Article contents
Selective immobilization of bacterial light-harvesting proteins and their photoelectric responses
Published online by Cambridge University Press: 17 August 2018
Abstract
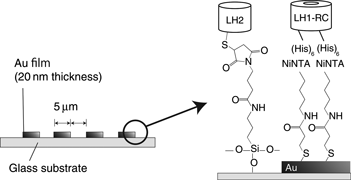
With the aim of understanding the excitation energy transfer mechanism in natural photosynthetic membranes, light-harvesting (LH)2 and LH1-reaction center, which are pigment-protein complexes separated from Rhodobacter sphaeroides, were aligned on a planar electrode surface in stripe patterns at 5 µm intervals. Observation of the absorption spectrum and fluorescence microphotographs revealed selective immobilization and conservation of the pigments. Photocurrent signals were obtained when the electrode was illuminated at either 880 or 800 nm. The fabricated structure was confirmed to function as a natural photosynthetic membrane with the highest photocurrent signal being obtained when using a co-immobilized substrate under excitation at 800 nm.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 1
- Cited by





