Article contents
Stimulation Electrodes Based on MnO2 Thin Films: Electrical Properties in Carbonate Buffered Saline
Published online by Cambridge University Press: 22 February 2011
Abstract
Hydrous manganese dioxide films are being investigated as a new redox material for the active surface of intracortical stimulation electrodes. The films are electrodeposited at room temperature on Pt substrates from a neutral solution of manganese acetate. The electrochemical properties of the films have been evaluated by cyclic voltammetry in neutral saline solutions buffered with bicarbonate. The films undergo a reversible charge injection reaction represented as
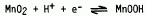
The results obtained in electrolytes of different buffer concentrations indicate the film is able to store the most charge in solutions having the lowest buffer capacity. The kinetics of the redox reaction thus favor operation in an acidic or basic environment in agreement with earlier published results. Two different mechanisms are proposed to explain the rate enhancement at the different pH's. In acidic electrolytes the reaction proceeds by dissolution of the film, while in alkaline solutions the rate determining step of proton diffusion in the solid film is increased. For neural stimulation, therefore, electrodes with manganese dioxide films should be pulsed cathodically to insure a neutral or alkaline environment around the electrode and minimize dissolution of the film.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1986
References
REFERENCES
- 2
- Cited by




