Article contents
Impact of angular deviation from coincidence site lattice grain boundaries on hydrogen segregation and diffusion in α-iron
Published online by Cambridge University Press: 29 August 2018
Abstract
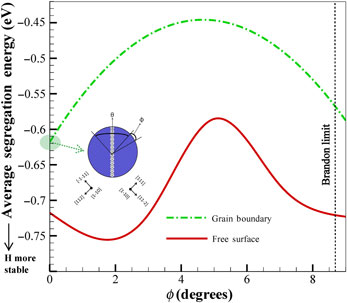
Coincidence site lattice (CSL) grain boundaries (GBs) are believed to be low-energy, resistant to intergranular fracture, as well as to hydrogen embrittlement. Nevertheless, the behavior of CSL-GBs are generally confused with their angular deviations. In the current study, the effect of angular deviation from the perfect  $\Sigma 3(111)[1\bar 10]$ GBs in α-iron on the hydrogen diffusion and the susceptibility of the GB to hydrogen embrittlement is investigated through molecular static and dynamics simulations. By utilizing Rice–Wang model, it is shown that the ideal GB shows the highest resistance to decohesion below the hydrogen saturation limit. Finally, the hydrogen diffusivity along the ideal GB is observed to be the highest.
$\Sigma 3(111)[1\bar 10]$ GBs in α-iron on the hydrogen diffusion and the susceptibility of the GB to hydrogen embrittlement is investigated through molecular static and dynamics simulations. By utilizing Rice–Wang model, it is shown that the ideal GB shows the highest resistance to decohesion below the hydrogen saturation limit. Finally, the hydrogen diffusivity along the ideal GB is observed to be the highest.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 4
- Cited by





