No CrossRef data available.
Article contents
Solid-State Reactions in Model Oxide Systems
Published online by Cambridge University Press: 15 February 2011
Abstract
The kinetics of thin-film solid-state reactions have been investigated in two model spinel forming oxide systems, NiO/Al2O3 and MgO/Fe2O3. In the NiO/Al2O3 system, thin-films of epitactic NiO were reacted with (0001), 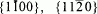 , and
, and  orientated Al2O3 (corundum). The kinetics of the spinel forming reaction for this system were found to be linear-parabolic in nature. Additionally, it was found that the kinetics of the spinel-forming reaction varied by nearly two orders of magnitude between the fastest and slowest diffusion couples. The substrate determines the orientation of the overlayers and thereby the structure of the phase boundaries. In the MgO/Fe-oxide system, thin films of epitactic Fe oxide were reacted with {001} MgO. The kinetics of this spinel forming reaction were parabolic in nature, indicative of diffusion control. In contrast to the N1O/Al2O3 system, the movement of phase boundaries are not the step controlling the reaction rate, but rather the diffusion of one of the cations across the reaction layer. In comparing the reaction rates for the two systems the activation energy for the formation of the spinel product in the MgO/Fe-oxide system was found to be almost a factor of 4 lower in comparison to the NiO/Al2O3 system.
orientated Al2O3 (corundum). The kinetics of the spinel forming reaction for this system were found to be linear-parabolic in nature. Additionally, it was found that the kinetics of the spinel-forming reaction varied by nearly two orders of magnitude between the fastest and slowest diffusion couples. The substrate determines the orientation of the overlayers and thereby the structure of the phase boundaries. In the MgO/Fe-oxide system, thin films of epitactic Fe oxide were reacted with {001} MgO. The kinetics of this spinel forming reaction were parabolic in nature, indicative of diffusion control. In contrast to the N1O/Al2O3 system, the movement of phase boundaries are not the step controlling the reaction rate, but rather the diffusion of one of the cations across the reaction layer. In comparing the reaction rates for the two systems the activation energy for the formation of the spinel product in the MgO/Fe-oxide system was found to be almost a factor of 4 lower in comparison to the NiO/Al2O3 system.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1997


