Article contents
Improvement of power–energy characteristic of the lithium-ion capacitor by structure modification of the graphite anode
Published online by Cambridge University Press: 08 May 2017
Abstract
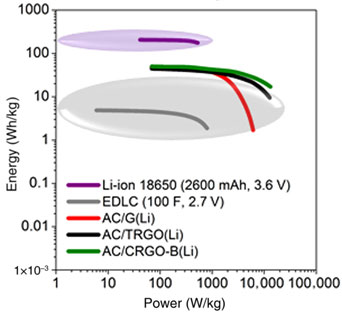
Lithium-ion capacitors utilizing graphite (G) and reduced graphite oxide (RGO) as negative electrode materials were investigated. Various reduction methods of graphite oxide were applied to compare properties of modified materials. The hybrid cells showed the energy density at mild current regimes of ca. 70–100 Wh/kg. However, at higher rates, only the capacitor with chemically RGO maintained this tendency. Moreover, the system demonstrated the energy density of 34 Wh/kg at a power density of 26 kW/kg. The electrochemical measurements were conducted in three-electrode systems to record responses of positive and negative electrodes separately.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 2
- Cited by



