within 4 h of dosing(Reference Block, Kakinami and Jonovich35). In the study, combination of ASA, and EPA/DHA, demonstrated increased effects in prolonging closure time, a measure of platelet function, in participants who were acutely resistant to ASA effects at the 81 mg dose. However, a positive correlation between prolonged closure time and ASA use alone showed that the adjunctive antithrombotic effects of fish oil were dependent on baseline ASA effects. Although peroxidation can occur with doses of EPA and DHA over 1 g/d and lead to increased risk for CVD, the combination with ASA, which has anti-inflammatory properties, can help negate these oxidative effects, further indicating the benefits of combination(Reference Lev, Solodky and Harel4,Reference Guillot, Caillet and Laville32,Reference Lagarde, Calzada and Guichardant36) . By focusing on patients who are at higher risk for CVD such as those with diabetes mellitus patients, the role of ASA, and EPA and DHA, becomes clearer. In thirty patients with diabetes mellitus who do not have known CVD, combination therapy for 7 d showed a significant reduction in TxB2 after 4 h of treatment, which was more effective than ASA alone in preventing stimulus-induced platelet activation and aggregation(Reference Block, Abdolahi and Smith37). Additionally, the combination of ASA and EPA and DHA reduced aggregation caused by arachidonic acid and collagen significantly and ADP modestly, further suggesting that the combination exerts a greater effect than ASA alone in preventing stimulus-induced platelet activation and aggregation(Reference Block, Abdolahi and Smith37).
Table 2A. Studies indicating ASA and omega-3 PUFA use in CVD: primary objective/outcome, sample size, dose of ASA and fish oil used, EPA and DHA content, and conclusions

Looking at LPC and LCA concentrations, which have been shown to increase risk for CVD, n3 PUFA ingestion for 28 d showed a significant reduction in the corresponding LPA analogues but not the LPC species palmitic acid, oleic acid and arachidonic acid (n = 567)(Reference Abdolahi, Georas and Thomas Brenna38). Further addition of combined dose of ASA to n3 PUFA ingestion for 7 d changed the lysolipid concentration, leading to an enhanced benefit of n3 PUFA on total LPC while slightly weakening the effects from total LPA. The addition of ASA to n3 PUFA led to an overall metabolic shift towards less inflammation. It was speculated that the conversion of LPC to LPA through autotaxin can be altered by n3 PUFA; however, this was demonstrated not to be the case(Reference Block, Duff and Lawrence17,Reference Abdolahi, Georas and Thomas Brenna38) . Serum LPA has also been correlated with acute myocardial infarction, and plasma concentrations of LPA are elevated in patients with cerebrovascular disease compared with healthy patients(Reference Gaire, Sapkota and Song39). Decreasing the concentration of LPA through chronic ASA and n3 PUFA dosing is a beneficial cardioprotective prevention strategy in patients who are deficient in EPA and DHA.
Oxidative stress is another factor in CVD development. A recent study in humans (n = 30) noted that an increase in telomerase activity correlated with a reduction in oxidative stress(Reference Holub, Mousa and Abdolahi40). Interestingly, ASA and n3 PUFA supplementation in combination for 7 d failed to further increase telomerase activity seen in n3 PUFA alone. This report explored the short-term effects of combination therapy on telomerase activity, but the long-term effects on telomerase activity and expression with oxidative stress were not observed. Although this contradicts the consideration of ASA with n3 PUFA use to reduce disease progression, these data also reinforce the importance of EPA and DHA concentrations within the body prior to and during ASA supplementation.
In patients with stable angina pectoris who received a percutaneous coronary intervention and were on dual antiplatelet therapy (n = 40), ASA (75 mg) and clopidogrel, the addition of EPA and DHA (Omacor, currently called Lovaza, 1 g/d) to the regimen for 30 d did not significantly alter any clinical outcomes(Reference Mizia-Stec, Mizia and Haberka41). The addition of these n3 PUFA did not cause an increased risk for bleeding or the narrowing of blood vessels after stent placement. However, because the combination of ASA, clopidogrel and EPA and DHA was only monitored for 1 month, the possible beneficial use of them in combination in these patients was not well established. In a similar study with patients who have coronary artery disease and required percutaneous coronary intervention (n = 54), the addition of n3 PUFA for 28 d changed the structure of the fibrin clots(Reference Gajos, Zalewski and Rostoff42). Participants who received n3 PUFA had clots that were less resistant to lysis. Additionally, with n3 PUFA supplementation, plasma 8-isoprostane concentrations were reduced, which indicated a reduction in dense fibrin clots(Reference Gajos, Zalewski and Rostoff42). n3 PUFA supplementation helped reduce thrombin, which improved the platelet response observed with clopidogrel administration. However, because these patients also received a statin, the effects observed with the altered clots could be a result from ASA, the statin or their combination. In a subset of coronary artery disease patients who were on a statin and ASA, EPA and DHA (4 g/d, Lovaza) or placebo was added. In this Slowing Heart Disease with Lifestyle and n3 Fatty Acid (HEARTS) clinical trial (n = 6), Lovaza ingestion for 1 year showed a restoration of RvD1, RvD2, RvD5 and RvE1 that was lacking in these patients(Reference Elajami, Colas and Dalli43). The restoration of RvD1 and RvD2 led to the prevention of superoxide production and pro-inflammatory gene activation, and the restoration of RvE1 led to decreased platelet aggregation to ADP receptors. Together with ASA-induced SPMs, AT-RvD1, AT-RvD3, AT-PD1 and AT-LXB4 increased macrophage phagocytosis of blood clots by ˜50 %, which leads to thrombus removal and thus prevents atherosclerotic events. This suggests that there is an association between the combined use of ASA and n3 PUFA and its beneficial effects in reducing atherosclerosis in coronary artery disease patients.
The ASCEND (A Study of Cardiovascular Events in Diabetes) trial showed that n3 PUFA (1 g/d, 460 mg EPA and 380 mg DHA) supplementation in diabetic patients (n = 15,341) for 6 months helped reduce serious vascular events or revascularisation regardless of ASA (100 mg) use in combination(Reference Bowman, Mafham and Stevens44,Reference Bowman, Mafham and Wallendszus45) . In the same trial, n3 PUFA alone showed a reduction in vascular death compared with placebo. However, the use of olive oil as the placebo in the ASCEND trial is questionable. Research in the Mediterranean diet that is rich in extra-virgin olive oil on cardiovascular risk has shown that olive oil has cardioprotective effects by reducing oxidative damage and inflammation biomarkers associated with CVD(Reference Hernáez, Sanllorente and Castañer46–Reference Schwingshackl, Christoph and Hoffmann49). As such, the comparison of n3 PUFA and ASA with a placebo that is associated with cardioprotection due to use of olive oil introduced a confounding variable that needs to be addressed in another trial.
In a similar justification, the results of the FAVOURED (Fish Oil and Aspirin in Vascular Access Outcomes in Renal Disease) trial may also be misrepresented because of the use of olive oil as the placebo. Nonetheless, the FAVOURED trial, which measured the usability of an arteriovenous fistula in haemodialysis (n = 567), showed that ASA (100 mg/d) and n3 PUFA (4 g/d, Omacor) supplementation for 1 year in a subset of the study reduced the intervention rates of failure of an arteriovenous fistula by 55 %(Reference Irish, Viecelli and Hawley50,Reference Viecelli, Polkinghorne and Pascoe51) . Combination use did not reach the primary outcomes in reducing overall arteriovenous fistula failure; however, in the secondary outcomes, ASA and n3 PUFA reduced rescue intervention rates. Using the secondary outcomes, the FAVOURED trial recommended 3 months of n3 PUFA supplementation with low-dose ASA to reduce the intervention rates of acute thrombosis in patients who have recently undergone an arteriovenous fistula(Reference Viecelli, Polkinghorne and Pascoe51).
Although both the ASCEND and FAVOURED trials contained large sample sizes (567 and 15 341, respectively), the use of olive oil as the placebo contributes to these results potentially being misleading. Additionally, because the effects of ASA and n3 PUFA depend on the baseline concentration of EPA and DHA, high-risk patients such as patients with diabetes mellitus may have decreased effects from the addition of ASA. The ‘V-shaped’ relationship between ASA and n3 PUFA could also play a role in not having an optimal response to the combination treatment(Reference Block, Abdolahi and Tu34).
ASA and n3 PUFA in colorectal adenomas and cancer
The use of ASA alone in the prevention of colorectal cancer has long been studied and has successfully prevented colorectal cancer through long-lasting COX-1 inhibition(Reference Patrignani, Sacco and Sostres52). In the Aspirin/Folate Polyp Prevention Study (AFPPS, n = 1121), ASA use alone for 3 years showed a reduction in the final metabolites, PGE, PGI and TxB2 secreted into the urine in a dose-dependent manner in adults with colorectal cancer(Reference Fedirko, Bradshaw and Figueiredo53). This shows ASA’s importance and relevance in preventing colorectal cancer. According to a statement provided by the United States Preventive Services Task Force published in 2016, the recommendation made to patients who are at risk for developing colorectal cancer between the ages of 50 and 70 years is to take low-dose ASA (81 mg) daily for 10 years(Reference Chubak, Whitlock and Williams54,Reference Whitlock, Burda and Williams55) . Although the use of ASA in colorectal cancer is well established, data on n3 PUFA use alone are less established. Nonetheless, the suggestion that combination therapy can help prevent colorectal adenomas is plausible and is worth studying.
In a study of 141 patients who were at high risk for developing colorectal adenomas, a subset of patients who were on ASA or non-steroidal anti-inflammatory drugs (NSAIDs) (dose not specified) with EPA and DHA (3 g/d, Lovaza) for 168 d showed significantly reduced PGE2 levels in patients who were not on ASA or NSAIDs(Reference White, Shrubsole and Cai56). However, baseline PGE2 levels in patients who were on ASA or NSAIDs who were also on olive oil as the placebo differed from the treatment group. Although this difference was not statistically significant, the difference may impact the results, suggesting that n3 PUFA alone was more effective in reducing PGE2, with this effect associated with reduced cancer development in patients.
On the contrary, the Seafood Polyp Prevention (seAFOod) trial demonstrated that, in patients with high risk for colorectal adenoma development (n = 709), ASA (300 mg/d enteric coated) and EPA (2 g/d EPA-free fatty acid) in combination led to a numerical decrease in certain types of colorectal adenomas in each individual compared with either ASA or EPA alone or with placebo after 12 months of treatment(Reference Hull, Sandell and Montgomery57,Reference Hull, Sprange and Hepburn58) . Additionally, the seAFOod trial highlighted the significance of ASA and EPA’s role in different subtypes of colorectal cancer. EPA alone was more effective in decreasing left-sided and conventional colorectal adenomas, whereas ASA was more associated with a significant decrease in conventional colorectal adenomas (Table 2B) and a decrease in right-sided and serrated lesions(Reference Hull, Sprange and Hepburn58). Therefore, the combinational use demonstrated chemoprotective effects in multiple locations and multiple colorectal cancer subtypes. Although the primary objective, reduced adenoma detection rate, was not reached with combination therapy, there are some shortfalls associated with using adenoma detection rate. Because the study was conducted using high-quality colonoscopies, this can lead to a higher adenoma detection rate and needs to be confirmed and compared with other studies. In the seAFOod trial, adenoma detection rate could be elevated due to the increased count of colorectal adenomas, leading to more severe disease. Instead, adenoma number is a better measurement for disease severity. Unfortunately, because the seAFOod trial was conducted using EPA and the study did not show the conversion of EPA to DHA in the patients, the generalisation to the combination of both EPA and DHA cannot be completed. Finally, the trial demonstrated that increased EPA concentrations in the rectal mucosa correlated with a lower colorectal adenoma count(Reference Hull, Sprange and Hepburn58). This suggests that, although combination treatment was unable to reduce adenoma detection rate, colorectal adenoma burden was reduced in these higher-risk patients (Table 2B).
Table 2B. The same metrics for studies that indicated ASA alone or ASA and omega-3 PUFA use in colorectal adenomas/cancer

ASA, acetylsalicylic acid, or aspirin; PUFA, polyunsaturated fatty acid; CVD, cardiovascular disease; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; MCP-1, monocyte chemoattractant protein-1; SPM, specialised pro-resolving mediators; HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; n3, omega 3; TxB2, thromboxane B2; LPC, lysophosphatidylcholine; LPA, lysophosphatidic acid; PCI, percutaneous coronary intervention; CAD, coronary artery disease; RvD, D-series resolvins; RvE, E-series resolvins; AVF, arteriovenous fistula; HD, haemodialysis.
* Values were normalised, and the doses given are their equivalent in humans.
** EPA and DHA content were determined experimentally; the used values are averages.
† EPA and DHA content were determined experimentally; the used values are averages.
‡ Patients in these trials were also on clopidogrel.
§ Patients in these trials were also on clopidogrel and a statin.
Future studies in colorectal cancer require the testing of ASA with both EPA and DHA to determine the complex interplay between these two compounds.
Role of ASA dose in CVD and colorectal adenoma
Research in ASA and EPA and DHA use in combination identified the importance of their concentrations on the function and effectiveness of ASA in preventing CVD and colorectal adenoma. As such, the dose-dependent nature of ASA in CVD and colorectal adenoma must be explored.
A study published by Chiang and colleagues of 128 patients showed that low-dose ASA (81 mg) compared with higher doses of ASA (325 mg or 650 mg) for 56 d did not make a significant difference in altering TxB2 concentrations(Reference Chiang, Bermudez and Ridker14). When compared with placebo, all doses of ASA significantly reduced TxB2, and a statistically significant increase in the ASA-triggered eicosanoid, 15-epi-lipoxin A4, occurred with the 81 mg dose but not with the higher doses. Additionally, when comparing between the groups, there was a levelling-off effect seen in TxB2 and 15-epi-lipoxin A4 with doses greater than 81 mg.
The efficacy and safety of ultra-low-dose ASA (<81 mg) use in CVD and colorectal adenomas have yet to be studied. Nonetheless, it has been well documented that 30 mg of ASA inhibits the production of TxA2 in platelets to the same extent as higher doses of ASA (300 mg) by the cumulative effects on COX-1(Reference van Gijn59). In a study of different doses of ASA (30 mg versus 283 mg), there was no difference in efficacy on death from vascular causes, non-fatal stroke and non-fatal myocardial infarction after a mean final follow-up of 31 months(Reference van Gijn59). Additionally, the 30 mg dose showed a lower incidence of minor bleeding and gastrointestinal discomfort. Because ASA is an irreversible inhibitor of COX-1 in platelets, once maximal platelet inhibition is reached, lower doses could be used to establish a similar effect with reduced side effects. Thus, it is recommended that a loading dose (120 mg) be used to reach maximum inhibition and that doses as low as 30 mg be used daily for the prevention of CVD and thrombosis(Reference van Gijn59). Although the study only monitored the patient up to 31 months on ultra-low-dose ASA, 81 mg of ASA have been used prophylactically for longer durations; thus, it can be reasonable to use ultra-low-dose ASA for longer than 31 months.
In the studies that explored the use of ASA with n3 PUFA, in general, low doses of 75–100 mg were used. Both the ASCEND and FAVOURED trials used 100 mg ASA with 1 g and 4 g of n3 PUFA, respectively. The studies did not indicate any increased risk for gastrointestinal side effects or bleeding with combination treatment compared with placebo(Reference Bowman, Mafham and Wallendszus45,Reference Viecelli, Polkinghorne and Pascoe51) . Higher doses of ASA are only associated with the additional impairment of PGE2- and PGI2-mediated gastrointestinal protective effects, leading to more side effects(Reference Patrono, Coller and FitzGerald60). As previously mentioned in Block and colleague’s research, because the additional effects of ASA on CVD prevention are dependent on baseline EPA and DHA concentrations, the use of 81 mg ASA must be considered when analysing the results with concurrent EPA and DHA supplementation(Reference Block, Abdolahi and Tu34). Nonetheless, the analysed literature on ASA and n3 PUFA use suggests the potential benefits of ultra-low-dose ASA under the circumstance that baseline EPA and DHA concentrations are achieved.
A meta-analysis that looked at ASA use in four key trials of patients with colorectal adenoma included the Aspirin/Folate Polyp Prevention Study (AFPPS, n = 1121), the Colorectal Adenoma Prevention Study (CALGB, n = 635), the United Kingdom Colorectal Adenoma Prevention (ukCAP, n = 939) Study, and the Association pour la Prevention par l’Aspirine du Cancer Colorectal (APACC, n = 272) Study. They showed that either lower-dose ASA (81 mg or 160 mg) or higher-dose ASA (300 or 325 mg) compared with placebo for 3 years did not have a significant difference in effects on adenomas (Table 2B)(Reference Benamouzig, Deyra and Martin2,Reference Baron, Cole and Sandler61–Reference Sandler, Halabi and Baron64) . In a direct comparison between low and high ASA dose, there was a statistically significant reduction in adenomas with the lower-dose ASA. However, higher doses of ASA were shown result in greater reduction in advanced lesions for patients who are at higher risk. Thus, low-dose ASA could be beneficial in preventing colorectal adenoma development in lower-risk individuals.
Interestingly, in the seAFOod trial, a higher dose of ASA (300 mg, enteric coated) was used and still showed efficacy in reducing the number of certain types of colorectal adenomas(Reference Hull, Sprange and Hepburn58). The use of a 300 mg dose in the seAFOod trial could be justified due to the patients having a higher risk for colorectal adenoma development. Nonetheless, based on results from the earlier clinical studies, perhaps a lower dose could be used to explore the additional effects of ASA with EPA supplementation in colorectal cancer (Table 2B).
Although the included studies show that lower doses of ASA with EPA and DHA supplementation could prevent CVD, and ASA with EPA supplementation could prevent colorectal adenomas, because there are no recent studies that explored the use of ultra-low-dose ASA, older studies were included to explore such a possibility, which is a weakness in this narrative review.
Role of EPA-to.DHA ratio in CVD and colorectal adenoma
n3 PUFA consists of both EPA and DHA, with a wide variety of downstream lipid mediators and SPM. Although the studies described earlier generally used a combination of EPA and DHA, the specific effects of EPA alone and DHA alone were not explored.
In the Comparing EPA to DHA (ComparED) trial (n = 154), high doses of EPA (2·7 g) or DHA (2·7 g) were used in patients with abdominal obesity for 70 d to determine the effects EPA and DHA separately on lipid levels and inflammatory markers(Reference Allaire, Couture and Leclerc65–Reference Vors, Allaire and Marin67).
Considering lipid characteristics, EPA when compared with placebo significantly increased LDL-C concentrations and decreased plasma triacylglycerol(Reference Allaire, Couture and Leclerc65). EPA also increased VLDL apoB100 catabolism and significantly decreased LDL apoB100 when compared with DHA(Reference Allaire, Vors and Tremblay66). DHA tended to increase the mean LDL particle size and the LDL peak particle size more than EPA(Reference Allaire, Vors and Tremblay66). DHA, when compared with placebo, significantly increased total cholesterol, LDL-C, total apoB and HDL-C while reducing serum triacylglycerol and the cholesterol-to-HDL-C ratio(Reference Allaire, Vors and Tremblay66). VLDL apoCIII was also greater after DHA supplementation when compared with EPA. Although PCSK9 concentrations were similar after EPA or DHA supplementation compared with placebo, the correlation with the concentrations of PCSK9, LDL-C and LDL apoB100 were different between the n3 PUFA(Reference Vors, Allaire and Marin67). Overall, triacylglycerol and LDL-C were increased more after DHA supplementation(Reference Allaire, Couture and Leclerc65,Reference Allaire, Vors and Tremblay66) . This was accomplished by reducing the production of VLDL apoB100 and increasing the conversion of VLDL to LDL apoB100, showing a net increase in LDL apoB100 concentrations. Supplementation with high-dose EPA showed an increase in VLDL apoCIII concentrations, whereas DHA tended to decrease apoCIII-containing lipoprotein concentrations when compared with the control(Reference Allaire, Vors and Tremblay66). This was speculated to be a cause for the increased conversion of VLDL to LDL apoB100 observed with larger LDL particles with DHA supplementation. A previous review demonstrated that small and dense LDL was correlated with an increased risk for myocardial infarction and CVD compared with patients with large LDL particles(Reference Hirayama and Miida68). The opposite effects of DHA on LDL-C and LDL particle size could potentially lower CVD risk. PCSK9 has also been shown to be involved in differential effects from DHA and EPA supplementation on the metabolic pathway of LDL due to its positive correlation with LDL apoB100 after supplementation with DHA but not with EPA(Reference Allaire, Vors and Tremblay66). Nonetheless, it can be hypothesised that DHA might have more potential in lowering CVD risk than EPA.
By comparing EPA and DHA, DHA alone had a greater increase in the Omega-3 Index as compared with EPA alone, suggesting a greater impact in lowering the risk for coronary heart disease and mortality. An increase of the Omega-3 Index negatively correlated with changes in triacylglycerol and positively correlated with LDL-C after DHA use only. The supplementation of EPA led to an up-regulation of ELOVL2 (elongation of very-long-chain fatty acids-like 2 gene), which is responsible for the conversion of EPA to docosapentaenoic acid (DPA)(Reference Allaire, Harris and Vors69). Although DPA concentrations are not traditionally considered in measuring Omega-3 Index, higher DPA levels have been correlated with lowering triacylglycerol and C-reactive protein concentrations, which could also contribute to cardioprotective effects seen for n3 PUFA. Further exploration is needed to consider DPA effects with EPA and DHA in CVD risk reduction.
Men tend to have a higher Omega-3 Index after EPA and DHA supplementation, and women accumulate more EPA in their erythrocytes after supplementation with EPA but not with DHA(Reference Allaire, Harris and Vors69). DHA supplementation increased LDL-C more than EPA in men but not in women(Reference Allaire, Couture and Leclerc65). Additionally, EPA and DHA supplementation activates PPARα in both sexes, but NFκB was only activated in men, which suggests different responses to n3 PUFA in different sexes(Reference Allaire, Couture and Leclerc65). However, this effect was not seen to impact clinical results in the studies described earlier, which might require further investigation.
Because DHA supplementation can increase LDL-C concentrations, in theory, DHA can lead to an increased risk for atherosclerotic plaque development. However, the effects of DHA in modulating inflammatory markers and HDL-C makes the generalisation of DHA on CVD difficult. Additionally, the ComparED trial included the use of maize oil as the placebo(Reference Allaire, Couture and Leclerc65–Reference Vors, Allaire and Marin67). Although maize oil demonstrated a decrease in total cholesterol and LDL-C that could have blunted the effects of EPA and DHA in comparison on study outcomes, the authors noted that the use of maize oil as placebo did not alter the outcomes. With these considerations, additional studies are needed to understand the role of DHA in CVD.
In a study on effects of EPA (3 g/d EPA) and DHA (3 g/d DHA) concentrations on blood pressure and sympathetic outflow (n = 90) after 84 d of treatment, DHA was shown to be responsible for reducing resting blood pressure and increasing muscle sympathetic outflow(Reference Lee, Notay and Klingel70). This is speculated to be a result of the arterial baroreflex arc that prevents reduction in prefusion pressure in normotensive patients. Previous research has also demonstrated the ability of DHA to reduce heart rate and total peripheral resistance when compared with EPA(Reference Mori, Bao and Burke71,Reference Rontoyanni, Hall and Pombo-Rodrigues72) .
In a subsequent DEFAT trial (n = 87) that looked at EPA (1·8 g divided over three doses EPA, Epadel) alone with EPA and DHA (2 g/d, Lotliga containing 0·93 g EPA and 0·75 g DHA) for 3 years showed that EPA and DHA were more beneficial in lowering triacylglycerol, arachidonic acid, remnant-like particle cholesterol, oxidised-LDL, cystatin-C, LDL and LDL-to-HDL ratio while increasing HDL when compared with EPA alone(Reference Sezai, Unosawa and Taoka3). However, the results from this trial could be skewed due to compliance issues in the EPA alone arm. This was because Epadel was dosed three times daily, whereas Lotliga was dosed once daily. Nonetheless, EPA and DHA showed more beneficial reductions in oxidative stress and increased CVD prevention than EPA alone.
The role of EPA or DHA with ASA showed that the effects of ASA on HDL-C and HDL ApoA1 exchange depended on DHA concentrations and not on EPA(Reference Block, Holub and Abdolahi73). Because ApoA1 exchange is critical in generating HDL responsible for cardioprotection, beneficial effects from ASA on CVD have, thus, been shown as dependent on having moderate plasma DHA concentration. This was not the case for EPA. In previous studies that looked at ASA with n3 PUFA in CVD, generally, EPA doses were higher than DHA doses. This was the case because FDA-approved n3-PUFA-containing products had more EPA per DHA. The outcomes of these studies could be enhanced if a lower EPA-to-DHA ratio was used. The evidence from the ComparED, DEFAT trials and subsequent studies suggest that DHA could be critical in CVD prevention, anti-inflammatory and blood-pressure-lowering effects.
In colorectal adenomas, the effect of the ratio of EPA and DHA on the reduction of adenomas has not been studied in detail. The seAFOod trial did not use DHA and only considered EPA alone in its findings; thus, the use of DHA in colorectal adenoma prevention cannot be recommended due to the lack of data(Reference Hull, Sandell and Montgomery57,Reference Hull, Sprange and Hepburn58) . Future studies looking into the effects of DHA alone and combined with n3 PUFA are needed to have a concrete recommendation for EPA and DHA use in combination with ASA in colorectal adenoma prevention.
Future perspective on ASA and EPA and DHA use
Current literature in ASA and EPA and DHA use in combination leads to many conclusions that suggest a possibility for combinational use. However, there are still many points to be considered. Assuming that ASA and these n3 PUFA could be used in combination, a possible formulation of a water-soluble ASA and a fat-soluble EPA and DHA must be considered. This is because medication adherence in these patients is critical and a combination dosage form can increase the patient’s willingness to take the medication to improve their outcomes. Recent advances in nano-pharmaceuticals and liposomal formulations suggest having ASA inside a shell of EPA and DHA to allow for the combination dose to be administered. A recent study published by Anuar and colleagues demonstrated the possibility for developing a nano-emulsion with ibuprofen to enhance the bioavailability of ibuprofen. The combination of olive oil, a surfactant and glycerol were identified as a possible mechanism in drug delivery(Reference Anuar, Sabri and Bustami Effendi74). Although there are no studies that suggest the use of EPA and DHA to replace olive oil in such a mechanism of drug delivery, perhaps further research can demonstrate the feasibility of this approach.
In an in vitro proof-of-concept study in HT-29 colorectal cancer cell lines, the use of DHA and α-linolenic acid (another n3 PUFA) encapsulated nanoparticles increased the incorporation into the cell lines, which demonstrates enhanced antineoplastic activity(Reference Serini, Cassano and Corsetto75). Although this technology is relatively new, this shows the possibility to enrich colonic cells with α-linolenic acid that in turn might increase the concentrations of EPA and DHA in individuals with colorectal cancer. This result provides a promising outlook in the use of n3 PUFA along with ASA to prevent colorectal adenomas and cancer.
Conclusions
Although there has been an increase in research focused on the combination of ASA with n3 PUFA in both CVD and colorectal adenomas and cancer, there are still controversial data regarding the use of such a combination in healthy adults. Results from currently published literature suggest a benefit with the combination of ASA and n3 PUFA in individuals who have diabetes mellitus or are at risk for severe CVD. Additionally, patients who are ASA resistant, or have low baseline EPA and DHA concentrations, should supplement with EPA and DHA to increase the effectiveness of ASA in combination as well as increase SPM that are responsible for resolving inflammation. The effects of ASA and EPA and DHA are not linear in relationship but rather are ‘V-shaped’ in some studies and require more research to understand the optimised doses of ASA and EPA and DHA. Our current understanding of ASA dose and the levels of EPA and DHA suggests that an ultra-low dose of ASA (30 mg/d) daily is beneficial in CVD and that DHA provides a more substantial effect in reducing inflammation compared with EPA. The combination of ultra-low-dose ASA with EPA and DHA must be researched to determine if such a combination is more optimal than the current standard.
In colorectal cancer, ASA and n3 PUFA (mainly EPA) have been shown to reduce colorectal adenoma burden based on adenoma location and subtype. Future studies that explore the relationship between ASA dose and DHA are required to understand if the beneficial effects of DHA seen in CVD can also be beneficial in colorectal cancer. If ASA and EPA and DHA can be beneficial in both CVD and colorectal adenoma prevention, a formulation for water-soluble ASA and fat-soluble n3 PUFA are required to increase adherence in these patients.
Acknowledgements
The authors thank Dr. Kelly A. Keating (Pharmaceutical Research Institute, ACPHS) for editing the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Authorship
Ivan E. Wang, planned, researched and wrote the article; Shana Yi, reviewed and added the role of ASA dose in CVD and colorectal adenomas; Robert C. Block, reviewed and supplemented with his articles in the development of the article; Shaker A. Mousa, planned, revised, reviewed and mentored the development of the article.